Keywords: nerve regeneration, peripheral nerve injury, brachial plexus injury, neuronal plasticity, supplementary motor area, premotor area, magnetic resonance imaging, motor preparation, motor imagery, clinical restoration of hand function, cortical remodeling, block design, neural regeneration
Abstract
Although some patients have successful peripheral nerve regeneration, a poor recovery of hand function often occurs after peripheral nerve injury. It is believed that the capability of brain plasticity is crucial for the recovery of hand function. The supplementary motor area may play a key role in brain remodeling after peripheral nerve injury. In this study, we explored the activation mode of the supplementary motor area during a motor imagery task. We investigated the plasticity of the central nervous system after brachial plexus injury, using the motor imagery task. Results from functional magnetic resonance imaging showed that after brachial plexus injury, the motor imagery task for the affected limbs of the patients triggered no obvious activation of bilateral supplementary motor areas. This result indicates that it is difficult to excite the supplementary motor areas of brachial plexus injury patients during a motor imagery task, thereby impacting brain remodeling. Deactivation of the supplementary motor area is likely to be a serious problem for brachial plexus injury patients in terms of preparing, initiating and executing certain movements, which may be partly responsible for the unsatisfactory clinical recovery of hand function.
Introduction
For patients suffering limb dysfunction because of severe peripheral nerve injury, the keys to restoring limb function are surgical repair of the peripheral nerve and functional brain remodeling (Moisello et al., 2008; Sun et al., 2014). Brachial plexus injury is a severe peripheral nerve deafferentation resulting from excessive stretching or tearing of the nerves that bridge the central nervous system and the upper limb (Xu et al., 2008). Although surgical techniques are advanced, it is hard to explain why brachial plexus injury patients do not have good clinical outcomes even after reinnervation. Despite the advances in surgical areas, it is believed that brain plasticity may play an important role in the recovery of hand function (Limthongthang et al., 2013; Sun et al., 2014). The beneficial effects of therapies such as mental practice have been investigated through motor imagery for training locomotor skills (Malouin and Richards, 2010; Liu et al., 2014). Previous studies have reported that cortical changes are mainly located in the primary motor cortex and sensory cortex. However, few studies have focused on the changes in advanced motor systems after peripheral nerve injury, such as the premotor area and the supplementary motor area. Currently, neuroscientists consider the ability of motor imagery to be vital in the restoration of hand function. Motor imagery is defined as an active process during which the representation of an action is internally reproduced within working memory without any overt output (Munzert et al., 2009; Vogt et al., 2013; Di Rienzo et al., 2014). The supplementary motor area is a region that contributes to the control of movement, and it is believed to be closely related to motor imagery that consists of both visual imagery and kinetic imagery (Solodkin et al., 2004). Existing work has focused on exploring the normal neural substrates of motor imagery and the physiological mechanisms underlying the supplementary motor area (Tak et al., 2015). Little is known about the change in the supplementary motor area during motor imagery tasks in brachial plexus injury patients. Whether the activation of supplementary motor area in brachial plexus injury patients is different from that of healthy ones during motor imagery tasks seems to be an interesting topic.
In this study, we explored brain activation when 10 patients with brachial plexus injury imagined completing certain motor tasks using their injured limb. The supplementary motor area lost its activation after brachial plexus avulsion injury. Long-term disuse of the injured limb and consequently disrupted motor output seemed to silence the activation of supplementary motor area. According to other previous studies, the inactivation of supplementary motor area produces great difficulty in the preparation, initiation and even the execution of movement (Deiber et al., 1996; Passingham, 1996; Lucci et al., 2014). This study might be an explanation for why patients cannot restore their distal limb motor function, even after peripheral nerve regeneration.
Participants and Methods
Participants
Ten patients with brachial plexus injury of the right limb scheduled for surgical treatment, aged 18–50 years with a mean age of 31.5 ± 6.5 years, were selected as the patient group from Huashan Hospital, Fudan University, China. A similar number of age- and sex-matched healthy people who underwent physical examination in Huashan Hospital were recruited publicly as the control group.
Inclusion criteria for the patient group were as follows: (1) complaints of paralysis of brachial plexus innervated area; (2) loss of sensory function of the upper limb; (3) diagnosis with physical examination, ultrasound scan, electromyography or surgical process; and (4) no history of other diseases.
Exclusion criteria for both groups were as follows: (1) confirmed or suspected history of diabetes mellitus, cervical spondylopathy, rheumatic disease, multiple sclerosis, chronic pain or cerebral diseases; (2) MRI contraindications; and (3) concurrent peripheral neuropathy; (4) within 1 year after injury.
The whole process of this study was supervised and approved by the Medical Ethics Committee of Huashan Hospital. All participants were aware of the process and purpose of the research, and provided their written informed consent to participate in this study.
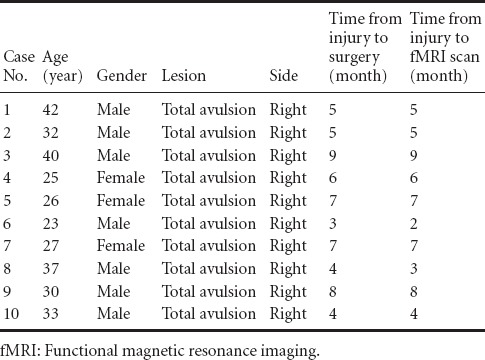
Clinical information about the patients is listed in Table 1. The patients enrolled in the study were unable to complete the designed motor task independently, including wrist and finger flexion.
Table 1.
Clinical information of enrolled patients with brachial plexus injury
Data acquisition
Participants lay supine in the GE Signa VH/I 3.0-T scanner (GE Healthcare, GE Asian Hub, Shanghai, China) with the head supported within a head coil. Their heads were fixed with the help of foam pillows that were belted over the forehead to minimize head movements. The gradient echo planar imaging sequence parameters used for acquisition of the fMRI images are listed below: repetition time = 3,000 ms; echo time = 35 ms; flip angle = 90°; field of view = 240 × 240 mm2; and acquisition matrix = 64 × 64, resulting in a voxel resolution of 3 mm × 3 mm × 3 mm. To ensure a steady state, we abandoned the images that were collected in the first 12 seconds. When the structural images were collected, a three-dimensional spoiled gradient-recalled acquisition sequence was used to acquire 1-mm-thick axial sections. The parameters are listed below: repetition time = 1,000 ms; echo time = 5 ms; flip angle = 200°; interslice space = 0 mm; field of view = 240 × 240 mm2; acquisition matrix = 256 × 256; number of excitations = 1.
Experimental paradigm
A resting-state scan was first arranged for all the participants, but the results of the resting-state scan were not relevant to this study. The participants were asked to imagine completing a unilateral hand movement. In the motor imagery task, the participants were directed to imagine flexing their five fingers repeatedly. The palm was to remain hollow, as if they were holding an egg in the palm. In the imagery task, they were required to concentrate and repeat this imaginary movement in their mind, with their eyes closed. The imaginary grasping task lasted for 30 seconds, alternating with another 30-second rest period. During the rest period, the participants were instructed to keep calm and avoid unnecessary movements in the MRI scanner. The participants were asked to relax their bodies completely with no muscle contraction. The whole scan lasted for 10 minutes.
Data analysis
After data collection, SPM8 software implemented in Matlab version 2010b (MathWorks., Inc, Natick, MA, USA) was used to analyze the MR images. First, we realigned the scans of each participant to the first image for each sequence separately. Then, they were transformed into the standard stereotactic anatomical space corresponding to the atlas of Talairach and Tournoux. The voxels were then smoothed with a full width at half-maximum of [8, 8, 8]. The original MR signal in each single voxel did not accord with a normal distribution. The signals were converted to Z scores with the method of Z-transformation, and thus were suitable for further statistical t-tests.
We underwent boxcar analysis for all sessions with a T-contrast, and retained only voxels with Z scores > 4.3946 and clusters > 100 voxels for an uncorrected threshold P < 0.001 for single-participant analysis. There was also a group analysis for each session using a one-sample t-test. Voxels only with T scores > 3.9296 and clusters >100 voxels for an uncorrected threshold P < 0.001 were retained.
After statistical analysis, the selected voxels that had a supra-threshold Z value were projected onto the standard CH2 brain template. Then, the number of activated voxels and the position of peak points in the brain regions, especially in the supplementary motor area, were listed.
Results
Activation of brain regions during imaginary grasping task in the control group
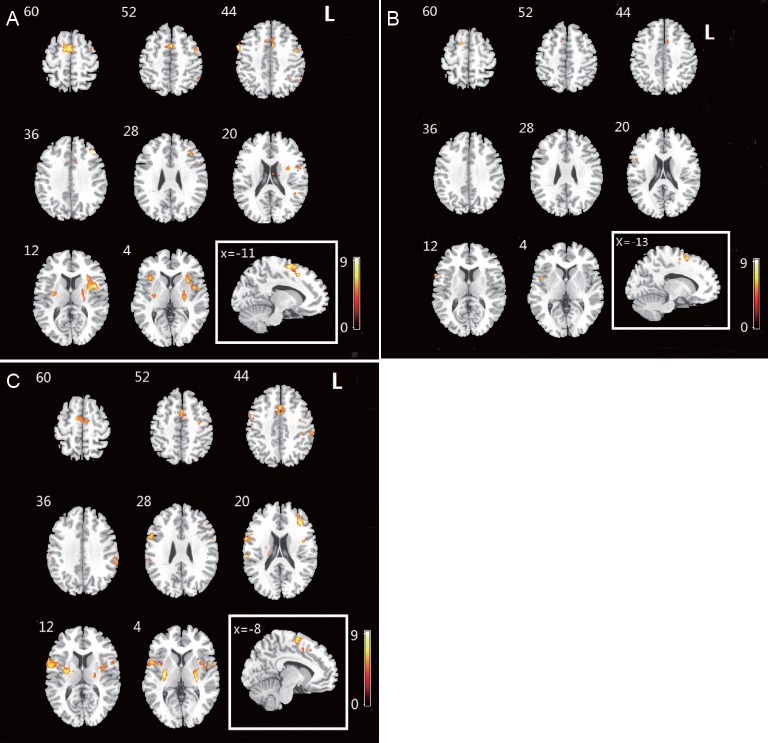
In the control group, obvious activations in the Brodmann 6 area that included premotor area and supplementary motor area were observed during the imaginary grasping task. Activation also existed in the frontal lobe. Additional sporadic activation could be found in both hemispheres (Figure 1, Table 2).
Figure 1.
Activation map of brain regions in control and patient groups during imagery grasping task.
(A) Activated brain regions in the control group. (B) Activated brain regions in the patient group on the affected side. (C) Activated brain regions in the patient group on the healthy side. The imaginary movement of the right hand generated activation in bilateral supplementary motor areas, right insula and inferior frontal lobe. The warm color bar is used to define the activation of the voxels. The red-yellow color suggests a positive activation in the brain area. The numbers in the figures correspond to the slice numbers in the CH2 brain template. L: Left.
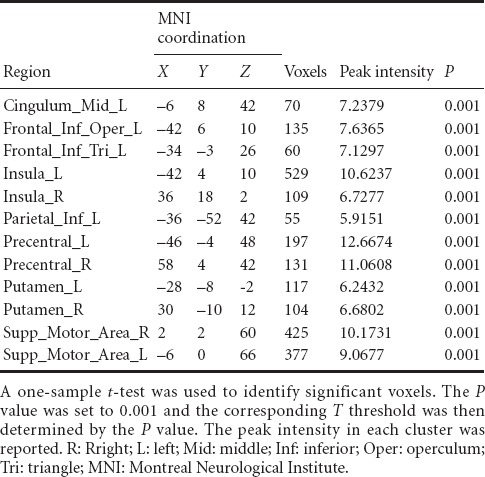
Table 2.
Brain regions activated in healthy participants during the imaginary grasping movement of the right hand
Activation of brain regions during the imaginary grasping task in the patients with brachial plexus injury
For the patients with brachial plexus injury, the imaginary grasp task of the healthy hand (left side) mainly activated the following brain regions: the cingulum, insula, supramarginal area and supplementary motor area (Figure 1, Table 3). However, the imaginary grasp task of the affected hand (right side) mainly caused the activation of the limbic system, which is situated in the left hemisphere. We found hardly any activation in the premotor area or supplementary motor area (Figure 1, Table 4). The results of the normal hand (left side) showed similar activation patterns to the control group. The most activated areas were concentrated in Brodmann area 6 (Figure 1 and Table 3).
Table 3.
Brain regions activated by the imaginary grasping movement of the left hand in the patients with brachial plexus injury
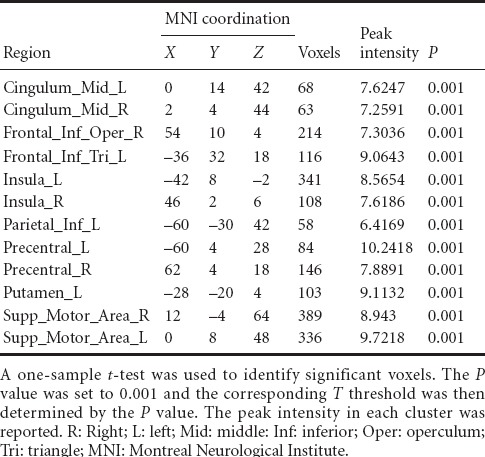
Table 4.
Brain regions activated in brachial plexus injury patients during the imaginary grasping movement of the right hand
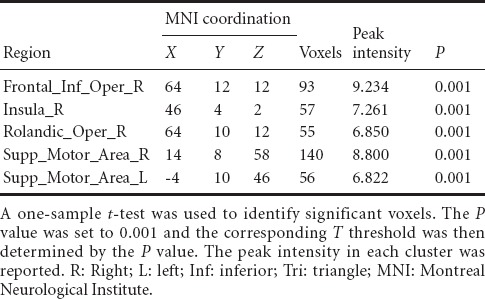
Group analysis
Based on the group analysis, the activated brain regions in the patient group during the imaginary grasping movement of the affected hand were different from those in the control group. Because of the deafferentation of the brachial plexus, the brain regions that were activated by the imaginary motion of the affected hand also differed greatly from those activated by the imaginary motion of the normal hand. Moreover, the global activation of the patient group was lower both in range and degree. No activation of the cerebellum was discovered in any participants, which suggests that there was no muscle contraction during the motor imagery task.
Discussion
Brachial plexus injury is a common dysfunction of the upper limbs because of traffic accident or industrial work. It mainly results in complete or incomplete motor paralysis. After peripheral nerve surgery, muscle contraction can easily be observed because of reinnervation (Terzis and Kostopoulos, 2007; Lee et al., 2015). However, it is difficult to restore meaningful and independent limb motor function. It is a common phenomenon that patients complain of unsatisfactory distal limb function. Up to now, many types of surgeries have been used to restore the motor and sensory function of the injured limb. However, the outcome is not always optimal, especially for the patients suffering from complete brachial plexus injury with root avulsion (Sammer et al., 2012). Previous work has suggested that both cerebral plasticity and cortical remodeling are responsible for the restoration of limb function (Navarro et al., 2007; Pagnussat et al., 2012). Besides anatomical repair, cortical factors may also play an important role. In the brain, the supplementary motor area is one of the most vital areas that contributes to the control of movement. Therefore, it is necessary to find out what exactly happens to motion-associated areas, such as the premotor area and the supplementary motor area. Their dysfunction may be one of the reasons responsible for the poor restoration of hand function.
In this study, the motor imagery task with the unaffected hand aroused the activation of most brain regions related to motor preparation. This is in accordance with previous studies on motor imagery (Decety et al., 1994). The activated brain regions in healthy participants were concentrated in the frontal lobe and bilateral supplementary motor areas. A similar activation pattern was also noted in the normal hands of patients. These results showcase a bilateral, instead of unilateral, activation in the supplementary motor area. The similarity of the activation pattern between healthy participants and the normal hands of brachial plexus injury patients implies that unilateral brachial plexus injury has no functional influence on the unaffected limb.
One of the unexpected results of the current study was the absence of superior parietal lobe (BA7) activation. According to previous position emission computed tomograrhy studies (Stephan et al., 1995) and classical physiological tests (Haaxma and Kuypers, 1974), during the imaginary motor task, activation in the superior parietal lobe can be observed. However, our findings differ from these previous studies, which may be because of the motor task design. The superior parietal lobe, the BA7 region, is believed to play an important role in visual-motor coordination. However, the visual oriented process was limited in our task design. The task required a simple grasp movement without emphasis on a particular position or direction. Moreover, the participants were also instructed to close their eyes throughout the whole scan. The activation of the BA7 region was probably suppressed.
One of the most vital discoveries of this study was the different activation pattern in the supplementary motor area of brachial plexus injury patients (affected hand task).First, the global activation of brachial plexus injury patients (affected hand task) greatly decreased compared with control participants, as shown in [Figure 3]. Second, the supplementary motor area of brachial plexus injury patients (affected hand task) displayed nearly no activation despite its anatomical integrity. Obviously, the supplementary motor area of brachial plexus injury patients was functionally disrupted, although the upstream neural network was never damaged. It is interesting that the exact same cerebral region regained its function when the motor imagery task of the normal hand was used.
In the present study, the lesion of all the patients was located in the C5–T1 root. Thus, the neural substrates superior to the lesion level were supposed to be anatomically undamaged. It has been thought that the disruption of motor output because of root avulsion does not influence the upstream neural activity. However, the results of our study show the opposite. The supplementary motor area and even the premotor area of the brachial plexus injury patients (affected hand task) presented far much less activation than those of control participants. The neural activity correlated with movement preparation and initiation was inhibited. These results lead to our assumption that the bilateral supplementary motor areas with either hand belong to a certain neural circuit, and they are involved in the preparation, initiation and execution of movements. When the motor output pathway connecting the supplementary motor area with a terminal motor unit was blocked, the neural circuit was functionally broken and the bilateral supplementary motor area of patients became silent when confronted with the affected hand imagery task.
This result is in accordance with one previous study that described functional connectivity changes between the bilateral supplementary motor areas of brachial plexus injury patients (Mao et al., 2014). The study reported that the brachial plexus lesion decreases the degree of functional correlation of the supplementary motor area between hemispheres. Together with our results, it seems that loss of motor output, such as long-term nonuse of the affected limb, has a great impact on the function of the supplementary motor area. The ability to initiate movement is destroyed by the long-term nonuse of the affected limb. It has been reported that dysfunction in the supplementary motor area abolishes spontaneous motion of the contralateral limbs (Nachev et al., 2008). In our study, we demonstrated that immobilization of limb functionally suppresses the activation of bilateral supplementary motor area, and might be partly responsible for the unsatisfactory clinical restoration of hand function. It has been already demonstrated that limb immobilization induces a decline of exercisability (Zanette et al., 2004) and a decrease in cortical thickness in the primary motor and somatosensory areas of contralateral limb (Langer et al., 2012). It is also predictable that brachial plexus injury patients suffer from cortical degeneration because of structural change after long-term limb disuse. This may explain why brachial plexus injury patients have poor motor function even when the terminal motor unit, such as the hand, is successfully reinnervated.
To some extent, our study shows the difficulty that brachial plexus injury patients have in restoring fine motor function with a functionally silent supplementary motor area. Immobilization of the limb prevents cortical activation. However, exercise may reverse this negative effect. Recent studies have reported that action observation is likely to prevent cortical suppression (Bassolino et al., 2014; Yang, 2014). The observation of certain actions promotes the motor facilitation of the corticospinal system. This facilitation applied to the precentral motor cortex helps to maintain the time course (Gangitano et al., 2001) and the muscle recruitment (Sato and Tanji, 1989; Borroni and Baldissera, 2008) of certain actions. Motor imagery is believed to have similar results, because these processes share almost the same neural substrates (Dahlin, 2013). Motor imagery tasks may help to reduce the negative impact of limb disuse and provide a new method of rehabilitation for those who are suffering from brachial plexus injury.
Here, we discovered that brachial plexus injury patients had difficulty in exciting the supplementary motor area during a motor imagery task. The supplementary motor area is a region closely associated with the preparation, initiation and execution of certain movements. We assumed that the silence of the supplementary motor area might partly be responsible for the unsatisfactory clinical restoration of hand function. Whether such treatment has long-term effects for hand function remains unpredictable, and requires further study.
Acknowledgments
We are grateful to Jia-wen Cao, the scanner operator of the Radiology Department from Huashan Hospital, China for her dedicated work in this study.
Footnotes
Funding: This study was supported by the Youth Researcher Foundation of Shanghai Health Development Planning Commission, No. 20124319.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Jackson C, Edanx QC, Wang J, Wang L, Li CH, Song LP, Zhao M
References
- Bassolino M, Campanella M, Bove M, Pozzo T, Fadiga L. Training the motor cortex by observing the actions of others during immobilization. Cereb Cortex. 2014;24:3268–3276. doi: 10.1093/cercor/bht190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni P, Baldissera F. Activation of motor pathways during observation and execution of hand movements. Soc Neurosci. 2008;3:276–288. doi: 10.1080/17470910701515269. [DOI] [PubMed] [Google Scholar]
- Dahlin LB. The role of timing in nerve reconstruction. Int Rev Neurobiol. 2013;109:151–164. doi: 10.1016/B978-0-12-420045-6.00007-9. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F. Mapping motor representations with positron emission tomography. Nature. 1994;371:600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Di Rienzo F, Collet C, Hoyek N, Guillot A. Impact of neurologic deficits on motor imagery: a systematic review of clinical evaluations. Neuropsychol Rev. 2014;24:116–147. doi: 10.1007/s11065-014-9257-6. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- Haaxma R, Kuypers H. Role of occipito-frontal cortico-cortical connections in visual guidance of relatively independent hand and finger movements in rhesus monkeys. Brain Res. 1974;71:361–366. doi: 10.1016/0006-8993(74)90979-2. [DOI] [PubMed] [Google Scholar]
- Langer N, Hanggi J, Muller NA, Simmen HP, Jancke L. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- Lee EY, Karjalainen TV, Sebastin SJ, Lim AY. The value of the tender muscle sign in detecting motor recovery after peripheral nerve reconstruction. J Hand Surg. 2015;40:433–437. doi: 10.1016/j.jhsa.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Limthongthang R, Bachoura A, Songcharoen P, Osterman AL. Adult brachial plexus injury: evaluation and management. Orthop Clin North Am. 2013;44:591–603. doi: 10.1016/j.ocl.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Liu H, Song L, Zhang T. Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study. Neural Regen Res. 2014;9:1474–1484. doi: 10.4103/1673-5374.139465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci G, Berchicci M, Spinelli D, Di Russo F. The motor preparation of directionally incompatible movements. NeuroImage. 2014;91:33–42. doi: 10.1016/j.neuroimage.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL. Mental practice for relearning locomotor skills. Phys Ther. 2010;90:240–251. doi: 10.2522/ptj.20090029. [DOI] [PubMed] [Google Scholar]
- Mao Y, Qiu TM, Chen L, Wu JS, Zhou LF. Sensorimotor cortical changes assessed with resting-state fMRI following total brachial plexus root avulsion. J Neurol Neurosurg Psychiatry. 2014;85:99–105. doi: 10.1136/jnnp-2013-304956. [DOI] [PubMed] [Google Scholar]
- Moisello C, Bove M, Huber R, Abbruzzese G, Battaglia F, Tononi G, Ghilardi MF. Short-term limb immobilization affects motor performance. J Motor Behav. 2008;40:165–176. doi: 10.3200/JMBR.40.2.165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 2009;60:306–326. doi: 10.1016/j.brainresrev.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Pagnussat AS, Simao F, Anastacio JR, Mestriner RG, Michaelsen SM, Castro CC, Salbego C, Netto CA. Effects of skilled and unskilled training on functional recovery and brain plasticity after focal ischemia in adult rats. Brain Res. 2012;1486:53–61. doi: 10.1016/j.brainres.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Functional specialization of the supplementary motor area in monkeys and humans. Adv Neurol. 1996;70:105–116. [PubMed] [Google Scholar]
- Sammer DM, Kircher MF, Bishop AT, Spinner RJ, Shin AY. Hemi-contralateral C7 transfer in traumatic brachial plexus injuries: outcomes and complications. J Bone Joint Surg Am. 2012;94:131–137. doi: 10.2106/JBJS.J.01075. [DOI] [PubMed] [Google Scholar]
- Sato KC, Tanji J. Digit-muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol. 1989;62:959–970. doi: 10.1152/jn.1989.62.4.959. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Sun G, Wu Z, Wang X, Tan X, Gu Y. Nerve transfer helps repair brachial plexus injury by increasing cerebral cortical plasticity. Neural Regen Res. 2014;9:2111–2114. doi: 10.4103/1673-5374.147939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Kempny AM, Friston KJ, Leff AP, Penny WD. Dynamic Causal Modeling for functional near-infrared spectroscopy. NeuroImage. 2015;111:338–349. doi: 10.1016/j.neuroimage.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzis JK, Kostopoulos VK. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:73e–92e. doi: 10.1097/01.prs.0000254859.51903.97. [DOI] [PubMed] [Google Scholar]
- Vogt S, Rienzo FD, Collet C, Collins A, Guillot A. Multiple roles of motor imagery during action observation. Front Hum Neurosci. 2013;7:807. doi: 10.3389/fnhum.2013.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gu Y, Xu J, Lin S, Chen L, Lu J. Contralateral C7 transfer via the prespinal and retropharyngeal route to repair brachial plexus root avulsion: a preliminary report. Neurosurgery. 2008;63:553–558. doi: 10.1227/01.NEU.0000324729.03588.BA. [DOI] [PubMed] [Google Scholar]
- Yang J. The influence of motor expertise on the brain activity of motor task performance: a meta-analysis of functional magnetic resonance imaging studies. Cogn Affect Behav Neurosci. 2014;15:381–394. doi: 10.3758/s13415-014-0329-0. [DOI] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–1275. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]