Keywords: nerve regeneration, inflammatory response, tumor necrosis factor alpha, nuclear factor-kappa B, neural regeneration
Abstract
Lipoxin A4 can alleviate cerebral ischemia/reperfusion injury by reducing the inflammatory reaction, but it is currently unclear whether it has a protective effect on diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury. In this study, we established rat models of diabetes mellitus using an intraperitoneal injection of streptozotocin. We then induced focal cerebral ischemia/reperfusion injury by occlusion of the middle cerebral artery for 2 hours and reperfusion for 24 hours. After administration of lipoxin A4 via the lateral ventricle, infarction volume was reduced, the expression levels of pro-inflammatory factors tumor necrosis factor alpha and nuclear factor-kappa B in the cerebral cortex were decreased, and neurological functioning was improved. These findings suggest that lipoxin A4 has strong neuroprotective effects in diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury and that the underlying mechanism is related to the anti-inflammatory action of lipoxin A4.
Introduction
Hyperglycemia, hyperlipidemia and hypertension are risk factors that appear to play major roles in the severity of stroke. Hyperglycemia has been documented to be associated with aggravating cerebral damage, with increased morbidity and mortality (Li et al., 1996; Meierhans et al., 2010). Furthermore, animal and human studies have linked hyperglycemia in the acute phase of ischemic stroke to worse clinical outcomes regardless of the presence of pre-existing diabetes (Bémeur et al., 2007). Inflammation is one of the main mechanisms by which diabetes mellitus (DM) aggravates ischemic cerebrovascular disease. It worsens cerebral ischemia and hypoxia, increases the cerebral infarct volume, and results in a higher disability rate in diabetic patients than in patients without DM (Di Carlo, 2009). Therefore, inhibition of the inflammatory response may be an important strategy for the treatment of cerebral ischemic injury.
Nuclear factor-kappa B (NF-κB) is the major cytokine participating in the inflammatory cascade (Prabhakar, 2013), and promoters and enhancers of various inflammatory mediators, such as tumor necrosis factor alpha (TNF-α), contain kappa B sites. NF-κB can be activated by the development of DM after cerebral ischemia/reperfusion (I/R) injury (Kim et al., 2014).
Lipoxin A4 (LXA4), a metabolite of arachidonic acid existing in human leukocytes, can alleviate cerebral I/R injury by intervening in the inflammatory reaction. It reduces the expression of inflammatory cytokines and adhesion molecules, and the adhesion between leukocytes and endothelial cells (Ye et al., 2010; Chen et al., 2013; Zhao et al., 2014). However, it is still unclear whether LXA4 has a protective effect on DM-aggravated I/R injury. In this study, we observed the effects of LXA4 on the expression of NF-κB and TNF-α in diabetic rats with focal cerebral I/R injury, and investigated the protective effects of LXA4 on DM complicated by focal cerebral I/R injury.
Materials and Methods
Ethics statement and experimental animals
The experimental protocol was approved by the Animal Ethics Committee of Guangdong Medical Laboratory Animal Center and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
A total of 36 adult male Sprague-Dawley rats, 5–6 months of age, specific pathogen-free level, weighing 250–280 g, were provided by Guangdong Provincial Medical Laboratory Animal Center of China (license No. SCXK (Yue) 2008-0002). Rats were randomly divided into three groups with 12 rats per group: sham (no surgery), I/R (DM + I/R), and LXA4 (DM + I/R + LXA4).
Induction of DM
Rat models of type 1 DM were established by intraperitoneal (i.p.) injection of streptozotocin (STZ; Sigma, St. Louis, MO, USA) at 20 mg/kg in the first 24 hours and at 35 mg/kg on the next day following 8 hours of fasting (Sun et al., 2005). STZ was dissolved in cold citrate buffer (pH 4.5) immediately before use and the solution was made fresh daily. The rats were allowed access to sucrose (5%) in drinking water during the next 24 hours to prevent hypoglycemia. Rats with blood glucose levels of more than 250 mg/dL were considered to be diabetic and were included in the study (Chaturvedi et al., 2009).
Establishment of rat models of focal cerebral I/R injury
After DM induction, I/R models were established once blood glucose levels remained stable for 7 days. Rats were anesthetized with chloral hydrate (350 mg/kg, i.p.) and subjected to middle cerebral artery occlusion as described previously with slight modifications (Longa et al., 1989). Briefly, the right common carotid artery, internal carotid artery (ICA), and external carotid artery (ECA) were exposed, and the ECA was dissected distally. A special nylon suture with a rounded tip (line diameter of 0.26 mm, head diameter of 0.36 ± 0.02 mm) was inserted into the ICA through the ECA stump and was gently advanced to occlude the middle cerebral artery (MCA). Animals that died after ischemia induction, or that had evidence of subarachnoid hemorrhage on extraction of brain tissue were excluded. After 2 hours of middle cerebral artery occlusion (MCAO), the suture was withdrawn to restore perfusion. Body temperature was monitored with a rectal probe and maintained at 37°C during the entire procedure. Rats in the sham group were manipulated in the same way but without MCAO.
LXA4 administration
The commercially obtained LXA4 (Cayman Chemical Company, Ann Arbor, MI, USA) was dissolved in ethanol, so we first tested the effect of ethanol in I/R rats during a preliminary study (data not shown). To match the ethanol concentration in the LXA4 working solutions, 5 mL of ethanol (0.09%) was cerebroventricularly administered. Neither infarct volume nor neurological deficits were affected by 5 mL of ethanol (0.09%). This result is in line with a previous study showing that treatment with 1.0 or 1.5 g/kg of ethanol reduced total infarct volume in rats, but 0.5 g/kg ethanol did not reduce brain injury (Krenz and Korthuis, 2012). In our present study, 5 mL of ethanol (0.09%) per rat is roughly equivalent to 0.000015 g/kg of ethanol, which is far below the level of 0.5 g/kg of ethanol previously established to have no effect on the measures under investigation. Thus, we considered it appropriate to use saline to replace ethanol as a vehicle for treatment.
LXA4 was stored in ethanol at −80°C. In the LXA4 group, 5 μL of LXA4 (0.2 mM) was administered into the lateral ventricles under stereotactic guidance at 24 hours after MCAO. The coordinates were as follows: anteroposterior, −0.8 mm; lateral, 1.5 mm; and dorsoventral, 3.8 mm from bregma (Paxinos and Watson, 2005). All surgical procedures were completed under sterile conditions and penicillin (200 000 U, i.m.v.) was injected to prevent infection. The rats in the sham and I/R groups received 5 μL of saline (i.c.v.) at 24 hours after reperfusion.
Neurological deficit evaluation
Neurological scores were assessed by a blinded observer at 24 hours after reperfusion. Scores from 0 to 5 were given based on a motor behavioral test as previously reported: 0, no neurological deficit; 1, failure to fully extend the contralateral forepaw; 2, decreased grip of the contralateral forelimb while the tail is pulled; 3, spontaneous circling or walking toward the contralateral side; 4, no spontaneous motor activity; 5, unresponsive to stimulation (Hunter et al., 2000).
Infarct volume
At 24 hours after reperfusion, the rats (n = 6 per group) were anaesthetized by chloral hydrate and decapitated. The brains were quickly removed and placed in cold saline for 5 minutes. Then, the tissues were cut at 2-mm intervals from the frontal pole to obtain five coronal sections that were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) for 30 minutes followed by overnight immersion in 10% formalin. The infarcted regions were quantified by assessing each section with Image J software (National Institutes of Health, Bethesda, Maryland, USA). The infarct size as a percentage of the whole brain was calculated (Frieler et al., 2011).
Western blot assay
At 24 hours after reperfusion, the rats (n = 6 per group) were anaesthetized by chloral hydrate (10%, stoted in 4°C refrigerator) and decapitated. The brains were quickly removed (stored in -80°C refrigerator). Total proteins in the cerebral cortex samples were extracted. Nuclear proteins were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Boster Company, Wuhan, China) according to the manufacturer's instructions. Protein concentrations were determined using bicinchoninic acid protein assay with bovine serum albumin as the standard (McConkey, 1984). Protein expression was detected as follows. In brief, total protein samples (40 mg) were separated on 12% sodium dodecyl sulfate-polyacrylamide gels and then transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST) and incubated overnight at 4°C with mouse anti-rat NF-κB p65 monoclonal antibody (1:600; Santa Cruz Biotechnology, Dallas, TX, USA) and rabbit anti-rat TNF-α monoclonal antibody (1:500; Santa Cruz Biotechnology). Rabbit anti-rat β-actin monoclonal antibody (1:500; Sigma) or mouse anti-rat Histone H3.1 monoclonal antibody (1:200; Santa Cruz Biotechnology) served as the internal control. After extensive rinsing with TBST, the membranes were incubated with goat anti-mouse or goat anti-rabbit IgG conjugated with horseradish peroxidase (1:7,000; LI-COR Biosciences, Lincoln, NE, USA) for 1 hour at 37°C. Bound antibody was detected using an enhanced chemiluminescence detection system (ECL, Pierce, Rockford, IL, USA) and exposed on X-ray films. The ratio of optical density value as protein expression in each sample was analyzed with Image J software.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL, USA) and the data are expressed as the mean ± SEM. Data from different groups were compared using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls tests (for normally distributed data). A value of P < 0.05 was considered statistically significant.
Results
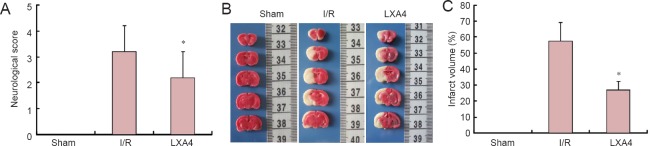
LXA4 improved the neurological function of diabetic rats with cerebral I/R injury
No rats in the sham group showed signs of impairment. The neurological deficits in the LXA4 group were significantly improved at 24 hours after reperfusion compared with the I/R group (P < 0.05; Figure 1A).
Figure 1.
Effect of lipoxin A4 (LXA4) on neurological deficits and brain infarct volume in diabetic rats with cerebral ischemia/reperfusion (I/R) injury.
(A) Neurological scores. The higher the score, the worse the neurological function. (B) Representative 2,3,5-triphenyltetrazolium chloride-stained brain sections (infarcted areas are white). Rats were subjected to 2 hours of middle cerebral artery occlusion followed by 24 hours of reperfusion. (C) Brain infarct volume. Data are expressed as the mean ± SEM (n = 6 rats per group). The data of different groups were compared by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls tests (for normally distributed data). *P < 0.05, vs. I/R. S: Sham group; I/R: diabetes mellitus + I/R injury group; LXA4: diabetes mellitus + I/R injury + LXA4 group.
LXA4 reduced the brain infarct volume in diabetic rats with cerebral I/R injury
TTC staining showed that at 24 hours after LXA4 administration no rats in the sham group had infarctions. The infarct volume was significantly decreased after 24 hours of reperfusion in the LXA4 group compared with the I/R group (P < 0.05; Figure 1B, C).
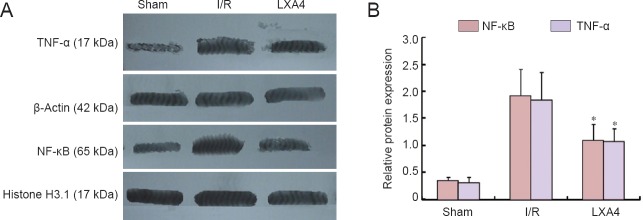
LXA4 decreased TNF-α and NF-κB expression in the cerebral cortex of diabetic rats with cerebral I/R injury
Western blot results showed that there were significant differences in the expression levels of TNF-α and NF-κB between the sham, I/R, and LXA4 groups (P < 0.001). The expression levels of TNF-α and NF-κB in the LXA4 group were significantly lower than those in the I/R group (P < 0.05; Figure 2).
Figure 2.
Effect of lipoxin A4 (LXA4) on expression levels of TNF-α and NF-κB in the cerebral cortex of diabetic rats with cerebral ischemia/reperfusion (I/R) injury (western blot assay).
Beta-actin and Histone H3.1 were used as internal controls. The results for TNF-α were expressed as the optical density ratio of TNF-α to β-actin, and the results for NF-κB were expressed as the ratio of the optical density of NF-κB to Histone H3.1. Each bar represents the mean ± SEM (n = 6 rats per group). The data of different groups were compared by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls tests (for normally distributed data). *P < 0.05, vs. I/R. S: Sham group; I/R: diabetes mellitus + I/R injury group; LXA4: diabetes mellitus + I/R injury + lipoxin A4 group.
Discussion
Studies have shown that the risk of stroke is 1.5–3 times higher, and the risk of recurrent stroke is doubled, in diabetic patients (Harada et al., 2009; Kostulas et al., 2009). However, the mechanism by which diabetes aggravates cerebral I/R injury is not fully understood. Research in recent years has shown that a strong inflammatory response may contribute to the aggravation of cerebral I/R injury caused by diabetes. For example, inflammatory cytokines contribute to the conversion of ischemic injury to inflammatory injury (Fan et al., 2012; Luan et al., 2013).
Lipoxins are endogenous anti-inflammatory lipid-based autacoids, resulting from the biosynthesis of prostaglandins and leukotrienes. They are produced endogenously in picogram to nanogram range in most murine species during inflammation and disease pathogenesis (Xu et al., 2012; Karra et al., 2015). It has been found that the lipid and its isomers, which are endogenous anti-inflammatory mediators, can inhibit the expression of many kinds of inflammatory cells, such as neutrophils, various proinflammatory cytokines and adhesion molecules (Chiang et al., 2005). Because lipoxin can inhibit the inflammatory response in many ways, it is referred to as the “brake signal” (Sobrado et al., 2009). The neuroprotective effects of lipoxin have been demonstrated in renal ischemia injury (Leonard et al., 2002), peritonitis (Mirakaj et al., 2014), pulmonary inflammatory response (Meng et al., 2015), ulcerative colitis (Yates et al., 2014) and cerebral I/R injury. The present study has shown that neurological deficit score and infarct size are significantly decreased in diabetic rats with cerebral I/R injury, which indicates that the nerve cell injury may be ameliorated by diabetes mellitus.
Our research group has previously demonstrated that NF-κB and TNF-α play important negative roles in cerebral I/R injury. Therefore, LXA4 can inhibit the activation of NF-κB and TNF-α and decrease the inflammatory reaction, which may be important for treating diabetes mellitus combined with cerebral I/R injury. The present study showed that TNF-α-induced NF-κB transcription activity can be inhibited by the lipoxin. It has been suggested that NF-κB is an important factor mediating the effect of lipoxin (Hao et al., 2015). Lipids can inhibit neutrophil and nuclear accumulation of NF-κB and transcription factor activator protein-1 (AP-1) in monocytes, and down-regulate expression of interleukin-8 (IL-8) (Huang et al., 2014). In addition, lipoxin can inhibit the up-regulation of gene expression stimulated by Salmonella typhimurium. These genes are mostly regulated by NF-κB (Wang et al., 2011b), and the change in gene expressionincreases NF-κB levels in the nucleus (Wu et al., 2010). Studies from Chen et al. (2013) have demonstrated that LXA4 can inhibit the activation of NF-κB, and the activation of proinflammatory cytokines, including TNF-α, IL-1, IL-6, IL-8 and platelet activating factor. Wang et al. (2011a) showed that LXA4, in the form of aspirin-triggered 15-epi-LXA4, can effectively inhibit the lipopolysaccharide-induced accumulation of NF-κB in leukocytes. In vitro experiments have confirmed that LXA4 can reduce the phosphorylation of NF-κBp65 in myosin and mesangial cells (Kure et al., 2010). Our results showed that the expression of NF-κB in diabetic patients with cerebral I/R injury was significantly decreased, indicating that the protective effect of lipoxin on cerebral I/R injury may be related to the inhibition, or decreased expression, of NF-κB.
In addition, Forsman and Dahlgren (2009) reported that lipoxin can inhibit the expression of TNF-α. Ye et al. (2010) showed that after injection of LXA4 in rats with focal cerebral I/R injury, TNF-α and IL-1 decreased significantly, thus inhibiting the inflammatory reaction and alleviating cerebral I/R injury. There is evidence that LXA4 can inhibit I/R injury caused by TNF-α in the gastrointestinal tract and other important organs. LXA4 may promote this recovery through regulating the signal transducer and activator of transcription-3 (STAT-3) and the protein kinase B (Akt1)/cyclin-dependent kinase inhibitor 1B (p27kip1) signal transduction pathways (Breckwoldt et al., 2008). Their results showed that the expression of TNF-α in diabetic patients with cerebral I/R injury was significantly decreased. This may indicate that the protective effect of lipoxin on cerebral I/R injury may be related to its inhibition, or decreased expression, of TNF-α.
In conclusion, LXA4 can inhibit inflammatory reactions in diabetic cerebral I/R injury and has a neuroprotective effect. Its mechanism may be related to the inhibition of TNF-α and NF-κB expression. However, the protective effect and mechanisms of LXA4 in cerebral I/R injury in rats require further study.
Footnotes
Funding: This study was supported by a grant from the Zhuhai Key Discipline Project of China, No. 200880.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Brooks W, Raye W, Yu J, Li CH, Song LP, Zhao M
References
- Bémeur C, Ste-Marie L, Montgomery J. Increased oxidative stress during hyperglycemic cerebral ischemia. Neurochem Int. 2007;50:890–904. doi: 10.1016/j.neuint.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A, Bhawani G, Agarwal PK, Goel S, Singh A, Goel RK. Antidiabetic and antiulcer effects of extract of Eugenia jambolana seed in mild diabetic rats: study on gastric mucosal offensive acid-pepsin secretion. Indian J Physiol Pharmacol. 2009;53:137–146. [PubMed] [Google Scholar]
- Chen Z, Wu Z, Huang C, Zhao Y, Zhou Y, Zhou X, Lu X, Mao L, Li S. Effect of lipoxin A4 on myocardial ischemia reperfusion injury following cardiac arrest in a rabbit model. Inflammation. 2013;36:468–475. doi: 10.1007/s10753-012-9567-x. [DOI] [PubMed] [Google Scholar]
- Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38:4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- Fan T, Jiang WL, Zhu J, Feng Zhang Y. Arctigenin protects focal cerebral ischemia-reperfusion rats through inhibiting neuroinflammation. Biol Pharm Bull. 2012;35:2004–2009. doi: 10.1248/bpb.b12-00463. [DOI] [PubMed] [Google Scholar]
- Forsman H, Dahlgren C. Lipoxin A4 metabolites/analogues from two commercial sources have no effects on TNF-α-mediated priming or activation through the neutrophil formyl peptide receptors. Scand J Immunol. 2009;70:396–402. doi: 10.1111/j.1365-3083.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- Frieler RA, Meng H, Duan SZ, Berger S, Schütz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42:179–185. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Xu F, Hao J, He YQ, Zhou XY, Dai H, Wu LQ, Liu FR. Lipoxin A4 suppresses lipopolysaccharide-induced hela cell proliferation and migration via NF-κB pathway. Inflammation. 2015;38:400–408. doi: 10.1007/s10753-014-0044-6. [DOI] [PubMed] [Google Scholar]
- Harada S, Fujita WH, Shichi K, Tokuyama S. The development of glucose intolerance after focal cerebral ischemia participates in subsequent neuronal damage. Brain Res. 2009;1279:174–181. doi: 10.1016/j.brainres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Huang YH, Wang HM, Cai ZY, Xu FY, Zhou XY. Lipoxin A4 inhibits NF-κB activation and cell cycle progression in RAW264. 7 cells. Inflammation. 2014;37:1084–1090. doi: 10.1007/s10753-014-9832-2. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Karra L, Haworth O, Priluck R, Levy BD, Levi-Schaffer F. Lipoxin B₄ promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol. 2015;8:852–862. doi: 10.1038/mi.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostulas N, Markaki I, Cansu H, Masterman T, Kostulas V. Hyperglycaemia in acute ischaemic stroke is associated with an increased 5-year mortality. Age Ageing. 2009;38:590–594. doi: 10.1093/ageing/afp120. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, Fujita T, Kutsumi H, Arita M, Azuma T, Yoshida M. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Ther. 2010;332:541–548. doi: 10.1124/jpet.109.159046. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Hannan K, Burne MJ, Lappin DWP, Doran P, Coleman P, Stenson C, Taylor CT, Daniels F, Godson C, Petasis NA, Rabb H, Brady HR. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- Li PA, Kristián T, Shamloo M, Siesjö K. Effects of preischemic hyperglycemia on brain damage incurred by rats subjected to 2. 5 or 5 minutes of forebrain ischemia. Stroke. 1996;27:1592–1601. doi: 10.1161/01.str.27.9.1592. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- McConkey B. C reactive protein concentrations during long distance running. Br Med J (Clin Res Ed) 1984;289:1696. doi: 10.1136/bmj.289.6459.1696-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierhans R, Béchir M, Ludwig S, Sommerfeld J, Brandi G, Haberthür C, Stocker R, Stover JF. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care. 2010;14:R13. doi: 10.1186/cc8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Mambetsariev I, Tian Y, Beckham Y, Meliton A, Leff A, Gardel ML, Allen MJ, Birukov KG, Birukova AA. Attenuation of lipopolysaccharide-induced lung vascular stiffening by lipoxin reduces lung inflammation. Am J Respir Cell Mol Biol. 2015;52:152–161. doi: 10.1165/rcmb.2013-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211:1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; 2005. [Google Scholar]
- Prabhakar O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:705–710. doi: 10.1007/s00210-013-0871-2. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernández-López D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yang G, Zhao H, Savelkoul HF, An L. Multidose streptozotocin induction of diabetes in BALB/c mice induces a dominant oxidative macrophage and a conversion of TH1 to TH2 phenotypes during disease progression. Mediators Inflamm. 2005;2005:202–209. doi: 10.1155/MI.2005.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther. 2011a;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, Yuan SY, Shang Y, Yao SL. Aspirin-triggered lipoxin A(4) attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J Neuroinflammation. 2011b;8:95. doi: 10.1186/1742-2094-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Liu B, Dong L, Wu HJ. NF-κB is involved in inhibition of lipoxin A4 on dermal inflammation and hyperplasia induced by mezerein. Exp Dermatol. 2010;19:e286–e288. doi: 10.1111/j.1600-0625.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhao F, Lin F, Chen J, Huang Y. Lipoxin A4 inhibits the development of endometriosis in mice: the role of anti-inflammation and anti-angiogenesis. Am J Reprod Immunol. 2012;67:491–497. doi: 10.1111/j.1600-0897.2011.01101.x. [DOI] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, Yao SL. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–183. doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- Zhao BS, Liu Y, Gao XY, Zhai HQ, Guo JY, Wang XY. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19:16925–16936. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]