Keywords: nerve regeneration, spinal cord contusion, senegenin, thinleaf milkwort root, motor function, apoptosis, electrophysiology, neural regeneration
Abstract
Senegenin has been shown to inhibit neuronal apoptosis, thereby exerting a neuroprotective effect. In the present study, we established a rat model of spinal cord contusion injury using the modified Allen's method. Three hours after injury, senegenin (30 mg/g) was injected into the tail vein for 3 consecutive days. Senegenin reduced the size of syringomyelic cavities, and it substantially reduced the number of apoptotic cells in the spinal cord. At the site of injury, Bax and Caspase-3 mRNA and protein levels were decreased by senegenin, while Bcl-2 mRNA and protein levels were increased. Nerve fiber density was increased in the spinal cord proximal to the brain, and hindlimb motor function and electrophysiological properties of rat hindlimb were improved. Taken together, our results suggest that senegenin exerts a neuroprotective effect by suppressing neuronal apoptosis at the site of spinal cord injury.
Introduction
Direct or indirect physical trauma to the spinal cord can result in hemorrhage, edema, neuronal apoptosis, and the interruption of nerve conduction (Yang et al., 2014; Guan et al., 2015; Zhang et al., 2015a). The key to restoring function in the injured spinal cord is improving neuronal survival and promoting axonal growth (Agrawal et al., 2010; Peng et al., 2011; Chen et al., 2013; Liu et al., 2013).
Thinleaf milkwort root can calm the heart and mind, dispel phlegm, wake a patient from unconsciousness, and reduce inflammation. Senegenin, the main bioactive ingredient in thinleaf milkwort root, has been shown to effectively protect neurons and inhibit apoptosis (Pi et al., 2011; Xu et al., 2012; Zhao et al., 2014; Horiuchi et al., 2015; Wang et al., 2015b). In the present study, we examined the effects of senegenin on neuronal apoptosis and motor function following spinal cord contusion injury. We also evaluated the effects of the medicine on somatosensory-evoked potentials (SEPs) and motor-evoked potentials (MEPs) after injury.
Materials and Methods
Ethics statement
All experimental protocols were approved by the Animal Ethics Committee, Nankai Hospital, China. The animal studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Animals
Sixty-four clean female Sprague-Dawley rats aged 3 months and weighing 250–300 g were purchased from the Animal Laboratory of Tianjin Medical University of China (animal license No. SCXK (Jin) 20070005).
Establishment of a rat model of spinal cord contusion injury
Forty-four rats were intraperitoneally anesthetized with 10% chloral hydrate, 350 mg/kg, and then fixed on the table in the prone position. After shaving, a median incision was made on the back, with the T8–9 spinous process as the center, to fully expose the T7–10 spinous processes and vertebral laminae. The T8–9 spinous process and a portion of the vertebral lamina were removed, and the intact dura mater was exposed. In accordance with a modified Allen's method (Li et al., 2010), a 10-g object was dropped from a height of 2.5 cm, which directly impacted the dura mater and spinal cord. Paralysis of the lower limbs was observed along with tail swinging and spasms. These responses confirmed successful establishment of the model. The wound was washed with hydrogen peroxide, and the tissue was sutured layer by layer. Extrusion was conducted 2–3 times daily to assist with urination until the micturition reflex recovered. Model establishment failed in three rats, and one rat was excluded because of death. The remaining 40 rats were equally and randomly assigned to the spinal cord contusion group and the senegenin group. An additional 20 rats were used in the sham surgery group (exposure of the spinal cord only).
Treatment with senegenin
Three hours after injury, rats in the senegenin group were injected with senegenin 30 mg/g (30 mg senegenin was dissolved in 10 mL physiological saline; Shanghai Chenglin Biotechnology Co., Ltd., Shanghai, China) via the tail vein for 3 consecutive days. Rats in the spinal cord contusion and sham surgery groups were injected with 10 mL of physiological saline via the tail vein.
Evaluation of motor function
Motor functions were assessed before injury, 1 and 3 days after injury, and 1, 2, 3 and 4 weeks after injury in the three groups.
The Basso-Beattie-Bresnahan (BBB) locomotor rating scale (Yin and Xu, 2012) was used to score locomotor function. The scale ranges from 0 to 21, where 0 = complete paralysis and 21 = normal. Main outcome measures included the number and range of joint movements, weight bearing capacity, limb coordination and tail activity.
The inclined plane test (Luo et al., 2015) was also used to evaluate locomotor function. Rats were placed on a smooth tiltboard. The angle was increased in increments of 5°, and the maximum angle at which the rat remained on the board for 5 seconds was recorded.
Terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labelling (TUNEL) assay
Three days after injury, five rats from each group were anesthetized with chloral hydrate. The chest was opened and aortic cannulation was carried out through the left ventricle, followed by fixation with 4% paraformaldehyde. With the injury site as the center, a 2-cm-long piece of spinal cord was excised and fixed in paraformaldehyde. The TUNEL assay was conducted in accordance with instructions in the TUNEL kit (Roche, Mannheim, Germany). 5-μm-thick sections were washed with phosphate buffered saline (PBS), incubated with 20 μg/mL proteinase K at 37°C for 30 minutes, washed with PBS, and then dried. All samples were incubated with TUNEL reaction mixture in the dark at 37°C for 60 minutes, and washed with PBS. Six non-overlapping 200× fields at the injury site were randomly selected using a fluorescence microscope (Shanghai SYAOO Instrument Equipment Co., Ltd., Shanghai, China). The number of TUNEL-positive cells was quantified and an average value was calculated.
Reverse transcription-polymerase chain reaction (RT-PCR)
Three days after injury, five rats from each group were intraperitoneally anesthetized with 10% chloral hydrate, 350 mg/kg. A 50-mg sample of spinal cord was obtained from the injury site. Total RNA was extracted from the spinal cord in accordance with instructions included with the Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA content was measured with an ultraviolet spectrophotometer (He, 2006). Using a two-step RT-PCR kit (TaKaRa, Dalian, China), mRNA was reverse-transcribed into cDNA, and cDNA was amplified using PCR. Primer sequences are listed in Table 1. Based on Genbank data (http://www.ncbi.nlm.nih.gov/genbank), optimal primers were identified with Primer 5.0 software (Premier Biosoft International, Vancover, Canada). Primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China after BLAST analysis. Amplified products were electrophoresed. PCR reaction conditions were as follows: denaturation at 94°C for 2 minutes; 35 cycles of 94°C for 45 seconds, annealing at 62°C for 1 minute and extension at 72°C for 1 minute; final extension at 72°C for 8 minutes. Electrophoresis results were analyzed using a gel image analysis system (Shanghai Qiaofeng Industrial Co., Ltd., Shanghai, China). The ratio of the integrated optical density of Bcl-2, Bax or Caspase-3 to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to quantify relative expression levels.
Table 1.
Primer sequences
Western blot assay
The spinal cord sample was centrifuged at 300 × g for 30 minutes and the supernatant was collected. Total protein concentration was measured using the Bradford protein assay (Yan et al., 2006). Samples were denatured by sodium dodecyl sulfate polyacrylamide gel electropheresis and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked at 37°C for 2 hours, and then washed three times for 10 minutes each. Afterwards, the membrane was incubated with rabbit anti-rat Bax, Bcl-2 or Caspase-3 monoclonal antibody (1:800; Guangrui Biological Technology Co., Ltd., Shanghai, China) and rabbit anti-rat GAPDH monoclonal antibody (1:1,000; Beijing Baiao Laibo Technology Co., Ltd., Beijing, China) at 4°C overnight, followed by four washes with tris-buffered saline/Tween-20 (TBST) for 5 minutes each. The membranes were then incubated with goat anti-rabbit IgG (1:700; Beijing Baiao Laibo Technology Co., Ltd.) at 37°C for 1.5 hours, followed by four washes with TBST for 5 minutes each. The samples were visualized with 3,3′-diaminobenzidine. Images were analyzed using Quantity One analysis software (Bio-Rad, Hercules, CA, USA). The optical denstiy ratio of Bax, Bcl-2 or Caspase-3 to that of GAPDH was used to determine relative expression levels.
Hematoxylin-eosin staining
Four weeks after injury, five rats from each group were anesthetized with 10% chloral hydrate, 350 mg/kg. The chest was opened to expose the heart, and the animal was transcardially perfused with physiological saline and fixed with 4% paraformaldehyde. A 1-cm section of spinal cord tissue at the injury site was dissected out, dehydrated through a graded alcohol series, and sliced into 20-μm-thick longitudinal frozen sections. Afterwards, the sections were stained with hematoxylin for 5 minutes, washed with running water, differentiated with hydrochloric acid in ethanol for 10 seconds, washed with running water for 10 minutes, stained with eosin for 7 minutes, washed with running water, dehydrated through a graded alcohol series, permeabilized with xylene, mounted with neutral resin, and observed with a light microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Nerve integrity as detected by fluorogold retrograde tracing
Four weeks after injury, five rats from each group were anesthetized, and the sciatic nerve was exposed along the vastus lateralis. The sciatic nerve was then contused with tissue forceps (Shanghai Zhili Medical Equipment Factory, Shanghai, China). 0.4 μL of 2% fluorogold (Beijing Bohui Innovation Technology Shareholding Co., Ltd., Beijing, China) was injected at multiple points at the site of injury with a microsyringe (Shanghai Bolige Trade Co., Ltd., Shanghai, China) in the dark at a rate of 0.1 μL/min. The needle was maintained in place for 5 minutes at each point. The incision was sutured layer by layer. After 1 week of normal feeding postoperatively, samples at the injury site were collected for histological examination, stored in 30% sucrose and placed at 4°C overnight. The samples were subsequently embeded in optimal cutting temperature compound at –18°C, and then sliced into 20-μm-thick coronal frozen sections. Five sections from each rat in each group were selected to observe the distribution of fluorogold-labeled cells in the spinal cord proximal to the brain with the fluorescence microscope. The number of fluorogold-positive fibers per field was quantified at 200× magnification.
Detection of SEPs and MEPs
Four weeks after injury, SEPs and MEPs in five rats from each group were measured with a Keypoint 4 Evoked Potential System (Beijing Weidi Kangtai Medical Instrument Co., Ltd., Beijing, China).
SEP: The rats were intraperitoneally anesthetized with 10% chloral hydrate, 350 mg/kg, and then positioned horizontally. A stimulating electrode was placed on the hindlimbs. A recording electrode was placed on the scalp over the hindlimb cortical sensory area (i.e., intersection of the coronal and sagittal sutures). A reference electrode was placed 0.5 cm posterior to the hindlimb cortical sensory area. Electrical pulses (direct current, square wave) were given until the hindlimb displayed a slight tic. The parameters were as follows: current intensity of 5–15 mA, pulse width of 0.2 ms, frequency of 3 Hz; superimposed 50–60 times. SEP latency and amplitude were recorded.
MEP: The rats were intraperitoneally anesthetized with 10% chloral hydrate, 350 mg/kg. The stimulating electrodes were placed 2 mm anterior to the coronal suture and 2 mm lateral to the sagittal suture on the scalp (i.e., motor cortex). The stimulation parameters were as follows: stimulus intensity of 40 mA, pulse width of 0.1 ms, frequency of 1 Hz, sensitivity of 5 μV, scanning speed of 5 ms; superimposed 300–500 times. MEP latency and amplitude were recorded.
Statistical analysis
All data were expressed as the mean ± SD and analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Repeated measures analysis of variance and Tukey's test were used to assess intergroup differences. P < 0.05 was considered statistically significant.
Results
Senegenin improved hindlimb motor function in rats with spinal cord contusion injury
There was no significant difference in BBB locomotor rating scale scores or inclined plane test scores among the rats prior to injury (P > 0.05). Motor function was significantly impaired in the spinal cord contusion group compared with the sham surgery group at 1–4 weeks after injury (P < 0.05). Motor function was significantly improved in the senegenin group compared with the spinal cord contusion group at 1–4 weeks after injury (P < 0.05; Table 2).
Table 2.
Effects of senegenin on hindlimb motor function in rats with spinal cord contusion injury
Senegenin suppressed apoptosis in rats with spinal cord contusion injury
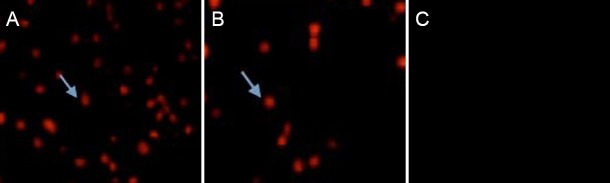
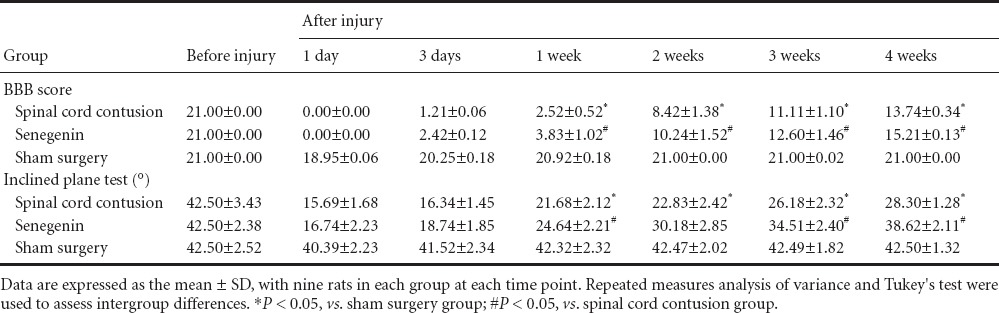
Three days after injury, no apoptotic cells were observed in the sham surgery group, but a large number of apoptotic cells were visible in the spinal cord contusion group (28.45 ± 5.72/200× field), as demonstrated by TUNEL assay. The number of apoptotic cells was significantly less in the senegenin group than in the spinal cord contusion group (12.82 ± 3.48/200× field; P < 0.05; Figure 1).
Figure 1.
Effects of senegenin on apoptosis after spinal cord contusion (TUNEL assay, fluorescence microscope, × 200).
Three days after injury, apoptotic cells (arrows) were distributed throughout the site of injury, and apoptotic cells were visible on the margins of the site of injury in the spinal cord contusion group (A) and in the senegenin group (B). Nuclei of apoptotic cells exhibited a red color. No apoptotic cells were identified in the sham surgery group (C). The number of apoptotic cells was lower in the senegenin group than in the spinal cord contusion group. TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
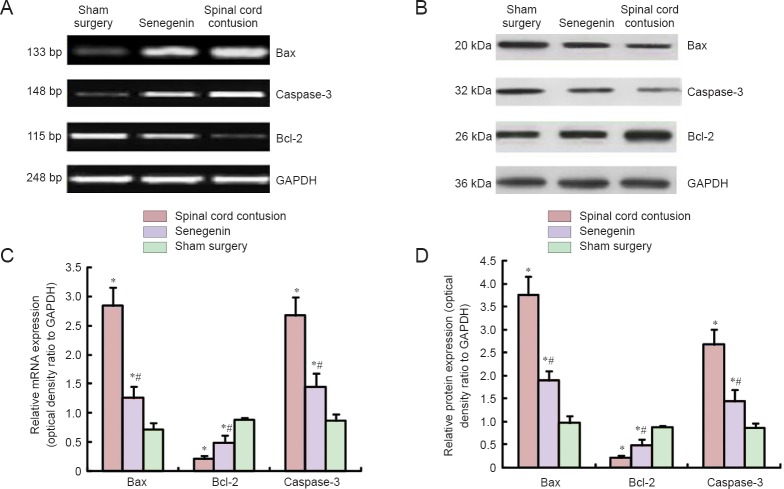
Effects of senegenin on apoptosis-related mRNA and protein expression levels in the injured rat spinal cord
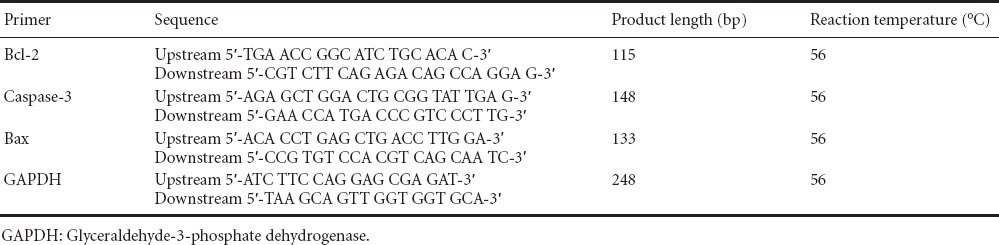
RT-PCR and western blot assay revealed that, 3 days after injury, Bax and Caspase-3 mRNA and protein expression levels were higher, but Bcl-2 mRNA and protein expression levels were lower, in the spinal cord contusion group compared with the sham surgery group (all P < 0.05). Compared with the spinal cord contusion group, Bax and Caspase-3 mRNA and protein expression levels were lower, but Bcl-2 mRNA and protein expression levels were higher, in the senegenin group (all P < 0.05; Figure 2).
Figure 2.
Effects of senegenin on apoptosis-related mRNA (A) and protein (B) expression in the injured spinal cord of rats.
Data are expressed as the mean ± SD, with five rats in each group. Repeated measures analysis of variance and Tukey's test were used to assess intergroup differences. *P < 0.05, vs. sham surgery group; #P < 0.05, vs. spinal cord contusion group.
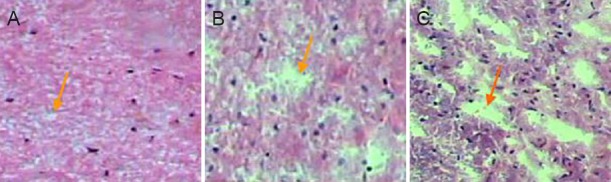
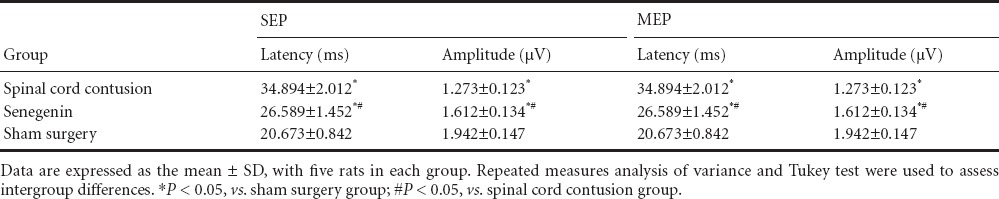
Effects of senegenin on pathological changes in rats with spinal cord contusion
Four weeks after injury, hematoxylin-eosin staining showed a complete and clear structure of the spinal cord, with no cavity, in the sham surgery group. In contrast, in the spinal cord contusion group, the spinal cord tissue exhibited a loose structure, with a visible cavity. Loose spinal cord tissue and a small cavity were visible in the senegenin group (Figure 3).
Figure 3.
Effects of senegenin on pathological changes in rats with spinal cord contusion (hematoxylin-eosin staining, × 40).
Four weeks after injury, no cavity was visible in the sham surgery group (A). A small cavity was seen in the senegenin group (B). A substantial cavity was identified in the spinal cord contusion group (C). Arrows point to the cavity.
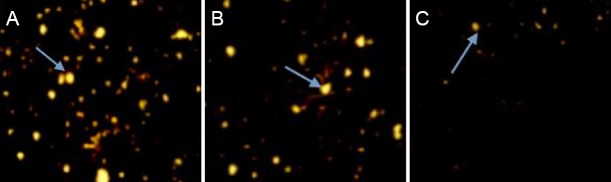
Senegenin promoted nerve regeneration in rats with spinal cord contusion
Fluorogold retrograde tracing revealed many fluorogold-labeled pyramidal cells and axons in the spinal cord proximal to the brain in the sham surgery group. Four weeks after injury, the number of fluorogold-positive fibers was lower in the spinal cord contusion group (8.63 ± 2.67/200× field) than in the sham surgery group (43.42 ± 5.82/200× field) (P < 0.05). Compared with the spinal cord contusion group, the number of fluorogold-positive fibers was greater in the senegenin group (26.46 ± 4.74/200× field; P < 0.05; Figure 4).
Figure 4.
Effects of senegenin on nerve regeneration in rats with spinal cord contusion (fluorogold retrograde tracing, × 200).
(A) Sham surgery group; a large number of fluorogold-positive fibers are visible. (B) Senegenin group; many fluorogold-positive fibers are visible 4 weeks after injury. (C) There is a reduced number of fluorogold-positive fibers in the spinal cord contusion group. Arrows point to fluorogold-positive fibers.
Senegenin promoted the recovery of neurophysiological function in rats with spinal cord contusion
Four weeks after injury, the evoked potential waveform disappeared in the senegenin and spinal cord contusion groups. In the senegenin group, SEP and MEP latencies were shorter, and amplitudes were higher, compared with the spinal cord contusion group (Table 3).
Table 3.
Effects of senegenin on neurophysiological functioning in rats with spinal cord contusion injury
Discussion
Senegenin has been shown to inhibit oxidation, scavenge free radicals, improve cell survival, decrease lactate dehydrogenase leakage, reduce malondialdehyde levels, and increase superoxide dismutase activity (Zhang et al., 2011, 2015b; Hu et al., 2015; Lu et al., 2015). Senegenin can improve learning and memory abilities in Alzheimer's disease mice by lowering oxidative stress (Li et al., 2014; Lin et al., 2014; Zhang and Wang, 2014). Furthermore, senegenin upregulates Bcl-2 expression, decreases Bax expression, maintains mitochondrial membrane permeability, inhibits cytochrome C release from mitochondria into the cytoplasm, lessens secondary nerve injury, and promotes the proliferation and differentiation of endogenous stem cells by maintaining the mitochondrial membrane potential and preventing the uncoupling of oxidative phosphorylation (Hou et al., 2014, 2015; Li et al., 2015a, b; Mao et al., 2015; Wang et al., 2015a). Although there are numerous studies on the antioxidative properties of senegenin, the effects of senegenin on apoptosis and its impact on the electrophysiological characteristics of the hindlimb after spinal cord contusion remained unclear. Therefore, in this study, we addressed these unresolved issues using a rat model of spinal cord contusion injury. We found that senegenin treatment substantially improved motor function and reduced the number of apoptotic cells in rats with spinal cord contusion injury. Furthermore, Bax and Caspase-3 mRNA and protein expression levels were diminished, while Bcl-2 mRNA and protein expression levels were increased, in the injured spinal cord after senegenin treatment. This indicates that senegenin may inhibit apoptosis by regulating the expression of apoptotic proteins.
In summary, our findings show that senegenin improves the electrophysiological properties of the rat hindlimb, and effectively promotes functional recovery after spinal cord contusion injury. Thus, senegenin may have substantial therapeutic potential in the treatment of spinal cord injury.
Footnotes
Funding: This research was supported by a grant from the Science and Technology Development Plan of Jilin Province of China, No. 2011084.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Patel B, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Agrawal G, Sherman D, Maybhate A, Gorelik M, Kerr DA, Thakor NV, All AH. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J Clin Neurosci. 2010;17:1159–1164. doi: 10.1016/j.jocn.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Chen HQ, Huang H, Gu J, Zhang X, Ye SL. The effects of hyperbaric oxygen preconditioning on the mitochondrial apoptosis of spinal cord neurons. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2013;35:82–85. [Google Scholar]
- Guan X, Dong MY, Yu Y, Gao B. The changes of apoptosis and the expression of endoplasmic reticulum stress classical markers in spinal cord neurons of rats with ischemia reperfusion injury. Shandong Yiyao. 2015;55:27–29. [Google Scholar]
- He R. Experument and research on rehabolotation of injured peripheral nerves by adhering sutures with plasmid of GDNF gene. Chongqin, China: Chongqing Medical University; 2006. [Google Scholar]
- Horiuchi H, Oshima Y, Ogata T, Morino T, Matsuda S, Miura H, Imamura T. Evaluation of injured axons using two-photon excited fluorescence microscopy after spinal cord contusion injury in yfp-h line mice. Int J Mol Sci. 2015;16:15785–15799. doi: 10.3390/ijms160715785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Zhang L, Zhang L, Tang P. Acute spinal cord injury in rats should target activated autophagy. J Neurosurg Spine. 2014;20:568–577. doi: 10.3171/2014.1.SPINE13237. [DOI] [PubMed] [Google Scholar]
- Hou ZY, Chen Z, Wei JS. Effects of buyang huanwu decoction on neural cell apoptosis after acute spinal cord injury in rats. Zhejiang Zhongyiyao Jiehe Zazhi. 2015;25:8–11. [Google Scholar]
- Hu J, Zeng L, Huang J, Wang G, Lu H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. doi: 10.1016/j.brainres.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Li CM, Xie SJ, Wang T, Du WB, Yang ZB, Quan RF. Effects of electro-acupuncture on neuronal apoptosis and associative function in rats with spinal cord injury. Zhongguo Gushang. 2015a;28:733–738. [PubMed] [Google Scholar]
- Li L, Guo JD, Wang HD, Shi YM, Yuan YL, Hou SX. Prohibitin 1 gene delivery promotes functional recovery in rats with spinal cord injury. Neuroscience. 2015b;286:27–36. doi: 10.1016/j.neuroscience.2014.11.037. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Y, Liu P, Zhu X, Chen M, Wang H, Lu D, Qi R. Senegenin inhibits hypoxia/reoxygenation-induced neuronal apoptosis by upregulating RhoGDIá. Mol Neurobiol. 2014;52:1561–1571. doi: 10.1007/s12035-014-8948-6. [DOI] [PubMed] [Google Scholar]
- Li YF, Chen D, Zhang DW, Xue F, Liu JM. Improvement and eletrophysiology assessment of the rats allen's model for acute spinal cord injury. Zhongguo Shiyan Zhenduan Xue. 2010;14:1169–1172. [Google Scholar]
- Lin L, Yan L, Zhang H, Li X, Zhang J, Dou H, Shen M, Yin X, Qu C, Ni J. Simultaneous analysis of polygala acid, senegenin and 3, 6′-disinapoylsucrose in rat plasma by liquid chromatography-tandem mass spectrometry: application to a pharmacokinetic study after oral administration. Biomed Chromatogr. 2014;28:594–600. doi: 10.1002/bmc.3076. [DOI] [PubMed] [Google Scholar]
- Liu YD, Chen XM, Yu ZS, Guan H. Apoptosis of neuron in cerebral motor cortex after spinal cord injury in rat. Zhongguo Jizhu Jisui Zazhi. 2013;23:546–552. [Google Scholar]
- Lu YF, Wu C, Liu J, Klassen CD. Genetically graded activation of Nrf2 protects mice against carbon tetrachloride-induced hepatotoxicity. Zunyi Yixueyuan Xuebao. 2015;38:6–14. [Google Scholar]
- Luo SH, Xiong WG, Liang ZQ, Chi HX. Treadmill exercise test and head-up tilt test in screening the cause of syncope. Jiangxi Yiyao. 2015;50:614–616. [Google Scholar]
- Mao M, Zhang T, Liu Y, He GQ, Wang KJ. Different spinal cord damage on apoptosis of rat secondary impact study. Zhongguo Mianyixue Zazhi. 2015:1461–1464. [Google Scholar]
- Peng LW, Yang GF, Ji JG, Wei XS, Li P, Zhao JR, Zhang RL, Feng X. Effects of serum of patients with acute motor axonal neuropathy on expression of apoptosis-related proteins in rat spinal motor neuron. Shanghai Jiaotong Daxue Xuebao: Yixue Ban. 2011;31:580–583. [Google Scholar]
- Pi T, Xue XY, Lin LF, Su JN, Cheng X, Luo HM. Neurotrophic effects of senegenin on the cultures of newborn rat cortical neurons. Zhongguo Yaoli Xue yu Duli Xue Zazhi. 2011;25:40–44. [Google Scholar]
- Wang DQ, Zhang PP, Li ZJ, Liu Y. Effects of mecobalamin on Bax and Bcl-2 in neurons after peripheral nerve injury. Zhonghua Laodong Weisheng Zhiyebing Zazhi. 2015a;33:841–843. [PubMed] [Google Scholar]
- Wang J, Hu B, Cao F, Sun S, Zhang Y, Zhu Q. Down regulation of lncSCIR1 after spinal cord contusion injury in rat. Brain Res. 2015b;1624:314–320. doi: 10.1016/j.brainres.2015.07.052. [DOI] [PubMed] [Google Scholar]
- Xu KL, Chen Q, Liu W, Yao YY, Xia XX, Zhang BL, Li YF. Effect of tenuigenin on tau protein phosphorylation at Ser396 site in neurons of AD rats induced by Aβ1–40. Zhongguo Bingli Shengli Zazhi. 2012;28:1605–1609. [Google Scholar]
- Yan P, Yang Q, Wang HZ, Xie J, Liu DW, Niu B. Comparison of Lowry's modified assay with Bradford's dye-binding assay for determining proteins in earthworm extract. Shanxi Yike Daxue Xuebao. 2006;37:9–11. [Google Scholar]
- Yang JL, Duan HW, Ma GP. Effects of platelet-activating factor administered intrathecally on the expression of neuronal apoptosis in spi-nal cord of rats. Zuzhong yu Shenjing Jibing. 2014;21:263–265. 293. [Google Scholar]
- Yin M, Xu YJ. Comparison study on BBB test with combined behavioral score after spinal cord injury in rats. Zhongguo Xueye Liubianxue Zazhi. 2012;22:398–400. [Google Scholar]
- Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015a;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Qi RB, Wang ZQ, Zhao YR, Wang HD, Lu DX. Effect of senegenin on H 2 O 2 -induced damage in hippocampal neurons of SD rats. Zhongguo Bingli Shengli Zazhi. 2011;27:1059–1065. [Google Scholar]
- Zhang P, Wang JM. The research idea of Polygala granule. Shijie Zuixin Yixue Xinxi Wenzhai: Dianzi Ban. 2014;14:114. [Google Scholar]
- Zhang X, Shi LL, Gao X, Jiang D, Zhong ZQ, Zeng X, Rao Y, Hu X, Li TZ, Li XJ, Li L, Chen JM, Xia Q, Wang TH. Lentivirus-mediated inhibition of tumour necrosis factor-á improves motor function associated with PRDX6 in spinal cord contusion rats. Sci Rep. 2015b;5:8486. doi: 10.1038/srep08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YD, Liu PH, Li XM, Lu F, Wang HD, Lu DX, Qi RB. Preliminary mechanism of senegenin against H/R-induced apoptosis of primary cortical neurons. Zhongguo Bingli Shengli Zazhi. 2014;31:1166–1171. [Google Scholar]