Amyothrophic lateral sclerosis (ALS) is a disorder characterized by the progressive degeneration of motoneurons and subsequent weakness of the skeletal muscles. Respiratory insufficiency is the most common cause of death for these patients, and it occurs approximately within three years. Riluzole is the only drug that has shown a survival benefit, although it is limited to 6 months and offers no improvement in clinical symptoms (Miller et al., 2007). Bone marrow mononuclear cells have been proposed as a treatment for ALS (Deda et al., 2009). They can provide a neurotrophic effect and modulate the immune host environment. The benefits of bone marrow cells infusion into the spinal cord parenchyma of a SOD1-mutant mice, a model of motoneuron degeneration, has been tested by our group. After laminectomy, the cells were injected at the L5–S1 level, into the anterior horn of the spinal cord through the posterior funiculus. The mice experienced functional improvement as well as an increase in the number of motoneurons in the anterior horn of the spinal cord when compared with sham operated mice. However, no neural differentiation of the transplanted cells was observed (Pastor et al., 2012). These data generated in animal models support the evidence that the major mechanism of action of the bone marrow stem cells is based on trophic support by the production of molecules. These trophic factors are important in ALS and other diseases characterized by degeneration of spinal motoneurons (Suzuki and Sevendsen, 2008). In the case of the familial ALS mouse model SOD1 G93A, the main neurotrophic mediator is the glial derived neurotrophic factor (GDNF). It is able to prevent motoneuron degeneration although it does not promote muscle reinnervation or recovery of muscle function (Suzuki et al., 2007). Further analysis of the mice experimental spinal cords showed that the grafted cells formed cellular nests surrounding the motoneurons and that they expressed GDNF at the mRNA and protein level. This graft-derived GDNF acted as a neurotrophic signal in the microenvironment of spinal motoneurons, increasing neuronal survival, and improving the functional performance of the mice after the transplant (Pastor et al., 2012), in contrast to Suzuki et al. observations.
We then conducted an open single arm phase I clinical trial. Autologous bone marrow mononuclear cells were infused into the posterior spinal cord funiculus of eleven patients. Safety was the primary endpoint and was defined as the absence of serious transplant-related adverse events. In addition, pulmonary function, breathing pattern, sleep studies, ALS-functional rating scale (ALS-FRS), Medical Research Council scale for assessment of muscle power (MRC), and Norris scales were assessed for 1 year after transplant. No acceleration in the rate of decline of FVC, ALS-FRS, Norris, or MRC, scales was observed (Blanquer et al., 2012). A decline in inspiratory or expiratory pressures in patients after procedure was not observed. No significant differences in the rate of decline of the neurological scales or the FVC measurements before and after infusion were found. The breathing pattern and sleep architecture did not change during the follow up. Although it is generally accepted that REM sleep shortening is present in ALS patients with diaphragmatic dysfunction as an attempt to avoid nocturnal hypoventilation, that was not observed in our patients (Ruiz-López et al., 2015). Only 5 patients needed ventilation in the course of their disease. 7 patients died at a median of 5.3 and 3 years from the diagnosis and the infusion of the cells respectively. 4 patients are still alive 8.4 years from the diagnosis and 7.5 years after infusion (Ruiz-López et al., 2015). The pathological studies performed in case of death showed that there was a higher number of motoneurons in the anterior horns of the transplanted spinal thoracic levels than in the upper and lower ones (Blanquer et al., 2012). A significant number of small basophilic CD90 spherical cells adjacent to and/or around the motoneurons were only observed at the grafted levels (Blanquer et al., 2012) and the morphology and arrangement of those cells were identical to that observed in our animal model in which we had demonstrated a GDNF-mediated neurotrophic effect on the host motoneurons (Pastor et al., 2012). Moreover, these motoneurons surrounded by CD90 cells had neither ubiquitin deposits nor any other morphological signs of degeneration, strongly suggesting that the cellular-mediated neurotrophic mechanism observed in the animal model was also reproduced in our patients (Blanquer et al., 2012).
Having demonstrated the safety of the procedure, it is necessary to consider two aspect of the therapy that may have some influence in its effectiveness:
The route of administration: In our trial, we evaluated the unilateral intramedullary injection in a particular segment in order to establish the safety of the intervention (Blanquer et al., 2012). Other trials with neural stem cells have evaluated the safety and tolerability of the lumbar to cervical spinal injection of cord segments closer to the diaphragmatic center targeting potentially clinically relevant objectives. The procedure required several vertebral laminectomies to expose the underlying spinal cord (Glass et al., 2012).
The intrathecal route has been used in humans, as a vehicle for infusion of proteins, drugs or neurotrophic factors. Some authors suggest that this route can be used for cell therapy in neurodegenerative diseases: it is a routine technique in medical practice, with mild and transient secondary effects, and has a smaller potential for relevant complications than the intraspinal injection (Slavin et al., 2008).
It is therefore important to determine whether these two routes are effective. Our group has completed the recruitment of a clinical trial (NCT01254539) comparing the safety and efficacy of the intrathecal or intraspinal injection of bone marrow mononuclear cells and the normal saline intrathecal injection in patients with ALS.
Another route evaluated by our group was the infusion of bone marrow stem cells in the femoral quadriceps of SOD1-mutant mice. GDNF transcription was increased in the transplanted quadriceps, as compared to the non-transplanted ones. The transplant also increased the number of motoneurons in the anterior horn of the dependent segments as compared to the contralateral horn and control mice. Furthermore, GDNF was found in the motoneurons innervating the treated quadriceps, whereas it was absent in the ones innervating non-treated muscles. This finding was consistent with an increase in the amount of GDNF in those spinal segments. However, no increase in GDNF transcription was found. The GDNF concentration increase in the spinal cord was caused by the motoneurons uptake of GDNF at the neuro-muscular plaque and its retrograde transport to the soma, preventing their degeneration. The cerebral cortex of the brain was analyzed in both the control and transplanted mice. An increase in GDNF was detected in the transplanted mice, specifically in the caudal area of the primary motor and somatosensory areas of the side contralateral to the grafted muscle. That motor area corresponds to the area of the hindlimb cortical pyramids, which innervate the anterior horn of the contralateral side (Pastor et al., 2013). This mechanism of retrograde spinocortical interaction may trigger plastic changes in the corticospinal system that might have the capability to produce changes in motor activity and functional recovery in transplanted mice.
Muscle transplantation of bone marrow stem cells is an attractive route with a possible neurotrophic effect on specific spinal neuronal groups and the cerebral cortex (Figure 1). Also, it would allow the repetition of the therapy. The effectiveness of the intramuscular infusion of autologous bone marrow cells in patients with Amyothrophic Lateral Sclerosis is being evaluated by our group in a clinical trial (NCT02286011).
Figure 1.
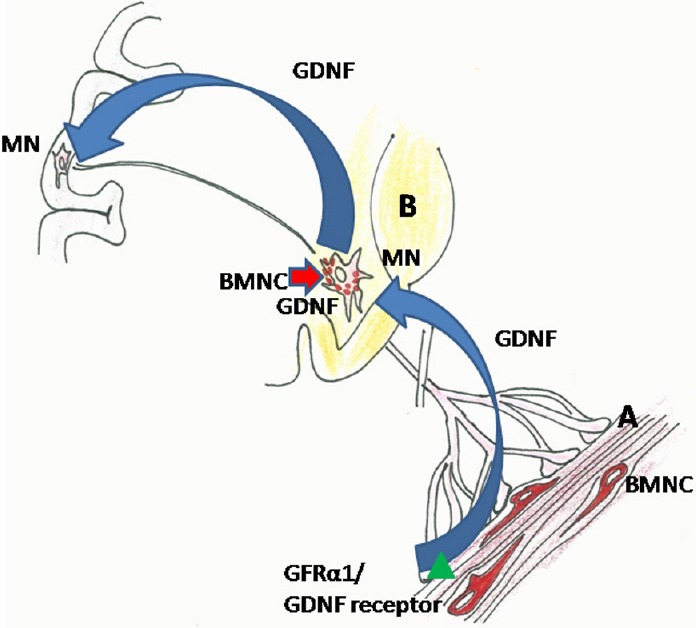
Retrograde transport of glial derived neurotrophic factor (GDNF) from the muscle to the cerebral cortex after transplanting bone marrow mononuclear cells (BMNC) and influence of transplanted cells on spinal cord motorneuron (MN).
(A) Transplanted cells (red) in muscle. The cells were detected between the muscle fibres. The cells present an elongated morphology, with multiple prolongations. (B) Transplanted cells (red) in spinal cord. The cells were detected surrounding the motorneurons.
ALS phenotypical expressions: ALS is a pleomorphic disease that can be classified by the site of its initial symptoms. This feature tends to correlate with a particular disease evolution. The bulbar onset patients present a rapid disease progression with early respiratory symptoms that make them less likely to benefit from this therapy.
Primary lateral sclerosis or progressive muscular atrophy are also two phenotypical expressions that would probably require a slower evolution of the disease to allow the neurotrophic effect to take place. A sniff nasal-inspiratory force less than 40 cmH2O (significantly related to nocturnal hypoxemia) or a FVC < 50% are predictive of high levels of mortality at six months and are markers of advanced disease (Morgan et al., 2005).This potential lack of efficacy in rapid progressing forms or advanced stages of the disease is due to the fact that there are less motoneurons to be rescued by the neurotrophic effect. Congruently, in patients with end-stage lower limb disease, there were no changes before and after the transplant of neural stem cells (Glass et al., 2012).
In our first trial the patients had to maintain the inclusion criteria for 6 months before the infusion: FVC had to be > 50% of that predicted, and a below 90% fall in oxygen saturation (T90) occurring in < 2% of sleep time without change in central drive, REM sleep or total sleep time (Blanquer et al., 2012; Ruiz-López et al., 2015).
Our data demonstrate that the intraspinal administration of autologous bone marrow stem cells is safe in patients with a non-advanced disease and stable lung function. Moreover, these cells can exert a neurotrophic effect. Assessing the real effectiveness of this treatment, alternative administration routes of the cells and whether a repeated treatment is convenient, are the next challenges that we face in our research.
References
- Blanquer M1, Moraleda JM, Iniesta F, Gómez-Espuch J, Meca-Lallana J, Villaverde R, Pérez-Espejo MÁ, Ruíz-López FJ, García Santos JM, Bleda P, Izura V, Sáez M, De Mingo P, Vivancos L, Carles R, Jiménez J, Hernández J, Guardiola J, Del Rio ST, Antúnez C, et al. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. 2012;30:1277–1285. doi: 10.1002/stem.1080. [DOI] [PubMed] [Google Scholar]
- Deda H, Inci MC, Kürekçi AE, Sav A, Kayihan K, Ozgün E, Ustünsoy GE, Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11:18–25. doi: 10.1080/14653240802549470. [DOI] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2007;1:CD001447. doi: 10.1002/14651858.CD001447.pub2. [DOI] [PubMed] [Google Scholar]
- Morgan RK, McNally S, Alexander M, Conroy R, Hardiman O, Costello RW. Use of Sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2005;171:269–274. doi: 10.1164/rccm.200403-314OC. [DOI] [PubMed] [Google Scholar]
- Pastor D, Viso-León MC, Botella-López A, Jaramillo-Merchan J, Moraleda JM, Jones J, Martínez S. Bone marrow transplantation in hindlimb muscles of motoneuron degenerative mice reduces neuronal death and improves motor function. Stem Cells Dev. 2013;22:1633–1644. doi: 10.1089/scd.2012.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor D, Viso-León MC, Jones J, Jaramillo-Merchán J, Toledo-Aral JJ, Moraleda JM, Martínez S. Comparative effects between bone marrow and mesenchymal stem cell transplantation in GDNF expression and motor function recovery in a motorneuron degenerative mouse model. Stem Cell Rev. 2012;8:445–458. doi: 10.1007/s12015-011-9295-x. [DOI] [PubMed] [Google Scholar]
- Ruiz-López FJ, Guardiola J, Izura V, Gómez-Espuch J, Iniesta F, Blanquer M, López-San Román J, Saez V, De Mingo P, Martínez S, Moraleda JM. Breathing pattern in a phase I clinical trial of intraspinal injection of autologous bone marrow mononuclear cells in patients with amyotrophic lateral sclerosis. Respir Physiol Neurobiol. 2016;221:54–58. doi: 10.1016/j.resp.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Slavin S, Kurkalli BG, Karussis D. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg. 2008;110:943–946. doi: 10.1016/j.clineuro.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Svendsen CN. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008;31:192–198. doi: 10.1016/j.tins.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]


