Myelination is an essential feature of the vertebrate nervous system that provides electrical insulation to axons, thereby facilitating the transmission of nerve impulses. Deficiencies in myelination in diseases such as multiple sclerosis (MS) lead to serious neurological disorders. Most MS patients initially exhibit a relapsing-remitting disease course that eventually converts to a secondary progressive form of the disease with incomplete recovery. Therefore, it is important to elucidate not only the causal reason for failure to remyelinate, but also the intrinsic regulatory mechanism(s) underlying successful myelination in the central nervous system (CNS).
Dysregulated protein tyrosine phosphorylation has been implicated in the development of a large number of human diseases, including cancer, diabetes, and inflammation. Protein tyrosine phosphorylation is dynamically regulated by two enzyme superfamilies: protein tyrosine kinases (PTKs) that catalyze the addition of phosphate, and protein tyrosine phosphatases (PTPs) that remove phosphate groups from substrates. Oligodendrocyte precursor cells (OPCs) are the principal source of myelinating oligodendrocytes. Several lines of evidence have indicated that protein tyrosine phosphorylation is involved in the signal transduction pathway leading to the differentiation of OPCs to oligodendrocytes and myelin formation.
The Src PTK family member, FYN kinase is the most prominent family member involved in oligodendrocyte differentiation and myelin formation. Fyn-knockout mice exhibit marked hypomyelination in the brain, and FYN activity is up-regulated during oligodendrocyte differentiation. FYN induces the tyrosine phosphorylation of p190RhoGAP, a GTPase-activating protein (GAP) for Rho GTPase, thereby suppressing Rho/ROCK signaling and leading to the maturation of oligodendrocytes and myelination (Wolf et al., 2001). These findings suggest that the down-regulation of PTP activity for p190RhoGAP promotes oligodendrocyte differentiation and myelination or remyelination in demyelinating lesions.
One such candidate is protein tyrosine phosphatase receptor type Z (PTPRZ) (also called PTPζ), a receptor-type PTP (RPTP), which is the most abundant RPTP molecule in A2B5-positive human white matter progenitor cells (WMPCs). A previous study reported that a lentiviral short-hairpin RNA for PTPRZ abolished the expansion of undifferentiated WMPCs and promoted their oligodendrocyte differentiation in cell cultures (Sim et al., 2006). Three isoforms have been generated by alternative splicing from the single PTPRZ gene: two transmembrane isoforms, PTPRZ-A and PTPRZ-B, and the secretory isoform PTPRZ-S (this isoform is also known as phosphacan) (Figure 1A). The three isoforms expressed in the CNS are highly glycosylated by chondroitin sulfate (Chow et al., 2008). Regarding downstream signaling pathways, we developed a genetic method to screen for PTP substrates named the “yeast substrate-trapping system” based on the yeast two-hybrid system and successfully identified several substrates for PTPRZ (Kawachi et al., 2001). Among these molecules, we found that PTPRZ preferentially dephosphorylates the consensus sequence motif, E/D-E/D-E/D-X-I/V-pY-X (X is not an acidic residue) in its physiologically relevant substrates (Fujikawa et al., 2011), such as p190RhoGAP at Y1105, paxillin at Y118, G protein-coupled receptor kinase-interactor 1 (GIT1) at Y554, and membrane-associated guanylate kinase WW and PDZ domain-containing 1 (MAGI1) at Y373. PTPRZ receptor proteins undergo metalloproteinase- and γ-secretase-mediated proteolytic processing on the cell surface, and are ultimately converted to the cytoplasmic PTPRZ fragment (Z-ICF) (Chow et al., 2008). Very recently, we found that Z-ICF is highly detected in rat C6 glioblastoma cells, and the catalytic activity is associated with the malignancy of the glioblastoma cells (Fujikawa et al., 2016). In peripheral tissues, gastric mucosal cells also express a nonproteoglycan form of PTPRZ-B though at lower levels, where PTPRZ functions as the receptor of VacA, a cytotoxin secreted by Helicobacter pylori for gastric ulcer (Fujikawa et al., 2003).
Figure 1.
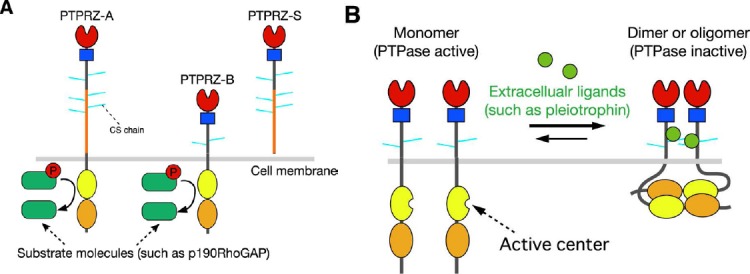
Protein tyrosine phosphatase receptor-type Z (PTPRZ).
(A) Schematic representation of PTPRZ isoforms. The three isoforms expressed in the central nervous system (CNS) are highly glycosylated in the extracellular region by chondroitin sulfate (CS) chains. Domains of the core proteins are highlighted in different colors: CAH (red), carbonic anhydrase-like domain; FNIII (blue), fibronectin type III domain; PTP-D1 (yellow) and PTP-D2 (orange), protein tyrosine phosphatase domain. The catalytic activity is retained in the membrane-proximal PTP-D1, but not in the distal PTP-D2. (B) Ligand-induced dimerization model of PTPRZ. In this model, receptors exist in equilibrium between monomer and dimer conformations. Binding of extracellular ligands including pleiotrophin, midkine, and interleukin-34 induces dimerization (or olimerization) of the receptors, thereby causing the inactivation of the cytoplasmic enzyme. The CS moiety of PTPRZ is important for achieving the high-affinity binding of pleiotrophinto PTPRZ (Maeda et al., 1996; Chow et al., 2008; Kuboyama et al., 2015).
We recently revealed that PTPRZ functioned negatively in FYN-p190RhoGAP signaling by investigating Ptprz-deficient mice in which the first exon of the Ptprz gene is replaced by LacZ, being completely null for the expression of all PTPRZ isoforms (Kuboyama et al., 2012, 2015). In Ptprz-deficient mice, the amounts of myelin basic protein (MBP) and myelinated axons in the brain were significantly larger than those in wild-type animals at postnatal day 10 when myelination occurs, but were similar at 3 months, suggesting a suppressive role for PTPRZ in myelination during CNS development (Kuboyama et al., 2012). Consistent with the earlier onset of CNS myelination, the proportion of OPCs (NG2 proteoglycan-positive cells) in primary cultures obtained from the Ptprz-deficient newborn mouse brain was lower on in vitro day 6 than that in the wild-type control, while that of MBP-positive oligodendrocytes was markedly higher (Kuboyama et al., 2012).
Two animal disease models have been widely accepted for studying the clinical and pathological features of MS lesions. Experimental autoimmune encephalomyelitis (EAE) is a T-cell-mediated inflammatory CNS demyelination model that is generated by immunization with the myelin/oligodendrocyte glycoprotein (MOG), and the cuprizone model of demyelination is induced, particularly in the corpus callosum in the brain, by a T-cell-independent mechanism through feeding of the copper chelator cuprizone. Adult Ptprz-deficient mice were less susceptible to the induction of EAE than wild-type controls (Kuboyama et al., 2012), though the number of T-cells and macrophages/microglia infiltrating the spinal cord did not differ between the two genotypes. Furthermore, cuprizone-fed Ptprz-deficient mice exhibited severe demyelination and axonal damage in the corpus callosum, similar to wild-type mice, whereas remyelination in Ptprz-deficient mice after cuprizone-induced demyelination was significantly accelerated (Kuboyama et al., 2015). Accelerated remyelination was attributed to the higher differentiation potential of OPCs because the number of oligodendrocyte-lineage cells recruited to the demyelinated area was not altered (Kuboyama et al., 2015).
We and others previously identified growth factors such as pleiotrophin/heparin-binding growth-associated molecule (HB-GAM), midkine, and interleukin-34 as inhibitory ligands for PTPRZ (Figure 1B: Maeda et al., 1996; Fukada et al., 2006; Kuboyama et al., 2015). Among these ligands, only the expression of pleiotrophin was found to be induced in the brain upon demyelination, and gradually disappeared with remyelination (Kuboyama et al., 2015). Pleiotrophin was detected in affected cortex neurons and their axon fibers in the cuprizone model. Since pleiotrophin colocalized with the synaptic vesicle marker, synapsin 1, the relevant neurons are presumed to release pleiotrophin from their demyelinated axons. The treatment of a primary culture of wild-type mouse brain cells with pleiotrophin did not induce oligodendrocyte maturation by itself, but enhanced thyroid hormone-induced oligodendrocyte differentiation (Kuboyama et al., 2015). Of note, the differentiation of Ptprz-deficient cells was not further potentiated by pleiotrophin (Kuboyama et al., 2015). Pleiotrophin released from demyelinated fibers in vivo may stimulate the differentiation of OPCs recruited in the demyelinated area in a PTPRZ-dependent manner.
We established immature oligodendrocytes (OL1 cells) from p53-knocout mice. They were strongly positive for the two receptor isoforms of PTPRZ-A and PTPRZ-B with chondroitin sulfate chains; however, their expression gradually decreased with differentiation, with only PTPRZ-B being weakly detectable in mature oligodendrocytes (Kuboyama et al., 2015). The chondroitin sulfate moiety of PTPRZ is known to be essential for achieving the high-affinity binding of its ligands, pleiotrophinand midkine (Maeda et al., 1996). Pleiotrophin binding leads to the inactivation of the intracellular catalytic activity of PTPRZ by inducing receptor dimerization or oligomerization (Figure 1B: Fukada et al., 2006; Kuboyama et al., 2015). Consistent with these findings, the treatment of immature OL1 cells with pleiotrophin enhanced the phosphorylation of p190 RhoGAP at Y1105 (Kuboyama et al., 2015). We found that pleiotrophin reduced the expression of NG2 proteins in OL1 cells, and resultantly enhanced thyroid hormone-induced differentiation to oligodendrocytes. Therefore, it is conceivable that the catalytic activity of PTPRZ functions to maintain OPCs in an undifferentiated state, and the pleiotrophin-induced inactivation of PTPRZ releases this blockage (Kuboyama et al., 2015). Although the expression of extracellular ligands for PTPRZ other than pleiotrophin did not increase in the brain in the cuprizone demyelination model (Kuboyama et al., 2015), we found that midkine and interleukin-34 also enhanced OL1 differentiation in vitro, similar to pleiotrophin (unpublished observations).
First-line immunomodulatory treatments with interferon β and glatiramer acetate are the most common methods by which to reduce the frequency of relapses and slow the progression of the disabilities associated with MS. A recently approved therapy for relapsing MS is Fingolimod, the first oral sphingosine 1-phosphate receptor modulator to prevent the migration of selected lymphocyte subsets into CNS tissues (Figure 2; Brinkmann et al., 2010). These therapies effectively control CNS inflammation in patients with MS; however, none are effective against the chronic progressive process. OPCs are still present at the demyelinated sites of MS patients, even at the progressive stage, but fail to differentiate. Therefore, therapeutic compounds that enhance remyelination from this quiescent OPC population are anticipated. The rationale for this concept has been demonstrated by improvements in the recovery outcomes of demyelinating mouse models with the administration of some agents that promote OPC differentiation, including an antibody for LINGO-1, which is a negative regulator of FYN kinase in OPCs (Mi et al., 2007).
Figure 2.
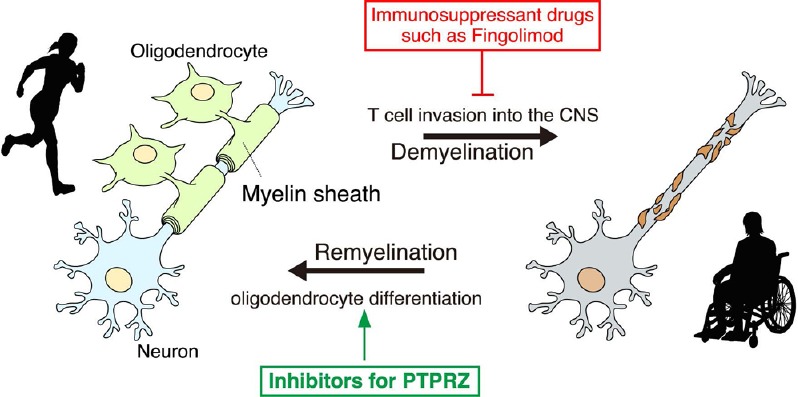
Protein tyrosine phosphatase receptor-type Z (PTPRZ) inhibitors are potential remyelination drugs.
The inhibition of PTPRZ stimulates the differentiation of oligodendrocyte precursor cells (OPCs) in the lesioned area and promotes remyelination. Therefore, the development of inhibitory compounds of PTPRZ enzymatic activity is promising and anticipated. For details, see the text.
Unfortunately, endogenous inhibitory ligands such as midkine reportedly trigger relapses of EAE by increasing the number of autoreactive T-helper cells, possibly by activating its other receptor molecules, such as anaplastic lymphoma kinase, integrins, or low-density lipoprotein receptor-related proteins (Kadomatsu et al., 2013). Of note, serum levels of midkine are reportedly lower in MS patients than in healthy controls (Shaygannejad et al., 2014). The risk of the side effects of pleiotrophin and midkine to induce autoreactive T-cell responses may be reduced by their coadministration with immunosuppressant drugs such as Fingolimod and Natalizumab (Shaygannejad et al., 2014). However, the development of selective inhibitors (chemical drugs) for the catalytic PTP domain of PTPRZ may be a more plausible and effective therapeutic strategy for demyelinating diseases (Figure 2).
As one of the more ambitious future strategies, the transplantation of human stem cells (such as induced pluripotent stem cells, iPSCs)-derived OPCs may have potential applicability to MS patients for remyelination. However, transplanted OPCs may not fully differentiate because of the difficulty of their survival and deficiency in inhibitory ligands for PTPRZ in MS patients. Therefore, the pretreatment of iPSC-derived OPCs with an extracellular PTPRZ ligand such as pleiotrophin prior to transplantation may be useful for stimulating the differentiation of OPCs to oligodendrocytes.
References
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Chow JP, Fujikawa A, Shimizu H, Suzuki R, Noda M. Metalloproteinase- and gamma-secretase-mediated cleavage of protein-tyrosine phosphatase receptor type Z. J Biol Chem. 2008;283:30879–30889. doi: 10.1074/jbc.M802976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa A, Fukada M, Makioka Y, Suzuki R, Chow JP, Matsumoto M, Noda M. Consensus substrate sequence for protein-tyrosine phosphatase receptor type Z. J Biol Chem. 2011;286:37137–37146. doi: 10.1074/jbc.M111.270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa A, Nagahira A, Sugawara H, Ishii K, Imajo S, Matsumoto M, Kuboyama K, Suzuki R, Tanga N, Noda M, Uchiyama S, Tomoo T, Ogata A, Masumura M, Noda M. Small-molecule inhibition of PTPRZ reduces tumor growth in a rat model of glioblastoma. Sci Rep. 2016;6:20473. doi: 10.1038/srep20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, Fukamachi H, Noda M. Protein tyrosine phosphatase receptor type Z deficient mice are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- Fukada M, Fujikawa A, Chow JP, Ikematsu S, Sakuma S, Noda M. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006;580:4051–4056. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kadomatsu K, Kishida S, Tsubota S. The heparin-binding growth factor midkine: the biological activities and candidate receptors. J Bio Chem. 2013;153:511–521. doi: 10.1093/jb/mvt035. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Fujikawa A, Maeda N, Noda M. Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase by the yeast substrate-trapping system. Proc Natl Acad Sci U S A. 2001;98:6593–6598. doi: 10.1073/pnas.041608698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Suzuki R, Noda M. Inactivation of protein tyrosine phosphatase receptor type Z by pleiotrophin promotes remyelination through activation of differentiation of oligodendrocyte precursor cells. J Neurosci. 2015;35:12162–12171. doi: 10.1523/JNEUROSCI.2127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Masumura M, Suzuki R, Matsumoto M, Noda M. Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination. PLoS One. 2012;7:e48797. doi: 10.1371/journal.pone.0048797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase æ/RPTPβ, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES, Yuan Q, Wong WM, Wang L, Su H, Chu TH, Guo J, Zhang W, So KF, Pepinsky B, Shao Z, Graff C, Garber E, Jung V, Wu EX, Wu W. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–1233. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- Shaygannejad V, Montazeri S, Jamshidian A, Tahani S, Gharagozloo M, Ashtari F, Vesal S, Hasheminia SJ, Dehghani L. Correlation of midkine serum level with pro- and anti-inflamatory cytokines in multiple sclerosis. Iran J Immunol. 2014;11:134–138. [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Waldau B, Roy NS, Schwartz TE, Pilcher WH, Chandross KJ, Natesan S, Merrill JE, Goldman SA. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]


