Keywords: nerve regeneration, peripheral nerve injury, nerve conduit, selective nerve regeneration, chemotaxis, human umbilical cord blood stem cell, stem cell transplantation, neural regeneration
Abstract
Given the anatomic complexity at the bifurcation point of a nerve trunk, enforced suturing between stumps can lead to misdirection of nerve axons, thereby resulting in adverse consequences. We assumed that Y-tube conduits injected with human umbilical cord stem cells could be an effective method to solve such problems, but studies focused on the best type of Y-tube conduit remain controversial. Therefore, the present study evaluated the applicability and efficacy of various types of Y-tube conduits containing human umbilical cord stem cells for treating rat femoral nerve defects on their bifurcation points. At 12 weeks after the bridging surgery that included treatment with different types of Y-tube conduits, there were no differences in quadriceps femoris muscle weight or femoral nerve ultrastructure. However, the Y-tube conduit group with longer branches and a short trunk resulted in a better outcome according to retrograde labeling and electrophysiological analysis. It can be concluded from the study that repairing a mixed nerve defect at its bifurcation point with Y-tube conduits, in particular those with long branches and a short trunk, is effective and results in good outcomes.
Introduction
The current gold standard for treating nerve defects is treatment with autologous nerve grafting, which requires more surgery time and leads to donor site morbidity. Additionally, the direct nerve suturing methods can inevitably cause misdirection of newly grown axons, which results in inadequate functional recovery (Shapira et al., 2015). Although the development of microsurgical techniques has greatly improved the accuracy of peripheral nerve suturing after injury, total functional recovery has never been achieved (Eser et al., 2009). This is likely because the regenerating axons cannot reach their prospective target organs (Brushart et al., 1998). Because of the anatomic complexity at the bifurcation point of a nerve trunk, regenerating axons from the nerve trunk can easily grow into the wrong pathways of the distal nerve stump, resulting in poor outcomes (de Ruiter et al., 2013).
As the theory of chemotactic peripheral nerve regeneration has become widely accepted, some scholars have suggested that nerve defects bridged by nerve conduits with a little space between the stumps may accelerate the selective and more accurate axonal regeneration that would lead to better functional recovery (Jiang et al., 2006). Injecting stem cells into the nerve conduits can provide the necessary neurotropic support and accelerate axonal regeneration after peripheral nerve injury (Mosahebi et al., 2002). Human umbilical Wharton jelly mesenchymal stem cells (hWJMSCs) have been shown to provide regenerating axons with the necessary cellular and neurotropic support in in vivo and in vitro studies (Koh et al., 2008).
It is assumed that if peripheral nerve injury is bridged by Y-tube conduits at the bifurcation point, neurotrophic factors secreted from a specific distal nerve stump can be concentrated to a relatively high level to better guide axons sprouting from the proximal nerve stump and regain functional recovery of the target tissue. However, to the best of our knowledge, few studies have focused on the therapeutic efficacy of Y-tube conduits on peripheral nerve injury at the bifurcation point in terms of chemotactic nerve regeneration. To determine whether Y-tube conduits can facilitate peripheral nerve regeneration after injury, as well as to establish a better Y-tube conduit design to bridge peripheral nerve defect, the current study tested the efficacy of different Y-tube conduits to repair the rat femoral nerve following its bifurcation point.
Materials and Methods
Preparation of Y-tube conduits and hWJMSC culture
Silicon Y-tube conduits with an inner diameter of 1.0 mm, outer diameter of 1.2 mm, 4-mm-long nerve trunk, and 4-mm-long branches were provided by YTGX Biotechnology Ltd., Beijing, China. The Y-tube conduits were cut into two different shapes: (1) a 4-mm-long nerve trunk with 3-mm-long branches; (2) a 3-mm-long nerve trunk with 4-mm-long branches (Figure 1). hWJMSCs were isolated from a mature umbilical cord retracted from the medical waste of the Fifth Affiliated Hospital of Xinjiang Medical University in China. All procedures were approved and monitored by the Ethics Committee of the Fifth Affiliated Hospital of Xinjiang Medical University, and the procedures conformed to the guidelines of the Helsinki Declaration. Briefly, with the consent of the parents, the umbilical cord of a healthy baby born at full term was washed in Hank's Balanced Salt Solution, the umbilical cord artery and vein were removed, and the Wharton jelly was cut into 1 mm × 1 mm × 1 mm tissue pieces. The tissue was then cultured in a culture dish with 10% fetal bovine serum (FBS, Lonza, Verviers, Belgium) in Dulbecco's Modified Eagle's medium (DMEM) in a 5% CO2 incubator at 37°C. The culture medium was changed every 3–4 days. When the cells reached 80–90% confluency, they were detached using PBS with 0.05% trypsin (Life Technologies, Grand Island, NY, USA) and 0.04% EDTA (Sigma-Aldrich, St. Louis, MO, USA), and the cells were re-plated at 5,000 cells/cm2. Passage 3 cells were used for transplantation.
Figure 1.
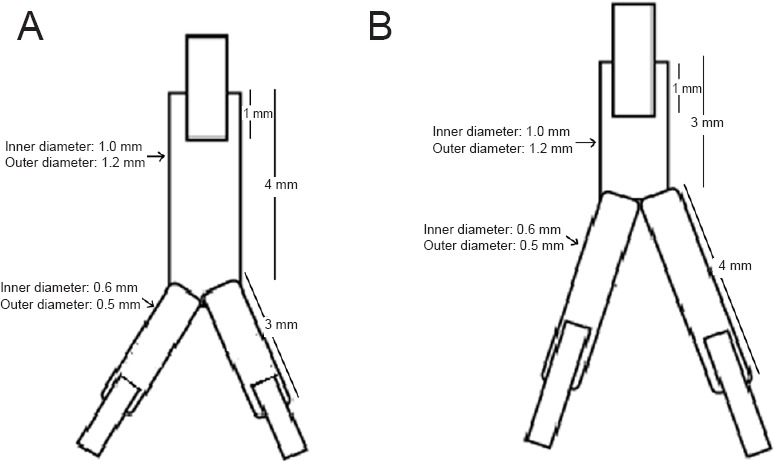
Sketch map of two types of Y-tube conduits.
(A) The 4-mm-long nerve trunk with 3-mm-long branches; (B) the 3-mm-long nerve trunk with 4-mm-long branches.
Ethics statement and experimental animals
The animal studies were approved by the Ethical Committee of Tsinghua University in China and performed in accordance with the Guide for the Care and Use of Laboratory Animals, the National Institutes of Health. Precautions were taken to minimize the number of animals used in each experiment and their suffering. A total of 50 female, specific pathogen-free, Sprague-Dawley rats (8 weeks old, 250–300 g) were housed with standard rodent food and unlimited water for 1 week before surgery. Experimental animals were provided by the Experimental Animal Center of Tsinghua University (License No. SYXK (Jing) 2009-0022).
Preparation of a rat model of femoral nerve injury and nerve injury treatment
The rats were anesthetized with 10% chloral hydrate (0.3 mL/100 g) and were placed on an operating table in a supine position. The right femoral nerve was exposed, and the rats were treated with different methods and divided into different experimental groups accordingly. Group A (n = 10): sham surgery was performed, and no injury was brought to the femoral nerve and its branches. Group B (n = 20): a 5-mm-long nerve defect was created by cutting 3 mm of the femoral nerve trunk and 2 mm of each of its branches with microscissors. The defect was bridged using a Y-tube conduit with a 4-mm-long nerve trunk and 3-mm-long branches by inserting each nerve stump 1 mm into the conduit (Figure 1A). A total of 5 μL passage 3 hWJMSCs (5 × 107/mL) (Figure 2) was injected into the conduit with a microsyringe. Group C (n = 20): a 5-mm-long nerve defect was created by cutting 3 mm of femoral nerve trunk and 2 mm of each of its branches with microscissors. The defect was bridged using a Y-tube conduit with a 3-mm-long nerve trunk and 4-mm-long branches by inserting each nerve stump 1 mm into the conduit (Figure 1B). A total of 5 μL hWJMSCs was injected into the conduit using a microsyringe.
Figure 2.
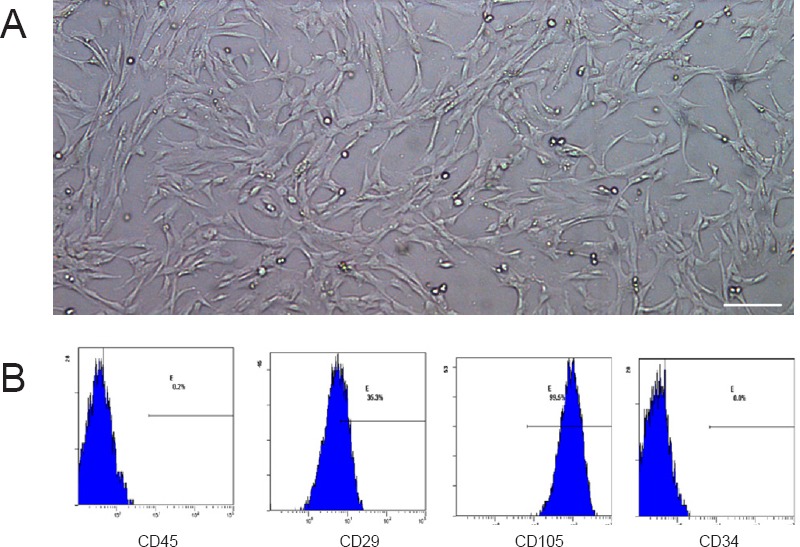
Morphology and identification of hWJMSCs at passage 3.
hWJMSCs at passage 3 were injected into the nerve conduit to support axonal growth. (A) Morphology of passage 3 hWJMSCs under an inverted microscope showing spindle-shaped cells. Scale bar: 100 µm. (B) Flow cytometry results showed expression percentages of CD45, CD29, CD105, and CD34 as 0.2%, 35.3%, 99.5%, and 0.0%, respectively. Therefore, the cells were identified as hWJMSCs. hWJMSCs: Human umbilical Wharton jelly mesenchymal stem cells.
Following surgery, the incisions were sutured and the rats were housed in standard conditions for 12 weeks. All animals were intraperitoneally injected with cyclimycin A (YTGX Biotechnology, Beijing, China) starting from 3 days before surgery until 2 weeks after surgery.
Twelve weeks after surgery, one animal died in group B and one in group C because of incision infection. The remaining rats were included in the final evaluation.
Electrophysiological testing
At 12 weeks after surgery, five animals from each group were selected and anesthetized with 10% chloral hydrate (0.3 mL/100 g). The femoral nerves in the normal and surgical sites were exposed and the motor-evoked potential of the quadriceps femoris muscle was measured. The stimulating electrode (RM6240, Chengdu Instrument Factory, Sichuan, China) was placed on the proximal end of the suture, and the recording electrode was pierced into the quadriceps femoris muscle. The latency period and amplitude of the evoked potential of quadriceps femoris were measured using the following electric stimulation parameters: 1.0 mA, 0.1 ms, and 1.0 Hz.
Weight of quadriceps femoris muscle
Following electrophysiological testing, the right and left quadriceps femoris muscles were excised and weighed on an electronic balance (Beijing Liu Yi Instrument Company, Beijing, China). The value obtained after dividing the weight of the right and left quadriceps femoris muscles was calculated.
Retrograde labeling
At 12 weeks after surgery, five animals from each group were selected. A 2% True Blue solution (Sigma-Aldrich) was prepared with distilled water and stored at 4°C, and a 5% Dil solution (Sigma-Aldrich) was prepared with dehydrated alcohol and stored at 4°C. The rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g) and were placed on the operation table in a supine position. Then, an incision was made on the right groin, and the right femoral nerve, its muscle branch, and the saphenous nerve braches were exposed. The muscle branch was cut from where it grew into the quadriceps femoris muscle, and the same length of saphenous nerve was cut to prepare for the next step. A 10-μL microsyringe was used to inject 5 μL of Dil and True Blue into an aseptic plastic chamber prepared beforehand. The nerve stumps of the muscle branches were inserted into the chamber filled with Dil, and the saphenous branch was inserted into the chamber of True Blue. The incision was then sutured. The rats were housed under standard conditions for 3 days. At 3 days after retrograde labeling, all animals were anesthetized using a previously described method (Shapira et al., 2015). The dorsal spinal canal was opened and the spinal cord was exposed. The L2–4 segment was carefully removed and placed on a –25°C freezing microtome (CE1900 Freezing Microtome, Leica, Heidelberg, Germany) until it froze, and then it was embedded and sagitally cut. Three 15-μm thick sections were cut after the spinal gray matter was reached. The sections were immediately observed under a fluorescence microscope with a photographic attachment (IX70 fluorescence microscope and photographic attachment, Olympus, Tokyo, Japan) for neurons in the ventral horn of spinal cord stained with Dil and True Blue. Images were collected to quantify the number of stained neurons. Axons sprouting from neurons that only entered the muscle branch were stained red (Dil), and the ratio of red-stained neurons within the total number of neurons in the ventral horn of spinal cord was calculated to estimate the accuracy of axonal regeneration.
Transmission electron microscopy examinations
At 12 weeks after surgery, five animals from each group were selected and deeply anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g). Then, the animals were sacrificed by cervical dislocation. The femoral nerves were harvested and fixed in 4% formalin for 24 hours. A 1-mm3 piece of femoral nerve was obtained 3 mm distal from the proximal end of the femoral nerve and was sent for transmission electron microscopy observation (HitachiH-7650, Hitachi High-Technologies, Tokyo, Japan). The nerve sheath thickness, axonal density, and fiber diameter were measured using Image-Pro Plus 6.0 image analyzing software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Data were analyzed using SPSS17.0 software (SPSS, Chicago, IL, USA) with one-way analysis of variance followed by the least significant difference test, and values were recorded as the mean ± SD. A level of P < 0.05 represented significance.
Results
Effect of different types of Y-tube conduits injected with hWJMSCs on quadriceps femoris muscle weight in rats with a femoral nerve defect
At 12 weeks after surgery, various degrees of quadriceps femoris muscle atrophy were identified in all animals in groups B and C. The value obtained by dividing the right quadriceps femoris muscle weight by the left quadriceps femoris muscle weight was 0.61 ± 0.18 in group B and 0.65 ± 0.20 in group C. No significant difference was found between the two groups (P > 0.05).
Effect of different types of Y-tube conduits injected with hWJMSCs on electrophysiological function of rats with a femoral nerve defect
Results from electrophysiological testing showed that, at 12 weeks after surgery, the latency period of the quadriceps femoris muscle in group C was significantly shorter than that in group B (P < 0.05; Figure 3A), although the amplitude was significantly greater than that in group B (P < 0.05; Figure 3B).
Figure 3.
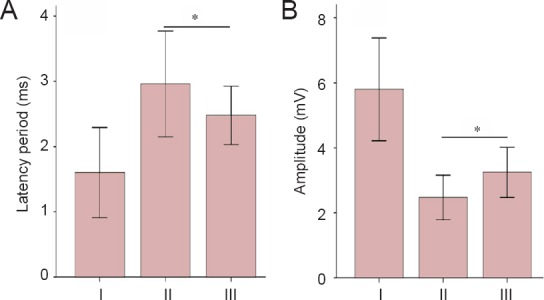
Effect of different types of Y-tube conduits injected with hWJMSCs on electrophysiological function of rats with a femoral nerve defect.
(A, B) The latency (A) and amplitude (B) of the evoked potential of quadriceps femoris. Group A: Sham surgery was performed, and no injury was brought to the femoral nerve and its branches. Group B: The nerve defect was bridged using a Y-tube conduit with a 4-mm-long nerve trunk and 3-mm-long branches by inserting each nerve stump 1 mm into the conduit. A total of 5 μL passage 3 hWJMSCs was injected into the conduit. Group C: The nerve defect was bridged using a Y-tube conduit with a 3-mm-long nerve trunk and 4-mm-long branches by inserting each nerve stump 1 mm into the conduit. A total of 5 μL hWJMSCs was injected into the conduit. Data are expressed as the mean ± SD and were analyzed using one-way analysis of variance followed by the least significant difference tests. *P < 0.05. hWJMSCs: Human umbilical Wharton jelly mesenchymal stem cells. I–III: Groups A–C.
Effect of different types of Y-tube conduits injected with hWJMSCs on neuronal sprouting in rats with a femoral nerve defect
Retrograde labeling results showed a significantly greater ratio of axons sprouting from neurons only into the muscle branch of the femoral nerve in group C than in group B (P < 0.05; Figure 4).
Figure 4.
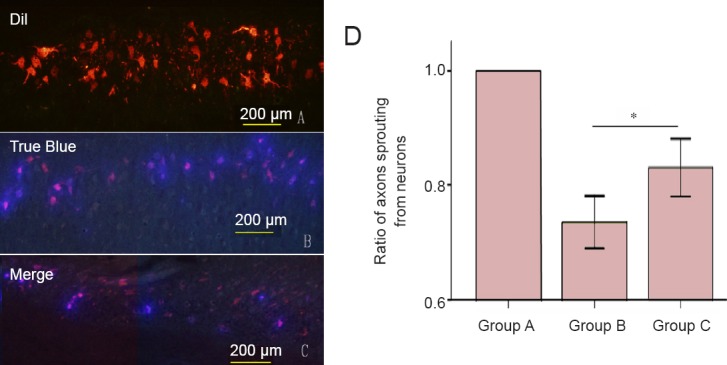
Effect of different types of Y-tube conduits injected with hWJMSCs on neuronal sprouting in the spinal cord of rats with a femoral nerve defect.
(A–C) Under a fluorescence microscope, red, blue and purple neurons are observed in the spinal cord of rats from groups A, B, and C. In rats from group A, neurons in the ventral horn of the spinal cord are stained with the red fluorescent marker Dil, indicating that axons sprouting from neurons only entered into the muscle branch of the femoral nerve. In rats from groups B and C, neurons in the ventral horn of the spinal cord are stained with red (Dil) and/or blue (True Blue) fluorescent markers, indicating that some axons sprouting from neurons entered into muscle and/or cutaneous branches of the femoral nerve. Scale bars: 200 μm. (D) The ratio of axons sprouting from neurons that only entered into the muscle branch in the ventral horn of the spine. Group A: Sham surgery was performed, and no injury was brought to the femoral nerve and its branches. Group B: The nerve defect was bridged using a Y-tube conduit with a 4-mm-long nerve trunk and 3-mm-long branches by inserting each nerve stump 1 mm into the conduit. A total of 5 μL passage 3 hWJMSCs was injected into the conduit. Group C: The nerve defect was bridged using a Y-tube conduit with a 3-mm-long nerve trunk and 4-mm-long branches by inserting each nerve stump 1 mm into the conduit. A total of 5 μL hWJMSCs was injected into the conduit. Data are expressed as the mean ± SD and were analyzed using one-way analysis of variance followed by the least significant difference tests. *P < 0.05. hWJMSCs: Human umbilical Wharton jelly mesenchymal stem cells.
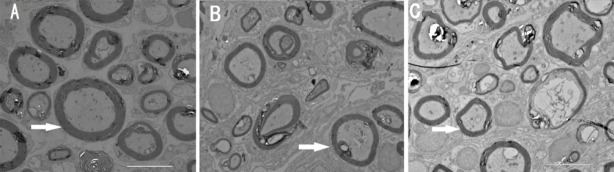
Effect of different types of Y-tube conduits injected with hWJMSCs on the ultramicrostructure of the femoral nerve in rats with a femoral nerve defect
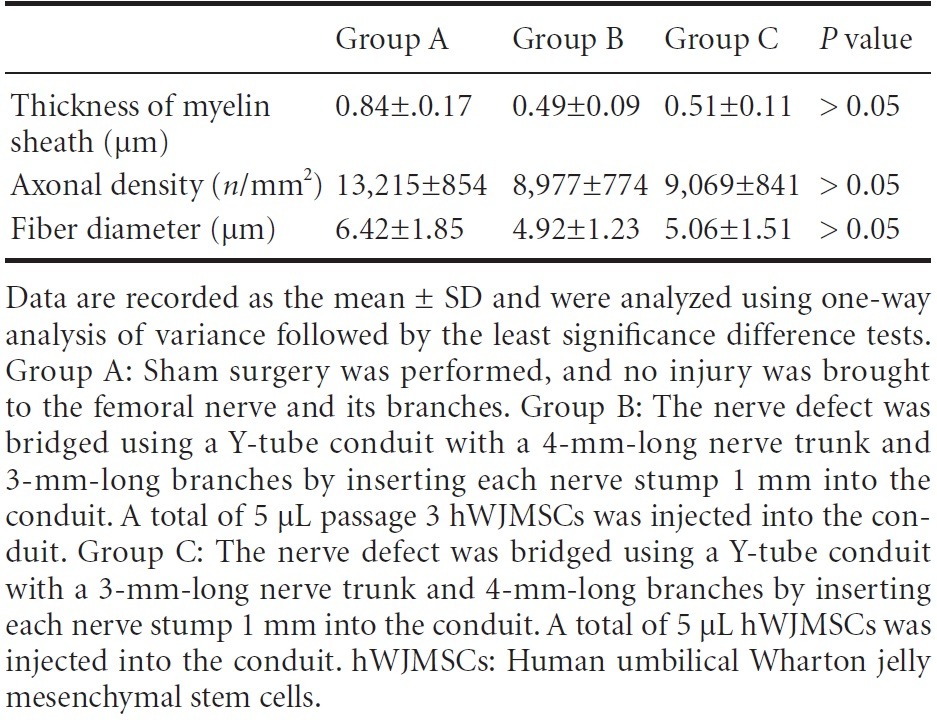
At 12 weeks after surgery, transmission electron microscope images revealed myelinated nerve fibers in the extracted nerves in all groups (Figure 5). No significant difference was found in myelin sheath thickness, axonal density, and fiber diameters between groups B and C (P > 0.05; Table 1).
Figure 5.
Effect of different types of Y-tube conduits injected with hWJMSCs on the ultramicrostructure of the femoral nerve in rats with a femoral nerve defect at 12 weeks after surgery.
(A–C) Transmission electron microscope images reveal well-myelinated nerve fibers in the extracted nerves from rats in groups A, B, and C. There are no significant differences in myelin sheath thickness (arrows), axonal density, and fiber diameters between groups B and C. Scale bars: 10 µm. hWJMSCs: Human umbilical Wharton jelly mesenchymal stem cells.
Table 1.
Effect of different types of Y-tube conduits injected with hWJMSCs on the femoral nerve in rats with a femoral nerve defect at 12 weeks after surgery
Discussion
Following peripheral nerve injury and treatment with nerve conduits, many factors affect the functional recovery, such as space between stumps and neurotropic and structural support of the conduits (Pfister et al., 2007; Zhao et al., 2007; Moradzadeh et al., 2008). Since Cajal (1928) proposed the hypothesis of chemotactic peripheral nerve regeneration, it has been gradually accepted by scholars that accurate axonal innervation is vital to achieving full functional recovery following peripheral nerve injury. Recent studies (Weber et al., 2000; Taras et al., 2005; Ashley et al., 2006) applied degradable nerve conduits and achieved satisfactory results. Brushart et al. (1998) showed that the microenvironment produced by nerve conduits is beneficial for accurate axonal innervation. The theory of chemotaxis has provided us with new tools to achieve more accurate axonal innervations (de Ruiter et al., 2008). If a small space is between the injured nerve stumps, nerve fibers growing from the proximal stump have been shown to selectively grow into their own pathways in the distal nerve and to their own target tissues (Höke et al., 2006). Another study (Jiang et al., 2006) used small gap sleeve bridging with nerve conduits to repair rat femoral nerve injury and achieved better functional recovery than autologous nerve grafting. The space created by the conduits can effectively accumulate the neurotropic factors secreted by the cells in the distal stump, prevent unnecessary interruption from the surrounding tissues, and can be helpful in the elective innervation of mixed nerves (Li et al., 2003; Chiu et al., 2004). We assume that the major reason why full functional recovery following injury to large nerve trunks at their bifurcation point, such as the brachial plexus nerve and femoral nerve, is unsuccessful, even with timely and accurate suturing, is because regenerating nerve axons cannot grow into their original nerve pathways and target organs. If a nerve conduit is used to reattach the injured nerve with a small gap between the stumps, it may create a favorable environment to allow axons to regenerate from the proximal stump to pathways in the distal stump, thereby achieving better functional recovery than direct nerve suturing. However, although many studies have applied the theory of chemotaxis to treat peripheral nerve injury (Li et al., 2003; Chiu et al., 2004; Höke et al., 2006; Jiang et al., 2006), few studies have made attempts to test the efficacy of Y-tube conduits to bridge peripheral nerve defects at the bifurcation point.
The current study made the first attempt to use the small gap bridging technique to achieve chemotactic nerve regeneration after peripheral nerve injury at the bifurcation point. Results showed that Y-shaped conduits to bridge the bifurcation point at the nerve injury are able to guide axons sprouting from the proximal nerve stump, ultimately regaining better functional recovery of the target tissues.
Accurate innervation of axons from the proximal nerve stump to the original nerve pathways and target organs can be affected by the space between the stumps (Muheremu et al., 2013). The distal stump and target tissues can guide the axons along a gradient of neurotropic factors. If the distance is too long, the chemotactic effect will become too weak to guide the axons. Conversely, if the distance is too short, axons will grow straight along the nearest pathway, which could be the wrong innervation pathway. Lundborg et al. (1982) found that when there is a distance of 6–10 mm between stumps of rat sciatic nerve, the chemotactic effect is most obvious. Politis et al. (1982) found that chemotactic nerve regeneration is most obvious in the gastrocnemius nerve when there is 4–5 mm distance between the stumps. In the current study, we used a gap of 4 mm between the stumps, because the femoral nerve and its saphenous nerve were much thinner than the sciatic nerve and closer to the gastrocnemius nerve. This method yielded satisfactory results.
In this study, two nerve conduits with different designs were applied to bridge a mixed nerve defect to the motor and sensory branches. To further promote axonal regeneration, we injected hUWJSCs to determine whether secreted growth factors promote neural regeneration following peripheral nerve injury (Troyer and Weiss, 2008). At 12 weeks after surgery, retrograde labeling and electrophysiological testing showed that Y-tube conduits with a shorter trunk and longer branches were more effective for selective nerve regeneration over the mixed nerve defect at the bifurcation point. This could be explained by a gradient of neurotropic factors secreted from the distal nerve stump nerve, although this was not measured in the current study. Further studies are needed to determine the appropriate concentrations of these neurotropic factors.
The present study used Y-tube conduits with different designs to bridge a peripheral nerve defect at the bifurcation point. The results showed that the Y-tube conduit effectively promoted accurate innervation when the branches were longer than the trunk of the conduit. These findings provide a basis for future clinical and animal studies to test the efficacy of Y-tube conduits for repairing mixed nerve defects (such as brachial plexus and femoral nerves) at the bifurcation point.
Footnotes
Funding: This study was funded by the National High Technology Research and Development Program of China (“863” Program, No. 2012AA020905), the National Natural Science Foundation of China (No. 81360194), and the National Basic Research Program of China (973 program, No. 2014CB542200).
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Rave A, Yu J, Wang L, Li CH, Song LP, Zhao M
References
- Ashley WW, Weatherly T, Park TS. Collagen nerve guides for surgical repair of brachial plexus birth injury. J Neurosurg Pediatr. 2006;105:452–456. doi: 10.3171/ped.2006.105.6.452. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Degeneration and regeneration of the nervous system. London: Oxford University Press; 1928. [Google Scholar]
- Chiu DT, Smahel J, Chen L, Meyer V. Neurotropism revisited. Neurol Res. 2004;26:381–387. doi: 10.1179/016164104225013815. [DOI] [PubMed] [Google Scholar]
- de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter GC, Spinner RJ, Verhaagen J, Malessy MJ. Misdirection and guidance of regenerating axons after experimental nerve injury and repair. J Neurosurg. 2013;120:493–501. doi: 10.3171/2013.8.JNS122300. [DOI] [PubMed] [Google Scholar]
- Eser F, Aktekin LA, Bodur H, Atan C. Etiological factors of traumatic peripheral nerve injuries. Neurol India. 2009;57:434–437. doi: 10.4103/0028-3886.55614. [DOI] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Zhang P, Zhang D, Fu Z, Yin X, Zhang H. Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:55–74. doi: 10.1080/10731190500430149. [DOI] [PubMed] [Google Scholar]
- Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, Kim HT, Kim SH. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- Li J, Jiang BG, Zhang DY, Zhang HB, Dang Y, Shang YG, Yang M. Using biological tube for bridging the peripheral nerve defect with a small gap: an experimental study. Zhonghua Shou Waike Zazhi. 2003;19:118–120. [Google Scholar]
- Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- Moradzadeh A, Borschel GH, Luciano JP, Whitlock EL, Hayashi A, Hunter DA, Mackinnon SE. The impact of motor and sensory nerve architecture on nerve regeneration. Exp Neurol. 2008;212:370–376. doi: 10.1016/j.expneurol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- Muheremu A, Wang Y, Peng J. Advances in experimental and clinical studies of chemotaxis. Can J Neurol Sci. 2013;40:292–298. doi: 10.1017/s0317167100014220. [DOI] [PubMed] [Google Scholar]
- Pfister LA, Papaloïzos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- Politis MJ, Ederle K, Spencer PS. Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 1982;253:1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Tolmasov M, Nissan M, Reider E, Koren A, Biron T, Bitan Y, Livnat M, Ronchi G, Geuna S, Rochkind S. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery. 2015 doi: 10.1002/micr.22418. doi: 10.1002/micr.22418. [DOI] [PubMed] [Google Scholar]
- Taras JS, Nanavati V, Steelman P. Nerve conduits. J Hand Ther. 2005;18:191–197. doi: 10.1197/j.jht.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Troyer DL, Weiss ML. Concise Review: Wharton's Jelly-derived cells are a primitive stromal cell population. Stem Cells (Dayton, Ohio) 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–1045. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Zhang PX, Jiang BG. Magnifying effect of conduit bridging in number of nerve fibers of broken peripheral nerves: experiment with rats. Zhonghua Yi Xue Za Zhi. 2007;87:1043–1047. [PubMed] [Google Scholar]