Spinal cord injury (SCI) causes disturbances in motor and sensory functions leading to paralysis, the severity of which depends on the spinal level of the injury. Traumatic lesions of spinal cord axon projection tracts are untreatable in human patients, although numerous research groups worldwide are studying putative treatment strategies. Both extrinsic factors in the environment of the axons as well as intrinsic factors in the neurons themselves play important roles in the regeneration process (Chew et al., 2012). The peripheral nervous system (PNS) provides a good example where the extrinsic and intrinsic factors play optimally together to allow regeneration. Schwann cells dedifferentiate and form new endoneurial tubes for the axons to grow through. Together with macrophages they clear the debris and produce growth factors and cytokines that positively stimulate the neurons. In parallel, the neurons intrinsically react to the injury by activating a regeneration-associated gene expression program. Most PNS axons produce a new growth cone and start growing within 3 hours (Bradke et al., 2012), eventually reinnervating their targets. In contrast, the projection neurons in the central nervous system (CNS) do not spontaneously activate regeneration-associated genes (RAGs) (van Kesteren et al., 2011). The axons first die back several hundreds of micrometers, tend to make retraction bulbs rather than growth cones, and seem unable to navigate in the correct direction (Bradke et al., 2012). Those CNS axons that do regenerate encounter a highly inhibitory scar that further blocks their growth (Fawcett et al., 2012). So, in the CNS both intrinsic and extrinsic mechanisms negatively influence regeneration. This is further corroborated by the observation that some spinal cord axons are able to regenerate through a peripheral nerve graft (van Kesteren et al., 2011) indicating again that the PNS environment is favorable to growth. However, the majority of injured neurons in the spinal cord do not regenerate spontaneously, so that peripheral nerve grafts still need to be combined with treatments such as cAMP, increasing the intrinsic regeneration capacity (Bunge, 2008). In this paper, I will address the extrinsic and intrinsic regeneration mechanisms with respect to treatments for SCI.
SCI is a complex disorder where many systems are involved. Axons of descending motor tracts and ascending sensory tracts are damaged (Figure 1). Motor tracts originate in the primary motor cortex (corticospinal tract, CST), the red nucleus (rubrospinal tract, RST), the locus coeruleus (noradrenergic fibers, NA) and Raphe nuclei (serotonergic fibers, 5-HT) (Schiwy et al., 2009; Zörner et al., 2014). The sensory ascending axons originate from the dorsal root ganglia (DRGs), whose peripheral axons regenerate very well. The central branches of the pseudounipolar DRG axon, however, have similar difficulties as their motor colleagues to regenerate after SCI (Bareyre et al., 2011). This illustrates again that the extrinsic and intrinsic mechanisms of regeneration are different for axons in the CNS or PNS environment.
Figure 1.
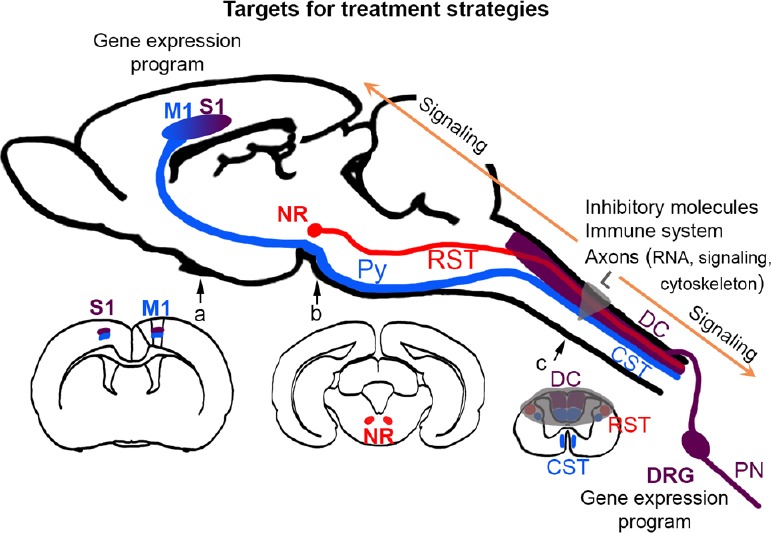
Extrinsic and intrinsic targets for treatment strategies.
Schematic representation of a sagittal section of mouse brain (modified from Paxinos & Watson, The Rat Brain in Stereotaxic Coordinates, 6th Edition) with three tracts of interest: in blue, the corticospinal tract (CST) arising in layer V of primary motor cortex (M1) and descending through the pyramidal tract (Py) to the spinal cord, in red, the rubrospinal tract (RST) arising from the red nucleus (NR), and in burgundy, the peripheral nerve (PN) arising in the dorsal root ganglia (DRG) that also send a central projection into the dorsal columns (DC) conveying information via the thalamus to the primary sensory cortex (S1). Since anteroposterior M1 and S1 locations are partially overlapping, the area is represented here by a gradient. Coronal sections of the forebrain (a), midbrain (b) and spinal cord (c) show the location of the tracts in the dorsal-ventral-lateral positions. The SCI lesion (L, in this example a dorsal hemisection) is displayed in transparent grey. The intrinsic and extrinsic factors that influence the regeneration of the tracts are indicated above their main location of action. Intrinsic regeneration mechanisms include the local translation of RNA in the axons, the reorganization of the cytoskeleton, the activation of signaling molecules that are transported retrogradely (arrows) to the cell bodies, where a gene expression program should start. In and around the lesion scar, extrinsic factors include inhibitory molecules and the immune system. Altogether, treatments for SCI should influence multiple factors, so that both the intrinsic as well as the extrinsic mechanisms of regeneration are targeted.
The extrinsic factors that negatively influence CNS axon regeneration are located in and around the lesion scar (Chew et al., 2012; Fawcett et al., 2012). Upon injury, astrocytes are activated and form a new glia limitans around the lesion area in order to re-establish the blood-brain barrier. Although the lesion area is usually referred to as a glial scar, it has a fibrous core surrounded by reactive astrocytes. This core is composed of infiltrating fibroblasts and pericytes that have migrated from the dura mater and blood vessels, respectively (Klapka et al., 2005; Fagoe et al., 2014). The fibrous core consists of a collagen IV-containing matrix that serves as a scaffold for axon growth inhibitory molecules, such as chondroitin sulfate proteoglycans (CSPGs), semaphorins, Ephrins and their Eph receptors, and tenascins (Klapka et al., 2005; Chew et al., 2012). We observed that the fibrous core, in addition to molecules produced by fibroblasts, contains inhibitors exclusively produced by astrocytes, which underscores the function of the extracellular matrix (ECM) as a scaffold for the soluble inhibitors (Vogelaar et al., 2015). Other inhibitors are not localized in the scar but in the white matter. Myelin components, like myelin-associated glycoprotein (MAG), Nogo, and oligodendrocyte myelin glycoprotein (OMgp) are very potent inhibitors of axon regeneration (Lee and Zheng, 2012). Due to conflicting data in various mutants of these inhibitors and their receptors, controversy exists as to whether blocking myelin inhibition affects regeneration of injured neurons or sprouting of intact uninjured axons. This is reviewed extensively by Lee and Zheng (2012) who conclude that the neuron-intrinsic lack of growth of injured axons may be masking the effects of removing myelin-associated inhibition.
There are numerous studies targeting the growth inhibitors to treat spinal cord injury (Chew et al., 2012). To name a few, antibodies or antagonists were applied to block MAG, Nogo or semaphorin 3A. However, these only target one component of the plethora of inhibitory factors. The chondroitinase ABC enzyme that digests the inhibitory glycosaminoglycan chains off the CSPGs targets a family of inhibitors, but others remain. Previously, we developed the anti-scarring treatment (AST), consisting of an iron chelator and cyclic AMP (cAMP). Chelating iron depletes the enzyme prolyl-4-hydroxylase from its cofactor, thus inhibiting the assembly of collagen IV. cAMP inhibits the production of collagen by fibroblasts as well as the proliferation of the latter. AST transiently suppressed scar formation for 2 weeks, leading to decreased accumulation of NG-2 (a CSPG) and increased CST axon regeneration through and beyond the scar (Klapka et al., 2005). The treatment protected the corticospinal neurons from death and resulted in partial recovery of locomotor function. In a follow up study, we found that AST promoted regeneration not only CST, but also RST, 5-HT, NA and ascending sensory axons (Schiwy et al., 2009).
Another neuron-extrinsic system influencing CNS regeneration is the immune system. Infiltrating T lymphocytes can be either detrimental or beneficial for regeneration (Jones, 2014). This is thought to depend on the type of T helper T (TH) lymphocytes, either the pro-inflammatory TH 1 or the anti-inflammatory TH 2 cells and on the influence of regulatory T-cells. These TH subtypes in turn are believed to drive macrophages derived from the blood or from residential microglia into the M1 or M2 phenotype. Although the concept of “protective autoimmunity” is relatively well accepted to date it is mostly not studied which TH cell phenotype is responsible for the neuroprotection (Jones, 2014). Recently, we have shown that local injection of TH 2, but not TH 1, lymphocytes in the spinal cord injury site was beneficial for both axonal regeneration and functional recovery (Walsh et al., 2015). We speculated that the TH 2 cytokine IL-4 might play a major role, since IL-4 knock out mice displayed worse SCI outcomes and IL-4 stimulated axon outgrowth in vitro.
Many studies on SCI treatments focused exclusively on the regeneration of axons through the lesion scar, but did not investigate the effects of the treatments on the CNS neurons themselves. The latter tend to undergo atrophy and fail to activate a gene expression program. Numerous studies on peripheral DRG neurons identified RAGs that are activated by peripheral injury, but not by damage of their central axons (van Kesteren et al., 2011). These RAGs can be used as potential modifiers of intrinsic CNS regeneration. Fagoe et al. (2014) reviewed the effects of gene therapy on axon regeneration. The overexpression of various RAGs, such as ciliary neurotrophic factor (CNTF), retinoic acid receptor β2 (RARβ2), signal transducer and activator of transcription 3 (STAT3), and cAMP responsive element binding protein (CREB), by injection of viral vectors into DRGs leads to enhanced regeneration of the DRG's central axons. A recent study on PCAF (p300/CBP-associated factor), a factor promoting histone acetylation, suggests that epigenetic modifications (Lindner et al., 2013) might also promote the regeneration of the CNS axons of DRG neurons (Fagoe et al., 2014). So, the CNS axons of PNS neurons are capable of regenerating when the neurons express certain RAGs. In relation to spinal cord projection neurons, only few such studies have been performed. The virus-induced overexpression of Krüppel-like factor 7 (KLF7), STAT3 and neuronal calcium sensor-1 (NCS-1) in cortical neurons increased sprouting and/or regeneration of CST axons after unilateral pyramidotomy, accompanied by functional improvement of forelimb fine motor functions (Fagoe et al., 2014). However, because of limitations in view of the number of genes and brain regions that can be transduced by injecting viruses, gene therapy is not optimal for clinical treatment of SCI. A better treatment choice would be a pharmacological therapy that by itself changes the intrinsic gene expression programs of the injured neurons. We studied the effects of AST on gene expression profiles in the CST neurons that are located in layer V of the sensorimotor cortex. We were able to show that AST activated a gene expression program pointing into the direction of axon outgrowth, axon guidance, cell survival, inhibiting genes involved in apoptosis and axon repulsion (Kruse et al., 2011).
The intrinsic properties of the axon itself are also an important factor to consider. The cell bodies of both central and peripheral long axon tracts are located far away (up to 1 meter in humans) from the actual lesion site. Therefore, recent studies suggest that the axon can translate proteins from local stores of mRNA. Axonal RNA translation in PNS axons plays a role in growth cone turning, retrograde signaling and regeneration of a new growth cone after injury (Vogelaar et al., 2009; Ben-Yaakov et al., 2012). Axonal RNA localization has been described for immature CNS axons, but is still a question of debate in mature CNS neurons (Bradke et al., 2012).
Finally, signaling plays a key role in regeneration. The concentration of cAMP and cGMP in the axon determines whether it turns away from or is attracted by extracellular molecules (Song, 1998). Therefore, it is a net balance between growth stimulators, e.g., neurotrophic factors and neuro-active cytokines, and growth inhibitors, that is decisive for the final “permeability” of the lesioned tissue. Signals integrate at the level of the cytoskeletal actin and microtubule networks, whose remodeling and stability determine the growth response of the axon (Bradke et al., 2012). Signaling molecules are transported from the axon to the cell body, where they can exert effects on cell survival and transcription. Indeed, a PNS lesion leads to the activation of signaling molecules, like STAT3, which are retrogradely transported to the DRG neurons, where it induces initiation of regeneration (Bareyre et al., 2011; Ben-Yaakov et al., 2012). Recent studies on PTEN, an inhibitor of Akt signaling, suggest that the inhibition of PTEN by knock out, shRNA or compounds increases CST axon regeneration, but with so far limited functional recovery (Lindner et al., 2013; Ohtake et al., 2015). The authors conclude their review that PTEN inhibition would be more successful when combined with other strategies targeting intrinsic and extrinsic regeneration mechanisms.
In summary, the damaged neuron has to activate a regeneration program and the axon has to form a growth cone (possibly via local translation) and regrow through a highly inhibitory environment, integrating negative and positive influences of molecular factors via the activation or inhibition of signaling pathways (Figure 1). For a treatment to achieve significant regeneration of long axon tracts after spinal cord injury, it has to influence more than one molecule, ideally both extrinsic and intrinsic factors. Making the scar more permissive for growth might make little difference when the neurons do not activate a regeneration-associated gene expression program and therefore limit their axonal re-growth. The challenge in this field of research is to find a treatment that stimulates the axon's and neuron's intrinsic regenerative capacity and at the same time attenuates most of the inhibitory properties of the scar. AST treatment may be such a multi-target strategy. We are currently working on the optimization of this treatment for better compatibility with the treatment of patients by using an alternative and clinically approved iron chelator (Vogelaar et al., 2015).
References
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci U S A. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31:262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew DJ, Fawcett JW, Andrews MR. The challenges of long-distance axon regeneration in the injured CNS. Prog Brain Res. 2012;201:253–294. doi: 10.1016/B978-0-444-59544-7.00013-5. [DOI] [PubMed] [Google Scholar]
- Fagoe ND, van Heest J, Verhaagen J. Spinal cord injury and the neuron-intrinsic regeneration-associated gene program. Neuromolecular Med. 2014;16:799–813. doi: 10.1007/s12017-014-8329-3. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Schwab ME, Montani L, Brazda N, Müller HW. Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- Jones TB. Lymphocytes and autoimmunity after spinal cord injury. Exp Neurol. 2014;258:78–90. doi: 10.1016/j.expneurol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP, Muller D, Zuschratter W, Muller HW. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- Kruse F, Bosse F, Vogelaar CF, Brazda N, Kury P, Gasis M, Muller HW. Cortical gene expression in spinal cord injury and repair: insight into the functional complexity of the neural regeneration program. Front Mol Neurosci. 2011;4:26. doi: 10.3389/fnmol.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol. 2012;235:33–42. doi: 10.1016/j.expneurol.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner R, Puttagunta R, Di Giovanni S. Epigenetic regulation of axon outgrowth and regeneration in CNS injury: the first steps forward. Neurotherapeutics. 2013;10:771–781. doi: 10.1007/s13311-013-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y, Hayat U, Li S. PTEN inhibition and axon regeneration and neural repair. Neural Regen Res. 2015;10:1363–1368. doi: 10.4103/1673-5374.165496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiwy N, Brazda N, Müller HW. Enhanced regenerative axon growth of multiple fibre populations in traumatic spinal cord injury following scar-suppressing treatment. Eur J Neurosci. 2009;30:1544–1553. doi: 10.1111/j.1460-9568.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- Song H. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- van Kesteren RE, Mason MR, Macgillavry HD, Smit AB, Verhaagen J. A gene network perspective on axonal regeneration. Front Mol Neurosci. 2011;4:46. doi: 10.3389/fnmol.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelaar CF, Gervasi NM, Gumy LF, Story DJ, Raha-Chowdhury R, Leung KM, Holt CE, Fawcett JW. Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol Cell Neurosci. 2009;42:102–115. doi: 10.1016/j.mcn.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelaar CF, Konig B, Krafft S, Estrada V, Brazda N, Ziegler B, Faissner A, Muller HW. Pharmacological suppression of CNS scarring by deferoxamine reduces lesion volume and increases regeneration in an in vitro model for astroglial-fibrotic scarring and in rat spinal cord injury in vivo. PLoS One. 2015;10:e0134371. doi: 10.1371/journal.pone.0134371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Gölz G, Rosenberger K, Kammertöns T, Vogt J, Vogelaar C, Siffrin V, Radjavi A, Fernandez-Castaneda A, Gaultier A, Gold R, Kanneganti TD, Nitsch R, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:2547. doi: 10.1172/JCI82458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zörner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Rothlisberger M, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem's contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014;137:1716–1732. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]


