Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability in individuals worldwide. Producing a clinically relevant TBI model in small-sized animals remains fairly challenging. For good screening of potential therapeutics, which are effective in the treatment of TBI, animal models of TBI should be established and standardized. In this study, we established mouse models of closed head injury using the Shohami weight-drop method with some modifications concerning cognitive deficiency assessment and provided a detailed description of the severe TBI animal model. We found that 250 g falling weight from 2 cm height produced severe closed head injury in C57BL/6 male mice. Cognitive disorders in mice with severe closed head injury could be detected using passive avoidance test on day 7 after injury. Findings from this study indicate that weight-drop injury animal models are suitable for further screening of brain neuroprotectants and potentially are similar to those seen in human TBI.
Keywords: nerve regeneration, traumatic brain injury, neurological severity score, passive avoidance, weight-drop injury model, C57BL/6 mice, neural regeneration
Introduction
In Malaysia, traumatic brain injury (TBI) has been one of the major causes of death (Moppett, 2007). Although the global magnitude of TBI is unknown due to lack of standardized data collection for TBI (Peeters et al., 2015), available data suggest that the number of TBI victims globally is rising sharply (Alves, 2014). For example, in Europe, TBI incidence has been increasing in last decade from 235 up to 326 per 100,000 population per year (Peeters et al., 2015). Moreover, according to World Health Organization, TBI is supposed to be the major cause of death in 2020 (Organization, 2006). Despite the prognosis for TBI victims has improved in recent years, many survivors of TBI suffer from emotional, cognitive and motor disturbances and a decreased quality of life.
Although many pre-clinical studies devoted to the treatment of TBI in animals was successful, the vast majority of clinical trials to date have failed (Kabadi and Faden, 2014). One of the major reasons is difficulties to produce TBI in animals that will be fully consistent with human pathophysiology of TBI. For instance, the size of the human brain is of importance. Basically, gyrencephalic aminals are more relevant for TBI modelling than lissencephalic as they are possible to be used to produce traumatic axonal injury (TAI), which is essential in human's TBI (Smith et al., 2013). However, the most popular TBI model for small rodents appears to be the controlled cortical impact which produces mainly focal injury of cortex rather than TAI (Xiong et al., 2013). On the other hand, Shohami weight-drop method seems to be more clinically relevant as it causes substantial TAI even in small animals, like mice or rats (Xiong et al., 2013). This fact attracted our attention as we were eager to establish a clinically relevant mouse model of TBI to perform drug tests. We found that animal modelling protocol using the Shohami weight-drop method is well-organized and very comprehensive (Flierl et al., 2009). However, during animal model establishment, we modified the method because we expected to assess not only a neurological impairment but a cognitive deficiency as well. Therefore, in this study, we aimed to produce a translational TBI in mice using Shohami weight-drop method with defined settings and to evaluate both neurological and cognitive deficiencies in animal models with the purpose of further screening of brain neuroprotectants.
Materials and Methods
Animals
Sixty-two male C57BL/6N mice (In Vivos®, Singapore), 11–13-week-old, weighing 25–28 g, were included in this study. These animals were kept in individually ventilated cages (Techniplast®, Italy; n = 3/cage) at 20–23°C under a 12-hour light/dark cycle with free access to water and food (Teklad Global Diets®, USA). All procedures performed on the laboratory animals were conducted in accordance with animal care and use committee guideline of the National Defence University of Malaysia.
Apparatus for TBI producing
A TBI device was designed and assembled at the Faculty of Engineering of the National Defence University of Malaysia in accordance with the recently established protocol (Flierl et al., 2009). The apparatus consists of three parallel horizontal planes joined by four parallel vertical metal bars (Figure 1A). The top and middle surfaces are made of acrylic glass and serve to hold a metal rod with round plastic tip (Figure 1B) that penetrates through them delivering the impact onto the animal's skull. The bottom platform is made of iron and mouse's head can be fixed on it to deliver falling weight into the certain area of the skull (Figure 1C). The device should be placed onto stone surface to decrease energy dissemination.
Figure 1.
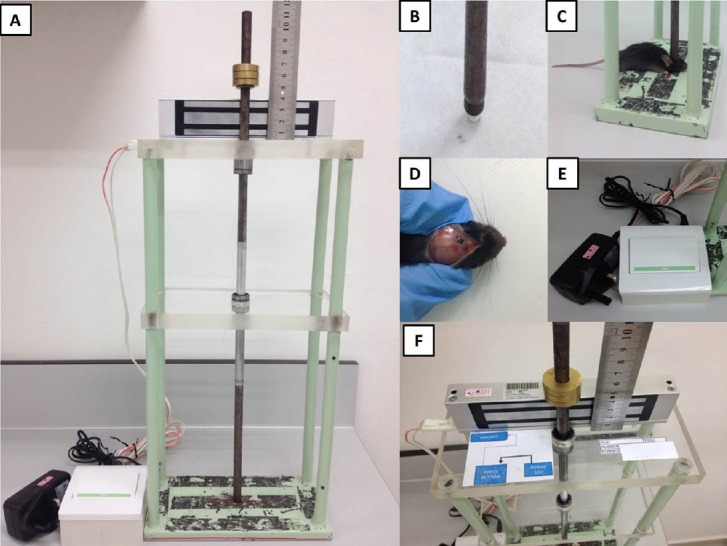
The apparatus causing traumatic brain injury (TBI) in small-sized rodents.
(A) The TBI device which performs weight-drop method to produce closed head trauma in mice; (B) plastic round 3 mm-diameter tip; (C) position of mice on the platform; (D) location of the area of impact; (E) pedal can be pressed by hand or foot, releasing the rod and causing the impact onto exposed mouse skull; (F) the setting, i.e., the certain weight on certain height can be adjusted using a ruler and an electromagnetic lock.
TBI procedure
After general anesthesia with intraperitoneal injection of 60 mg/kg sodium pentobarbital (Dorminal, Alfasan, Holland), a midline incision over the skull was performed, the skin retracted, and the skull exposed to locate the area of impact (Figure 1D). Mice were randomly divided into sham-operated, 200 g weight-drop, 250 g weight-drop and 300 g weight-drop groups.
The head was manually fixed on the bottom platform of the weight-drop device. The 3 mm diameter plastic tip of a metal rod weighting 200 g, 250 g or 300 g was gently advanced onto exposed mouse skull at the left hemisphere 2 mm lateral from the midline and 2 mm back from the coronal suture. The head was held in place manually while the rod was uplifted at the 2 cm height and held by a magnet (Figure 1F). The trauma was performed by pressing the pedal (Figure 1E) which switched off the magnet and the rod free-felt onto the mouse skull. Immediately, oxygen (4 liters O2 per minute) was being supplied using a pressurized cylinder to mice during 2 minutes to avoid respiratory depression. After intraperitoneal injection of 0.05 mg/kg fentanyl (Talgesil, Duopharma(M), Malaysia) mice were returned to their cages. Sham controls received anesthesia and skin incision only.
Neurological severity score (NSS)
To standardize the evaluation of neurological motor function deficit in mice after TBI, NSS has been established (Chen et al., 1996). This scale mimics the Glasgow Outcome Scale, which is a standard method clinically used to evaluate the functional neurological and cognitive outcomes of patients with TBI (King et al., 2005). Initially, NSS included 25 tasks to fulfill, but then the number of tasks was reduced to 10 (Chen et al., 1996; Flierl et al., 2009). The time points for NSS evaluation conventionally were chosen as 1 hour, 4 hours, 24 hours, 2 days, 3 days and 7 days post-injury (Flierl et al., 2009), however, there are many modifications of this (Sinson et al., 1997; Wu et al., 2014). NSS highly correlates with the data obtained using magnetic resonance imaging and histological examination (Tsenter et al., 2008) and thus reflects injury of brain tissue. Each NSS task is described as below.
Task 1: Exit circle. A mouse was placed in the center of the circle (Figure 2B) and the time needed for the mouse to exit the apparatus was monitored. A healthy mouse, as it has an intrinsic seeking behavior, usually leaves the circle within 3 minutes. If the mouse was still inside the circle after 3 minutes 1 point was given, otherwise 0 points were counted.
Figure 2.
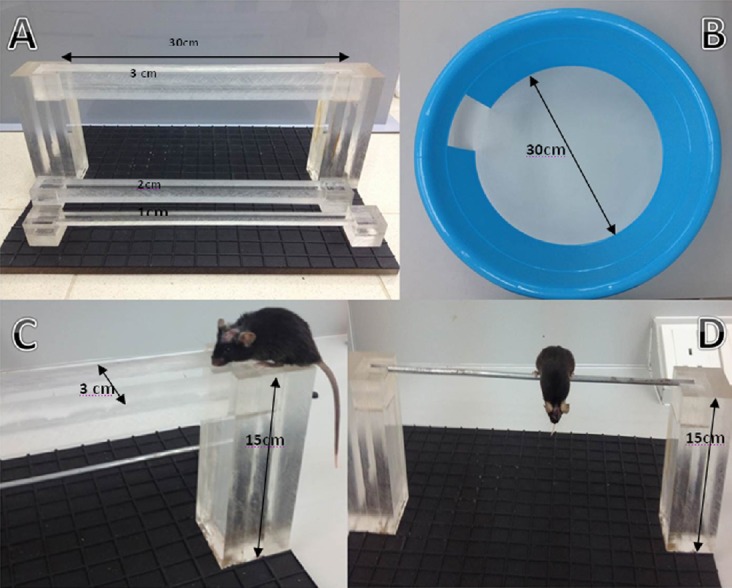
Illustration of the tools employed to evaluate neurological severity score (NSS) and an example of task assessment.
(A) “Beam walk”. To fulfill the task a mouse should cross the beam (3, 2, 1 cm) into opposite side; (B) “Exit circle”. A healthy mouse should find the exit out of circle within 3 minutes. (C) An example of “Beam walk” test; (D) “Round stick balance”. The task is considered successful if a mouse is able to balance with all four paws during 10 seconds.
Task 2: Seeking behavior. Again, the mouse was placed in the center of the circle (Figure 2B), but the exit should be closed. Ability of the mouse to explore the environment and sniffing behavior within 3 minutes were monitored. When the mouse did not perform seeking behavior 1 point was counted, and if seeking behavior was observed, 0 points were counted.
Task 3: Monoparesis/hemiparesis. For this task, anatomic forceps was used. Holding mice up by the tail, we touched the mouse paws by the forceps. If the mouse grips the forceps 0 points were counted, and if the mouse fails to grip or grips with very low intensity, 1 point was added.
Task 4: Straight walk. A mouse was placed onto empty surface to evaluate the ability of the mouse to walk straight as well as its alertness. If the mouse demonstrated impaired gait pattern and failed to actively explore environs, we counted 1 point. When the mouse displayed normal walk, 0 points were given.
Task 5: Startle reflex. If a mouse displayed reaction on the loud paw clapping 0 points were added, otherwise 1 point was given.
Task 6: Beam balance. If a mouse was able to balance on the beam (0.5 × 0.5 mm2) during 10 seconds, 0 points were counted, and if the mouse failed, then 1 point was given.
Tasks 7, 8, 9: Beam walk. A mouse was placed at the end of 3-, 2-, 1-cm-wide beam with 30 cm length (Figure 2A, C). A healthy mouse can easily cross all the beams due to intrinsic seeking reflex. If a mouse did not cross the beam or arrive at the opposite side within 3 minutes, 1 point was given, in case of successful arriving, a point of 0 was counted. If the mouse failed to cross 3-cm- (or 2-cm) wide beam, further tasks with 2-cm (or 1-cm) wide beam would not be carried out and 3 or 2 points were given, respectively.
Task 10: Round stick balance. If a mouse failed to balance during 10 seconds on the 5-mm-diameter round stick, 1 point was added, and if the mouse managed to balance, we counted 0 points (Figure 2D).
All points from 10 tasks should be sum up to get total NSS points.
Passive avoidance test
The passive avoidance test was carried out to assess cognitive deficit in mice with TBI. This is a step-through method, which is focused on learning by mouse to avoid from stepping through a guillotine-like door to a visibly securer dark chamber where the electric shock had been present earlier. To perform this task, a passive avoidance apparatus (Ugo Basile, Switzerland) was used (Figure 3). The latency to refrain from crossing into the punished compartment serves as an index of the avoidance ability giving opportunity to assess the impaired memory. The training place consists of a rectangular chamber divided into two compartments. The first compartment is bright as it is lightened by an overhead stimulus light and the second compartment is dark as it has black non-transparent walls which are always dark. The two compartments are parted by an automatic guillotine door and the floor of each chamber has a steel grid where an electrical foot shock can be provided. During the habituation trial, a mouse was placed in the lighted compartment, facing away from the black compartment and allowed to explore for 30 seconds. After 30 seconds, the door was opened and the mouse was allowed to explore dark space freely. Owing to intrinsic preference to the dark environment, the mouse immediately entered the dark compartment and the door was closed. During the training trial, the mouse was placed in the lighted compartment, facing away from the dark compartment and allowed to explore for 30 seconds before the guillotine door was lifted. When the mouse entered the dark compartment with all four paws, the guillotine door was closed, and the latency to enter the dark compartment was recorded (from the time the door is lifted). When the door had been closed for 3 seconds, a foot shock (0.3 mA, 2 seconds of duration) was applied. On the test day (24 hours after training), the mouse was returned to the lighted compartment, facing away from the dark compartment. Again, after 30 seconds, the guillotine door was lifted. When the mouse entered the dark compartment, the guillotine door was closed, and time required for the mouse to enter the dark compartment was measured as latency time for a maximum period (cut-off time) of 180 seconds. Then the mouse was removed and returned to the home cage.
Figure 3.
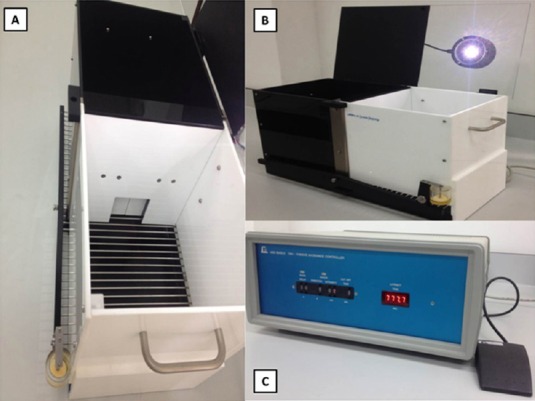
The passive avoidance test.
(A) A guillotine-like door to a visibly securer dark chamber. (B) A rectangular chamber is divided into two compartments: lightened by an overhead stimulus light, and dark. (C) Shock settings (0.3 mA, 2-second duration) and time evaluation (cut-off time 180 seconds).
Statistical analysis
Statistical analysis was performed using the GraphPadPrizm® (La Jolla, CA, USA). Two-way analysis of variance followed by Bonferroni post-hoc tests was used. All results are expressed as the mean ± SD. A level of P < 0.05 was considered statistically significant.
Results
Severity of brain trauma
To establish brain trauma models in mice, we used three different weight parameters: 200 g, 250 g and 300 g. The mortality rate of mice in group with 300 g falling weight was 50% (3 out of 6) that was extremely high, moreover, the NSS points at 1 hour in all mice from this group were more than 8. According to the protocol (Flierl et al., 2009), a mouse that obtains more than 8 NSS points should be withdrawn from the experimental study, so we sacrificed the rest 3 alive mice and stopped further investigation of this group. The mortality rate of mice in group 250 g falling weight falling weight was 14.3% (1 out of 7) and NSS points at 1 hour post-injury was 6.83. This indicates that 250 g falling weight causes severe brain trauma with acceptable mortality rate, as was stated in the established protocol (Flierl et al., 2009). Finally, there were no cases of mouse death (0 out of 7) in group with 200 g falling weight and NSS points were 3.83 that showed development of mild brain trauma.
Neurological impairment
Sham-operated animals gained 0 NSS points and successfully performed all the 10 tasks. In the group with 250 g falling weight, NSS, as was stated above, 1 hour post-injury achieved 6.83 ± 0.89 points, indicating severe brain trauma. NSS was almost the same at 4 hours post-injury and it began to gradually decrease from 24 hours post-injury and reached 4.00 ± 0.82 points on day 7 post-injury (Figure 4). In the group with 200 g falling weight we observed similar trend: initial NSS was 3.83 ± 0.68, indicating mild brain trauma, without any improvement at 4 hours post-injury (3.67 ± 0.47). Then gradual spontaneous recovery was seen as NSS dropped to 0.83 ± 0.68 points on day 7 post-injury.
Figure 4.
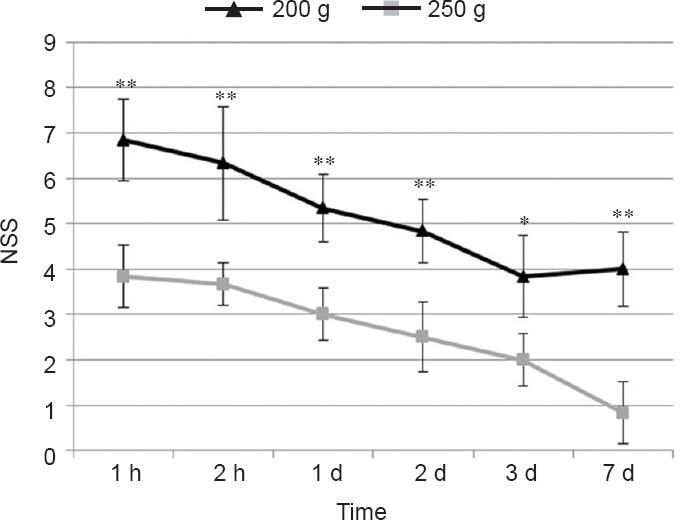
Neurological severity scores (NSS) in mice with traumatic brain injury caused by 250 g and 200 g falling weight.
Lower NSS represent better neurological function. Values are expressed as the mean ± SD (n = 6). Statistical analysis was carried out using twoway analysis of variance with Bonferroni post-hoc test. *P < 0.01, **P < 0.001, vs. 200 g falling weight.
Cognitive impairment
Passive avoidance test results are presented in Figure 5. Sham-operated animals demonstrated good ability to learn the task as they avoided coming into the chamber in which they had been shocked the day before. The mean latency was equal; the cut-off time was 180 seconds, as it was stated in methods. Interestingly, the same results were achieved in mice with TBI from both experimental groups on day 1 and day 4. However, on the 7th day, the mean latency was significantly shorter in mice with TBI caused by 250 g falling weight than in sham-operated mice and mice with TBI caused by 200 g falling weight. This indicates that at 1 week post-injury, mice with TBI caused by 250 g falling weight developed cognitive deficit.
Figure 5.
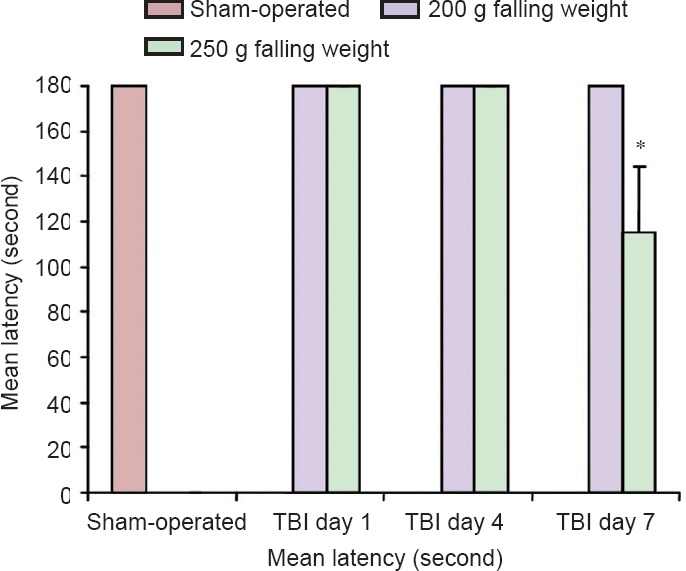
Cognitive function of mice with traumatic brain injury (TBI) using the passive avoidance test.
Values are expressed as the mean ± SD (n = 6). Statistical analysis was performed using two-way analysis of variance with Bonferroni post-hoc test. *P < 0.001, vs. 200 g weight-drop group.
Discussion
We found the optimal settings in our apparatus to perform severe TBI in mice. It is known that NSS reflects the severity of brain damage and correlates with the data obtained by magnetic resonance imaging and histological examination (Tsenter et al., 2008). Our NSS data at 1 hour post-injury are fully consistent with other experimental studies in C57BL/6 mice (Flierl et al., 2009; Semple et al., 2010), confirming that severe brain trauma can be established using 250 g falling weight from 2 cm height and mild brain trauma using 200 g falling weight from 2 cm height. We also observed a spontaneous recovery after TBI during 7 days. The same experience has been published elsewhere, and some differences in the extent of recovery have been noted (Beni-Adani et al., 2001; Yatsiv et al., 2005; Flierl et al., 2009). For instance, Flierl at el. (2009) showed that NSS in mice with TBI on day 7 post-injury was almost 1, while a recent study of Albert-Weiβenberger et al. (2012) demonstrated scoring on day 7 post-injury being around 2. Another study applying Shohami weight-drop method to cause TBI in male C57BL/6J mice revealed that NSS on day 7 post-injury in the control group was more than 3(Beni-Adani et al., 2001). We do hope that this particular method will be further studied to minimize the level of such a drawback because this animal TBI model is very valuable in view of translational TAI that closely mimics human TBI.
A typical clinical symptom in patients with TBI is usually associated with neurological and cognitive deficits. Mouse cognitive disorders have not been mentioned in an established protocol (Flierl et al., 2009). Basically, it is well known from the published literature that Morris water maze seems to be the most common and vigorous method used for evaluation of cognitive impairment and learning deficit in mice with TBI (Milman et al., 2005; Yu et al., 2012; Budinich et al., 2013; Tucker et al., 2015). Other methods, like novel object recognition test, Y-maze test or radial arm water maze test, were also used, but less extensively (Bachstetter et al., 2015; Baratz et al., 2015).
We have decided to employ passive avoidance test as it is a quick procedure for studying short- and long-term memory and an ideal test for first screening, simple to set up and use, and sensitive for both rats and mice (Tayebi Meybodi et al., 2005; Walker, 2010). Our findings reveal that severe TBI causes a cognitive impairment detected at 1 week post-injury, whereas animals with mild trauma do not have any cognitive deficit on day 7 post-injury. Another study which used the same TBI mouse models and evaluated cognitive disorders with passive avoidance test, demonstrated that the ability of mice with mild brain trauma to learn the task was significantly decreased on day 30, but not on day 7 post-injury (Milman et al., 2005). Importantly, that study was performed involving mild but not severe TBI mouse models.
Undoubtedly, more severe TBI causes greater changes which lead to earlier development of cognitive disorders that were demonstrated in our study. Our results also showed that no cognitive changes were observed in animals with mild TBI on 7 day post-injury. This indicates that our findings of cognitive disorders are fully consistent with the findings from other studies.
We successfully designed and set up the apparatus to produce brain trauma in mice. We also established the settings to produce severe brain trauma in C57BL/6 mice, namely 250 g falling weight and 2 cm height. Additionally, we demonstrated the trend of spontaneous improvement of mice with both severe and mild brain trauma during 1 week post-injury. Finally, we showed that cognitive disorders in mice with severe brain trauma presented on day 7 post-injury. Thus, we obtained clinically relevant animal models of traumatic brain injury on the basis of Shohami weight-drop closed head injury animal models. This model holds great potential in future use for pharmacological screening of neuroprotective agents.
Footnotes
Funding: The study was supported by a grant from the Ministry of Higher Education of Malaysia, No. RAGS/2013/UPNM/SKK/01/2.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Grimpe B, Gürese C, Shah ZA, Wong TW, Li CH, Song LP, Zhao M
References
- Albert-Weiβenberger C1, Várrallyay C, Raslan F, Kleinschnitz C, Sirén AL. An experimental protocol for mimicking pathomechanisms of traumatic brain injury in mice. Exp Transl Stroke Med. 2012;4:1. doi: 10.1186/2040-7378-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves JL. Blood-brain barrier and traumatic brain injury. J Neurosci Res. 2014;92:141–147. doi: 10.1002/jnr.23300. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Webster SJ, Goulding DS, Morton JE, Watterson DM, Van Eldik LJ. Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic. J Neuroinflammation. 2015;12:69. doi: 10.1186/s12974-015-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Wang JY, Rubovitch V, Luo W, Hoffer BJ, Greig NH, Pick CG. Transiently lowering tumor necrosis factor-alpha synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J Neuroinflammation. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, Eizenberg O, Trembolver V, Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Budinich CS, Tucker LB, Lowe D, Rosenberger JG, McCabe JT. Short and long-term motor and behavioral effects of diazoxide and dimethyl sulfoxide administration in the mouse after traumatic brain injury. Pharmacol Biochem Behav. 2013;108:66–73. doi: 10.1016/j.pbb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Kabadi SV, Faden AI. Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int J Mol Sci. 2014;15:1216–1236. doi: 10.3390/ijms15011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JT, Jr, Carlier PM, Marion DW. Early Glasgow Outcome Scale scores predict long-term functional outcome in patients with severe traumatic brain injury. J Neurotrauma. 2005;22:947–954. doi: 10.1089/neu.2005.22.947. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, Maas AI. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien) 2015;157:1683–1696. doi: 10.1007/s00701-015-2512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2 -/- mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinson G, Perri BR, Trojanowski JQ, Flamm ES, McIntosh TK. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J Neurosurg. 1997;86:511–518. doi: 10.3171/jns.1997.86.3.0511. [DOI] [PubMed] [Google Scholar]
- Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma. 2013;30:307–323. doi: 10.1089/neu.2012.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebi Meybodi K, Vakili Zarch A, Zarrindast MR, Djahanguiri B. Effects of ultra-low doses of morphine, naloxone and ethanol on morphine state-dependent memory of passive avoidance in mice. Behav Pharmacol. 2005;16:139–145. doi: 10.1097/00008877-200505000-00002. [DOI] [PubMed] [Google Scholar]
- Tsenter J, Beni-Adani L, Assaf Y, Alexandrovich AG, Trembovler V, Shohami E. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J Neurotrauma. 2008;25:324–333. doi: 10.1089/neu.2007.0452. [DOI] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT. Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J Neurotrauma. 2015 doi: 10.1089/neu.2015.3977. doi:10.1089/neu.2015.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA. Animal models. Adv Exp Med Biol. 2010;678:138–146. doi: 10.1007/978-1-4419-6306-2_18. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2006) Neurological disorders: public health challenges. Geneva: World Health Organization; [Google Scholar]
- Wu CH, Hung TH, Chen CC, Ke CH, Lee CY, Wang PY, Chen SF. Post-injury treatment with 7, 8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS One. 2014;9:e113397. doi: 10.1371/journal.pone.0113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhang Y, Chuang DM. Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J Neurotrauma. 2012;29:2342–2351. doi: 10.1089/neu.2012.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]


