Keywords: nerve regeneration, brain injury, in utero fetal distress, hypoxic-ischemic brain injury, electroacupuncture, real-time fluorescent quantitative PCR, NeuroD, nerve repair, Baihui (DU20) acupoint, non-acupoint, neural regeneration
Abstract
NeuroD plays a key regulatory effect on differentiation of neural stem cells into mature neurons in the brain. Thus, we assumed that electroacupuncture at Baihui (DU20) acupoint in newborn rats exposed to in utero fetal distress would influence expression of NeuroD. Electroacupuncture at Baihui was performed for 20 minutes on 3-day-old (Day 3) newborn Sprague-Dawley rats exposed to in utero fetal distress; electroacupuncture parameters consisted of sparse and dense waves at a frequency of 2–10 Hz. Real-time fluorescent quantitative PCR results demonstrated that mRNA expression of NeuroD, a molecule that indicates NeuroD, increased with prolonged time in brains of newborn rats, and peaked on Day 22. The level of mRNA expression was similar between Day 16 and Day 35. These findings suggest that electro acupuncture at Baihui acupoint could effectively increase mRNA expression of molecules involved in NeuroD in the brains of newborn rats exposed to in utero fetal distress.
Introduction
Fetal distress in utero, a major factor for hypoxic-ischemic encephalopathy, can cause permanent neurological disorders such as mental retardation, movement disorders, and even death (Group of Neonatology et al., 2005; Kadam et al., 2007; Pichiule et al., 2007). Previous transplantation studies have shown limited survival of neural stem cells. Those that do survive primarily differentiate into glial cells, as well as a small number of neurons (An et al., 2002; Jeong et al., 2003; Qun et al., 2010; Fan et al., 2013; Tu et al., 2014; Yan et al., 2014). The microenvironment greatly influences the proliferation and differentiation of neural stem cells (NSCs), and the manipulation of the microenvironment has become the focus of studies attempting to promote neuronal differentiation.
The microenvironment described in modern medicine is analogous to the “sea of marrow” in traditional Chinese medicine. Hypoxic-ischemic encephalopathy is considered a “deficiency of marrow-reservoir” in newborn infants. Baihui (DU20) acupoint is strongly associated with the brain, and its name, “One Hundred Meetings,” refers to the convergence of all yang meridians in the body; acupuncture at Baihui acupoint tonifies the marrow and brain. Electroacupuncture (EA) is the combination of traditional acupuncture, moxibustion, and electrical stimulation, and is a form of acupuncture where a small electrical current is passed between pairs of acupuncture needles, which induces a needling sensation. Recent studies have focused on electroacupuncture-induced differentiation of NSCs into neurons, and the effect of EA has been affirmed (Kang et al., 1995; Fumagall et al., 2009; Stone et al., 2011). Acupuncture has been shown to effectively mitigate hypoxic-ischemic brain injury by resisting free radicals, improving electrical activity in the brain and cerebral microcirculation, and affecting neurotrophic factor secretion and angiogenesis (Liu et al., 2006; Chung et al., 2007; Yang et al., 2008; Stone et al., 2011). Several studies have shown that EA has an anti-apoptotic effect, and can promote the formation of new mature neurons (Lee et al., 1995; Wang et al., 2011; Guo et al., 2012).
NeuroD is expressed throughout the peripheral nervous system and central nervous system (Lawler et al., 2012; Mu et al., 2014; Xu et al., 2014), and plays an important role in regulating NSCs differentiation into mature neurons (Lee et al., 1997; Roybon et al., 2009; Hu et al., 2013). We presumed that EA could regulate NeuroD expression by affecting the brain microenvironment. This study measured the effects of EA on NeuroD mRNA expression in the brains of newborn rats exposed to in utero fetal distress by using real-time fluorescent quantitative PCR.
Materials and Methods
Experimental animals
Ten specific pathogen-free, male Sprague-Dawley rats weighing 200 ± 20 g and 20 female rats weighing 220 ± 20 g were provided by the Experimental Animal Center of Fujian Medical University of China [license No. SCXK (Min) 2012-0001]. All rats were allowed to acclimatize to their new environment for 2 weeks prior to placement in the same cage. All rats were maintained and housed under controlled conditions at 22°C in a 12-hour reversed light/dark cycle with free access to food and water. All surgeries were performed under anesthesia, and all efforts were made to minimize pain and distress of the experimental animals. All animal experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986). The experiments were approved by the Animal Ethics Committee of Fujian Medical University of China (Approval No. 2014-12).
Establishment of a rat model of fetal distress in utero and EA intervention
Male and female rats were placed in the same cage at 18:00. Vaginal smears were used to detect sperm every morning at 7:00 a.m. Under a light microscope (Olympus, Tokyo, Japan), the first day of observing sperm was considered embryonic day 1 (E1). On E22, newborn rats delivered by natural birth were considered as the blank control group (n = 6). Pregnant rats on E22 were continuously anesthetized with an appropriate amount of anhydrous diethyl ether. Via cesarean section, a median incision was made on the abdomen to expose the uterus. Bilateral uterine arteries and bilateral ovarian arteries were occluded with a bulldog clamp for 20 minutes. The fetuses were then removed.
Newborn rats exposed to in utero fetal distress were randomly assigned to a 20-minute fetal distress group, 20-minute fetal distress + Baihui group, 20-minute fetal distress + non-acupoint group, and a blank control group, with six rats in each group. Newborn rats at the age of 3 days (day 3) in the 20-minute fetal distress + Baihui group and 20-minute fetal distress + non-acupoint group were treated with EA using an electroacupuncture stimulator (KWD808-I; Changzhou Yingdi Electronic Medical Device Co., Ltd., Changzhou, China). In accordance with an animal acupoint atlas from Experimental Acupuncture Science, the needle (0.5 cun, 1 cun = 3.33 cm; Suzhou Medical Sino-foreign Joint Venture Suzhou Hua Tuo Medical Instruments Co., Ltd., Suzhou, Jiangsu Province, China) was inserted into the Baihui, at a depth of 1 cm. The non-acupoint was 0.5–1.0 cm right to Baihui, with a needling depth of 1 cm. The EA parameters were as follows: sparse and dense waves with a frequency of 2–10 Hz; needle handle tremor verified that the electric current was stimulating muscle contraction and was used to determine the most effective intensity. The rats did not exhibit any stress. EA was performed at 17:00, once a day for 15 minutes, for 7 consecutive days.
Total brain RNA extraction
On Days 16, 22, and 35 (i.e., 7, 13 and 26 days after treatment), rats from each group were anesthetized with 10% chloral hydrate and sterilized with 75% ethanol. Brain tissue, except the cerebellum, was frozen at −80°C, and then triturated with liquid nitrogen. Trizol (1 mL/100 mg) was added and incubated at room temperature for 15 minutes without stirring. Then, 200 μL of chloroform/1 mL Trizol was added and further incubated at room temperature for 10 minutes without stirring. The samples were centrifuged at 4°C and 12,000 r/min for 15 minutes. The upper aqueous layer was removed to a new RNase-free Eppendorf tube, and an equal volume of precooled isopropanol was added. After shaking, the mixture was incubated at room temperature for 10 minutes, and centrifuged at 4°C and 12,000 r/min for 15 minutes. After removal of the supernatant, the precipitate was washed with 1 mL 75% ethanol and centrifuged at 7,500 r/min for 5 minutes. After removal of the supernatant, the precipitate was allowed to rest without stirring for 30 seconds, and 30–60 μL of RNase-free water was added and mixed with a pipette, followed by incubation in a 65°C water bath for dissolution. A small amount of total RNA was detected by 1% nondenaturing agarose gel electrophoresis. The ratio of absorbance at 260 nm and 280 nm (A260 nm/280 nm) was measured with an ultraviolet spectrophotometer (Nano-Drop 2000; Thermo, Waltham, MA, USA).
cDNA preparation and PCR assay for determining primer specificity
RT-PCR was performed in accordance with a TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, Beijing, China). Each reaction system contained 2 μg total RNA, 10 μL of 2× TS Reaction Mix, 1 μL of TransScript® RT/RI Enzyme Mix, 1 μL of genomic DNA. RNase-free water was added to a total volume of 20 μL. All reagents were incubated at 42°C for 30 minutes, followed by 85°C for 5 minutes. RNA was efficiently reverse transcribed into cDNA and stored in an iced bath. The cDNA was diluted five-fold and considered as a template. The system was as follows: 10 μL of 2× EasyTaq PCR SuperMix, 0.8 μL of upstream primer (10 μM), 0.8 μL of downstream primer (10 μM), and 1 μL of cDNA template. RNase-free water was added to a total volume of 20 μL. Reaction conditions were as follows: initial denaturation at 94°C for 5 minutes, 35 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 20 seconds, followed by extension at 72°C for 7 minutes. Primer specificity was detected by agarose gel electrophoresis with 5 μL of PCR products.
The primers used are as follows:

Real-time fluorescent quantitative PCR for NeuroD gene expression
The reaction system was composed of 10 μL of TransStart Tip Green qPCR SuperMix (2×), 0.4 μL of PCR Forward Primer (10 μM), 0.4 μL of PCR Reverse Primer (10 μM), 0.4 μL of Rox Reference Dye II (50×), and 2 μL of cDNA template. Double-distilled water was added to a total volume of 20 μL. Three parallel wells of internal reference gene and target gene from each sample were set for each group. The total reaction solution was prepared, and then placed separately. With an Applied Biosystems 7500 Real-Time PCR System (ABI, Foster, CA, USA), the reaction conditions were as follows: initial denaturation at 94°C for 30 seconds, 40 cycles of denaturation at 94°C for 5 seconds, annealing and extension at 60°C for 34 seconds. Melting curves at 60–95°C were analyzed. Relative changes in gene expression were analyzed using the 2–ΔΔCt method for relative quantitation.
Statistical analysis
Measurement data are expressed at the mean ± SD, and analyzed using SPSS 21.0 software (IBM, New York, NY, USA). One-way analysis of variance and the least significant difference t-test were used to compare differences in intergroup data. A value of P < 0.05 was considered statistically significant.
Results
Identification of total RNA in the brains of newborn rats exposed to in utero fetal distress
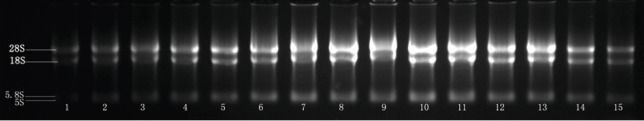
Agarose gel electrophoresis results revealed clear 28S rRNA and 18S rRNA bands. The brightness of 28S bands was one- or two-folds greater than the 18S bands (Figure 1). Spectrophotometry results demonstrated that the ratio of A260 nm/280 nm was 1.9–2.1. The results indicated intact RNA of a high purity from the brain material.
Figure 1.
Agarose gel electrophoresis results of total RNA in the brains of newborn rats exposed to in utero fetal distress.
1–3: Blank control group; 4–7: 20-minute fetal distress group; 8–11: 20-minute fetal distress + Baihui group; 12–15: 20-minute fetal distress + non-acupoint group. Gel imaging system shows distinct bands of total RNA from each sample.
Primer specificity as detected by real-time fluorescent quantitative PCR for NeuroD
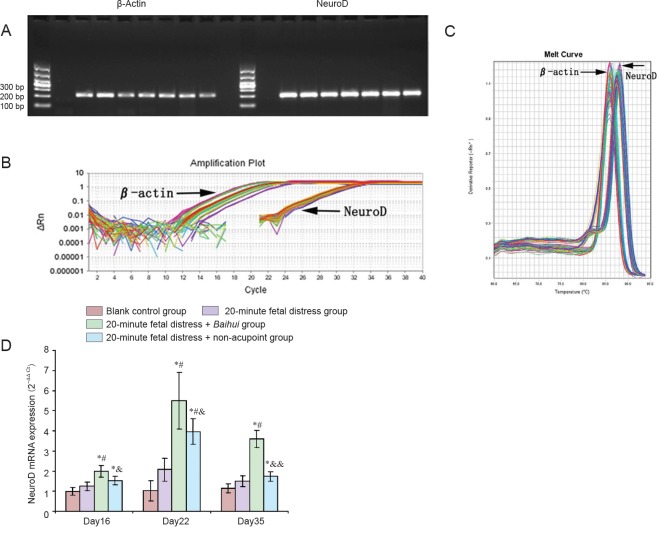
Gel electrophoresis results showed single bands of products amplified by β-actin primers on the left and products amplified by NeuroD primers on the right visible at 200 bp. The background was clear. Nonspecific bands and primer dimmers were not found (Figure 2A). Melting curve analysis results showed a single peak of each product (Figure 2C). S-shaped amplification curves presented with good fitting, and Ct values were similar (Figure 2B). These results confirmed good primer design, stable reaction system, uniform template allocation, and reliable outcomes.
Figure 2.
Effects of electroacupuncture at Baihui (DU20) acupoint on NeuroD mRNA expression in the brains of newborn rats exposed to in utero fetal distress.
(A) Agarose gel electrophoresis: single bands of products amplified by β-actin primers on the left and products amplified by NeuroD primers on the right are visible at 200 bp. Background is clear. (B, C) PCR amplification curves and melting curves. Left: amplification curves of β-actin; right: amplification curves of NeuroD. (D) NeuroD mRNA expression after electroacupuncture: NeuroD mRNA expression gradually increased after electroacupuncture, peaks on Day 22, and decreased on Day 35 to levels similar to Day 16. NeuroD mRNA expression was greater in the 20-minute fetal distress + Baihui group than in other groups on Days 16, 22 and 35. *P < 0.01, vs. blank control group; #P < 0.01, vs. 20-minute fetal distress group; &P < 0.05, &&P < 0.01, vs. 20-minute fetal distress + Baihui group (mean ± SD, n = 6, one-way analysis of variance and the least significant difference t-test). Day 16, 22, and 35 refers to 7, 13, and 26 days after electroacupuncture, respectively.
On Day 16, NeuroD mRNA expression was higher in the 20-minute fetal distress + Baihui group and 20-minute fetal distress + non-acupoint group compared with the blank control group (P < 0.01), and NeuroD mRNA expression was highest in the 20-minute fetal distress + Baihui group (P < 0.05). On Day 22, NeuroD mRNA expression reached a peak in each group. NeuroD mRNA expression was significantly different among groups: the blank control group < 20-minute fetal distress group < 20-minute fetal distress + non-acupoint group < 20-minute fetal distress + Baihui group (P < 0.01 or P < 0.05). On Day 35, NeuroD mRNA expression was significantly higher than in the blank control group (P < 0.05). NeuroD mRNA expression was highest in the 20-minute fetal distress + Baihui group (P < 0.01; Figure 2D). These findings suggested that EA induced a trend of increased NeuroD mRNA expression, which peaked and then decreased again. EA had a significant effect on the 20-minute fetal distress + Baihui group.
Discussion
This study established models of hypoxic-ischemic encephalopathy based on results from a previous study (Bjelke et al., 1991). In traditional Chinese medicine, the key for treating hypoxic-ischemic encephalopathy is Qi and blood support in the brain and spinal cord (Lei et al., 2011). In accordance with the animal acupoint atlas in Experimental Acupuncture Science and Science of Acupuncture and Moxibustion, Baihui, which is located at the right midpoint of the parietal bone, was selected in this study; the non-acupoint was 0.5–1.0 cm right to Baihui acupoint (Huang et al., 2013).
EA was used to treat 3-day-old newborn rats, and the effect of EA on NeuroD mRNA expression was examined. EA has been previously shown to improve collateral circulation, reduce tissue swelling, regulate cell metabolism, affect synthesis and release of neurotransmitters, signaling molecules, and apoptosis-related proteins, adjust phosphorylation levels, and induce neuronal recovery after hypoxia and ischemia (Kaneto et al., 2009; Ohira, 2011; Zhang et al., 2011). EA is used to rhythmically stimulate a specific acupoint by pulse current to accelerate meridian-QI and blood circulation (Wu, 2013).
In the present study, real-time fluorescent quantitative PCR results demonstrated that NeuroD mRNA expression was greatest in the 20-minute fetal distress + Baihui group on Days 16, 22, and 35. NeuroD mRNA expression was higher in the 20-minute fetal distress + non-acupoint group than in the 20-minute fetal distress group on Day 22. The effect of acupuncture at non-acupoint is not theoretically identical to that at an acupoint (such as Baihui). In the present study, the position of the non-acupoint (0.5–1.0 cm right to Baihui) was close to Baihui. The EA frequency at the non-acupoint was thought to affect Baihui acupoint, thereby exerting a weak effect on reinforcing marrow and benefiting brain. However, the precise mechanism remains unclear. Zhao et al. (2012) considered that two regions far from each other in the body (an acupoint and a special region of the body) had a functional connection. For example, acupuncture at Baihui acupoint could tonify the marrow and brain (Kuhn et al., 1999; Song et al., 2002; Jia et al., 2005).
EA has been shown to improve brain injury by changing the microenvironment, inhibiting apoptosis, and promoting neural regeneration, and early intervention results in better outcomes (Rui et al., 2011; Song et al., 2013; Zhu et al., 2013; Huang et al., 2014; Tao et al., 2014; You et al., 2014). Thus, we began treatment of rats at 3 days of age. Results showed that NeuroD mRNA expression was higher in the 20-minute fetal distress + Baihui group and 20-minute fetal distress + non-acupoint group than in the blank control group on Day 35. NeuroD plays a positive regulatory role in neuronal differentiation of NSCs (Lee et al., 1997; Roybon et al., 2009; Hu et al., 2013).
In summary, EA might effectively improve the brain microenvironment by affecting mechanisms related to neural regeneration and neuroprotection. Previous studies have shown that NeuroD contribute to neural regeneration and neuroprotection against hypoxic-ischemic brain injury, and results from the previous study showed that EA increased NeuroD mRNA expression. This study provided an important basis for clinical studies regarding the effects of EA on hypoxic-ischemic brain injury.
Acknowledgments
We are very grateful to Wei Guo from the Department of Human Anatomy, Histology and Embryology, School of Basic Medical Sciences, Fujian Medical University, China for guiding experimental procedure, and every teacher from the Department of Human Anatomy, Histology and Embryology, School of Basic Medical Sciences, Fujian Medical University, China for their suggestions and assistance on experiments.
Footnotes
Funding: This research was supported by the Natural Science Foundation of Fujian Province of China, No. 2015J01133; the Professor Academic Development Foundation of Fujian Medical University of China, No. JS11003.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Pack M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Amrein I, Lipp HP. Adult hippocampal neurogenesis of mammals: evolution and life history. Biol Lett. 2009;5:141–144. doi: 10.1098/rsbl.2008.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelke B, Andersson K, Ogren SO, Bolme P. Asphyctic lesion: proliferation of tyrosine hydroxylase-immunoreactive nerve cell bodies in the rat substantia nigra and functional changes in dopamine neurotransmission. Brain Res. 1991;543:1–9. doi: 10.1016/0006-8993(91)91041-x. [DOI] [PubMed] [Google Scholar]
- Chung JH, Lee EY, Jang MH, Kim CJ, Kim J, Ha E, Park HK, Choi S, Lee H, Park SH, Leem KH, Kim EH. Acupuncture decreases ischemia-induced apoptosis and cell proliferation in dentate gyrus of gerbils. Neuro Res. 2007;29:23–27. doi: 10.1179/016164107X172239. [DOI] [PubMed] [Google Scholar]
- Daval JL, Pourie G, Gean S, Lièvre V, Strazielle C, Blaise S, Vert P. Neonatal hypoxia triggers transient apoptosis followed by neurogenesis in the rat CA1 hippocampus. Pediatr Res. 2004;55:561–567. doi: 10.1203/01.PDR.0000113771.51317.37. [DOI] [PubMed] [Google Scholar]
- Ehninqer D. Kempermann G Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- Fan XY, Mothe AJ, Tator CH. Ephrin-B3 decreases the survival of adult rat spinal cord-derived neural stem/progenitor cells in vitro and after transplantation into the injured rat spinal cord. Stem Cells Dev. 2013;22:359–373. doi: 10.1089/scd.2012.0131. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Madaschi L, Caffino L, Marfia G, Di-Giulio AM, Racagni G, Gorio A. Acute spinal cord injury reduces brain derived neur- otrophic factor expression in rat hippocampus. Neuroscience. 2009;159:936–939. doi: 10.1016/j.neuroscience.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Group of Neonatology, Chinese Pediatric Society, Chinese Medical Association (2005) Diagnostic criteria for neonatal hypoxic-ischemic encephalopathy. Zhonghua Er Ke Za Zhi. 43:584. [PubMed] [Google Scholar]
- Guo QS, Zhu MY, Wang L, Fan XJ, Lu YH, Wang ZW, Zhu SJ, Wang Y, Huang Y. Combined transfection of the three transcriptional factors, PDX-1, NeuroD1, and MafA, causes differentiation of bone marrow mesenchymal stem cells into insulia-producing cells. Exp Diabetes Res 2012. 2012:1–10. doi: 10.1155/2012/672013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Feng F, Chen KP. Progress of studies on transcription factor atonal family. J Biol. 2013;30:81–84. [Google Scholar]
- Huang W, Jiang GD, Jia LI. Effect of ultra early acupuncture on Baihui (DU20), Shuigou (DU26) in GLUT1 and GLUT3 of rats with acute cerebral ischemia. Zhongguo Zhongyi Jizheng. 2014;23:51–53. [Google Scholar]
- Huang W, Li J, Bei CX, Xiang T, Wu S. Effects of acupuncture on content of ICAM-1 and VCAM-1 in hippocampus among rats with acute cerebral ischemia. Hunan Zhongyi Zazhi. 2013;29:129–131. [Google Scholar]
- Jia CW, Jiao JY, Sun YX, Zhao WF, Li GW. Affect on monoamine neurotransmitter in ad model sd rats with the “bushenyisui” acupuncture treatment. Zhongguo Zhongyi Jichuyixue Zazhi. 2005;11:853–855. [Google Scholar]
- Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;20:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Katakami N, Matsuhisa M. Combination of MafA, PDX-1 and NeuroD is a useful tool to efficiently induce insulin-producing surrogate beta-cells. Curr Med Chem. 2009;16:3144–3151. doi: 10.2174/092986709788802980. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman E. Long-lasting neurotrophin induced enhancement of synaptic transmis- sion in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Svendsen CN. Origins, functions, and potential of adult neural stem cells. Bioessays. 1999;21:625–630. doi: 10.1002/(SICI)1521-1878(199908)21:8<625::AID-BIES1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lawler PR, Lawler J. Molecular basis for the regulation of anglo-genesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:1–13. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE. NeuroD and neurogenesis. Dev Neurosci-Basel. 1997;19:27–32. doi: 10.1159/000111182. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lei S, Ma BX, Li HW, Zhang JK. Advances in clinical research on acupuncture treatment of cerebral palsy. Zhongyi Yanjiu. 2011;24:77–78. [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479:128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Xu JW, Guo W. Expression of neurogenic differentiation factor in the brain of transient hypoxia postnatal rats. J Anat. 2011;42:610–613. [Google Scholar]
- Liu ZH, Pan PG, Ma MM, Qian XG, Fu WJ, Zhang HY, Zhang CT. Effects of acupuncture on quality of life in children with spastic cerebral palsy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:214–216. [PubMed] [Google Scholar]
- Liu ZH, Pan PG, Qi Yan C, Zhao Y, Chai TQ, Tang CZ, Wang QY, Yang JJ, Lin JQ. Effect of electroacupuncture on neuronal apoptosis and protein expression of nerve growth factor in brain tissues of newborn rats with hypoxic-ischemic injury. Zhongguo Linchuang Kangfu. 2006;23:114–118. [Google Scholar]
- Mu GP, Zeng YC, Chen PD. Electro-acupuncture on neurological function and cellular apoptosis in cerebral ischemia rats. J Emerge Tradit Chin Med. 2014;3:48–50. [Google Scholar]
- Ohira K. Injury-induced neurogenesis in the mammalian forebrain. Cell Mol Life Sci. 2011;68:1645–1656. doi: 10.1007/s00018-010-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-in-ducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia-ischemia. J Biol Chem. 2007;282:36330–36340. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- Roybon L, Deierborg T, Brundin P, Li JY. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci. 2009;29:232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- Rui J, Pei HT. Effects of electroacupuncture on differentiation of neural stem cells of rats with cerebral ischemia. Qingdao Daxue Yixueyuan Xuebao. 2011;47:327–328. [Google Scholar]
- Shapiro EM, Gonzalez-Percz O, Manuel Garcia-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rode brain. Neuroimage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AQ, Zhang YP. Effect on vWF after cerebral ischemia reperfusion of electroacupuncture in middle cerebral artery occlusion rats. Chengdu Zhongyiyao Daxue Xuebao. 2013;36:39–72. [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, DeVito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Chen A, Lan L. A study on mechanism of anti-apoptotic effect of electroacupuncture in acute local cerebral ischemia-reperfusion rats. Zhongguo Kangfu Yixue Zazhi. 2014;10:19–20. [Google Scholar]
- Tu XS. Stem cell transplantation for human diseases: current status and progress in basic research. Zhongguo Zuzhi Gongcheng yanjiu. 2014;45:7364–7369. [Google Scholar]
- Wang L, Bluske KK, Dickel LK, Nakagawa Y. Basal progenitor cells in the embryonic mouse thalamus-their molecular characterization and the role of neurogenins and Pax6. Neural Dev. 2011;6:35. doi: 10.1186/1749-8104-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HX. Clinical study of acupuncture in the treatment of poststroke spastic hemiplegia. Zhongyi Xuebao. 2013;28:1761–1762. [Google Scholar]
- Xu XM, You YL, Zhou S. Research progress of acupuncture promoting angiogenesis after cerebral ischemia. Zhonghua Zhongyiyao Xuekan. 2014;32:63–65. [Google Scholar]
- Yan DM, Wang HL, Zhou PJ, San L. Study on clinical safety of human neural stem cells in treatment of cerebral palsy in children. Zhongguo Fuyou Baojian. 2014;29:22. [Google Scholar]
- Yang Z, Yu H, Rao X, Liu Y, Pi M. Effects of electroacupuncture at the conception vessel on proliferation and differentiation of nerve stem cells in the inferior zone of the lateral ventricle in cerebral ischemia rats. J Tradit Chin Med. 2008;28:58–63. doi: 10.1016/s0254-6272(08)60015-1. [DOI] [PubMed] [Google Scholar]
- You YM, Xue XH, Tao J, Yang SL, Ye XQ, Jiang YJ, Wu YY, Jiang X. Effect of electroacupuncture at Quchi and Zusanli acupoints on the regulation of neuronal mitochondrial apoptosis pathway in rats with cerebral ischemia-reperfusion injury. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2014;10:21–22. [Google Scholar]
- Zhang P, Li J, Liu YH, Chen X, Lu H, Kang Q, Li W, Gao M. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology. 2011;31:384–391. doi: 10.1111/j.1440-1789.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li LY, Yang J. Study on acupuncture effect of Hegu point(LI4)with resting-state fMRI. Zhongguo Zhongxiyi Jiehe Yingxiangxue Zazhi. 2012;10:97–100. [Google Scholar]
- Zhu G, Chen G, Shi L, Feng J, Wang Y, Ye C, Feng W, Niu J, Huang Z. PEGylated rhFGF-2 conveys long-term neuroprote- ction and improves neuronal function in a rat model of parkinson's disease. Mol Neurobiol. 2015;51:32–42. doi: 10.1007/s12035-014-8750-5. [DOI] [PubMed] [Google Scholar]
- Zhu YF, Zhang XJ, Zhu XL, Jia J, Wei HD, Wang Q, Xiong LZ, Chen SY. Role of glutamate transporter 2 in electroacupuncture preconditioning-induced reduction of global cerebral ischemia-reperfusion injury in mice. Zhonghua Mazuixue Zazhi. 2013;33:223–227. [Google Scholar]