Abstract
Despite the growing number of preclinical and clinical trials focused on immunotherapy for the treatment of malignant gliomas, the prognosis for this disease remains grim. Cancer immunotherapy seeks to recruit an effective immune response to eliminate tumor cells. To date, cancer vaccines have shown only limited effectiveness because of our incomplete understanding of the necessary effector cells and mechanisms that yield efficient tumor clearance. CD8+ T cell cytotoxic activity has long been proposed as the primary effector function necessary for tumor regression. However, there is increasing evidence that indicates that components of the immune system other than CD8+ T cells play important roles in tumor eradication and control. The following review should provide an understanding of the mechanisms involved in an effective antitumor response to guide future therapeutic designs. The information provided suggests an alternate means of effective tumor clearance in malignant glioma to the canonical CD8+ cytotoxic T cell mechanism.
Keywords: Immunotherapy, cytotoxicity, glioblastoma
I. INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults, with an annual incidence of over 17,000 new cases in the United States.1,2 The prognosis for this deadly disease is bleak, with a median survival of 18 to 21 months.1,3,4 Complete tumor resection is difficult owing to the diffusely infiltrative nature of the tumor.1 Combining postoperative radiation therapy with temozolomide chemotherapy has provided the greatest improvement in survival but only 2.5 additional months.5 Recognition and understanding of the biology of GBM and its interactions with the immune system have led to novel immunotherapeutic approaches, including a variety of tumor vaccines.
However, there are challenges with immunotherapy for GBM because of specific features of the central nervous system (CNS) that limit an immune response. The blood-brain barrier limits entry of most immune cells to the brain parenchyma from peripheral blood, classical lymphatic vessels and nodes are absent in the CNS, and there are few T lymphocytes and limited antigen (Ag) processing and presentation, which all help prevent overwhelming CNS inflammation on a daily basis. There are resident immune cells that provide immune surveillance of the brain. Microglia are tissue-resident macrophages that screen and remove cellular debris and foreign material from the interneuronal spaces in the brain parenchyma.6 Other macrophages and dendritic cells reside in the choroid plexus, perivascular spaces, and meninges. Cerebrospinal fluid that may contain specific Ags leaves the CNS through Virchow-Robin spaces to activate T lymphocytes in the cervical draining lymph nodes (DLNs).7,8 These activated T cells can then return to and patrol the CNS.
In addition to the limitations of the immune system within the CNS, GBMs and their micro-environment are immune suppressive. Tumor-elaborated soluble factors, such as transforming growth factor beta (TGF-β) and prostaglandin E2 (PGE2)9 act in the tumor or the DLNs to dampen T cell reactivity. Other mechanisms of immune suppression in the DLN include regulatory T cell–mediated killing of tumor Ag–presenting dendritic cells (DCs) T cell receptor nitration by myeloid-derived suppressor cells (MDSCs) and tolerogenic tumor-associated dendritic cells (TADCs).10 Distinct types of suppressive immune cells, MDSCs, type 2 macrophages (M2), TADCs, and T regulatory cells (Tregs), are recruited to the tumor micro-environment by PGE2-dependent tumor expression of chemotactic molecules such as CXCL12.11 Monocytes activated by cancer invasion signals migrate into the tumor and transform into tumor-associated macrophages (TAMs) with two distinct phenotypes.12,13 Type 1 TAMs facilitate tumor killing, but type 2 macrophages (M2) promote tumor growth and vascularization by secreting epidermal growth factor, fibroblast growth factor, and vascular endothelial growth factor.14–16 These immune suppressive cells in the tumor micro-environment induce tolerance and inactivate infiltrating T cells; TADCs, in particular, block an immune response by up-regulating the checkpoint inhibitor receptors PD-L1 and CTLA-4 and by expressing the inhibitory cytokines TGF-β and IL-10.17
Immunotherapy has the potential to greatly improve survival while providing good quality of life due to minimal toxicities. The majority of protocols rely primarily on the cytotoxic activity of CD8+ T cells to affect tumor regression. However, many other components of the immune system that have been largely overlooked may play important roles in tumor clearance. This report is a review of CD8-independent means of immunotherapy that may provide potent tumor control in human GBM patients.
II. NATURAL KILLER CELLS
Natural killer (NK) cells are lymphoid cells that participate in both innate and adaptive immune responses to pathogens and cancer cells.18–20 They use a variety of receptors to mediate different effects (Table 1). Examples of inhibitory receptors are major histocompatibility (MHC) class I ligands, some killer cell immunoglobulin-like receptors (KIRs), and CD94/ NKG2A, which suppresses NK cell cytotoxicity. In contrast, activating receptors, including natural cytotoxicity receptors (NKp30, NKp44, and NKp46), initiate NK cell cytolytic activity.20–22
TABLE 1.
Receptors used by NK cells to mediate different effector functions
| Activation receptors | Inhibitory receptors | Dual function receptors |
|---|---|---|
| NKG2D | KIR2DL1 | CD94-NKG2 |
| NKp30 | KIR2DL2 | 2B4 |
| NKp44 | KIR2DL3 | NTB-A |
| NKp46 | KIR3DL1 | KIR2DL4 |
| NKp80 | KIR3DL2 | |
| DNAM1 | LILRB1 [LIR-1] | |
| CD96 | KIRG1 | |
| CD16 [Fcg RIIIA] | NKR-P1A | |
| KIR2DS1 | Siglec7 and/or Siglec9 | |
| KIR2DS2 | ||
| KIR3DS1 | ||
| BY55 [CD160] | ||
| CD2 |
Note: Alternative receptor nomenclature is given in brackets. DNAM1 = DNAX accessory molecule 1; KIR2DL2 = killer cell immunoglobulin-like receptor (KIR), two domains, long cytoplasmic tail, 2; KIR2DS1 = KIR, two domains, short cytoplasmic tail, 1; KLRG1 = killer cell lectin-like receptor G1; LILRB1 = leukocyte immuno-globulin-like receptor, subfamily B, member 1; NKG2 = NK group 2; NKp = NK cell protein; NKR-P1A = NK cell receptor protein 1A; Siglec = sialic-acid-binding immunoglobulin-like lectin.
Healthy cells avoid attack by NK cells through the expression of MHC class I molecules and minimal expression of stress-induced self molecules.20,23,24 In contrast, virally infected or malignant cells become susceptible to NK cell–mediated lysis by up-regulating stress-induced molecules and/or down-regulating MHC class I molecules.25 To persist, tumors have evolved a variety of mechanisms to escape NK cell–mediated cytolysis. A few examples are secretion of immune regulatory molecules or immune suppressive modulators, which down-regulate NK cell effector functions, and modulation of NK cell receptor–ligand expression patterns.26,27 The latter includes aberrant expression of nonclassical human leukocyte antigen (HLA) class I Ags.
HLA-E and HLA-G are considered nonclassical HLA class I Ags because they are less polymorphic and bind more restricted peptide repertoires than the classical HLA-A, -B, and -C class I Ags.28,29 HLA-E has a broad tissue distribution, similar to the classical HLA class I Ags, while HLA-G expression is much more restricted. However, both are expressed by fetal trophoblasts and, as such, were thought to function physiologically by protecting the fetus from allorecognition by maternal NK cells.30 This belief holds true for HLA-E because it suppresses NK-mediated cytotoxicity via the inhibitory receptor CD94/NKG2A.31
Wischhuschen et al. first reported that HLA-E-mediated inhibition of NK function may contribute to the pathology of gliomas.32 They found that HLA-E is expressed by glioma cell lines and primary glioma cells and that, relative to normal CNS tissue, HLA-E expression is enhanced in low-grade gliomas and is enhanced to even higher levels in GBMs. Subsequent studies confirmed and extended these findings by showing HLA-E expression to be associated with a subset of glioma cells with tumor-initiating properties.33 HLA-E inhibited recognition and killing of these glioma-initiating cells by NK cells, interfering with HLA-E expression by siRNA-gene silencing restored NK killing.
The jury is still out on whether HLA-G suppresses NK responses. The long-held idea that HLA-G, like HLA-E, protects the fetus from maternal NK recognition is falling out of favor. Evidence shows (1) that HLA-G expression promotes HLA-E co-expression by stabilizing the HLA-E molecule upon binding of the HLA-G signal peptide, suggesting HLA-G may act indirectly through HLA-E to suppress NK responses; and (2) that, while HLA-G suppresses CD8+ cytotoxic T cell function, it in fact activates NK cells via the KIR2DL4 receptor, leading to pro-inflammatory and pro-angiogenic responses.29,30,34 The emerging paradigm for the role of HLA-G in pregnancy is that it indirectly inhibits NK allo-recognition through HLA-E and that it promotes uterine tissue remodeling, which is required for implantation. These findings suggest that we re-evaluate the reported immune inhibitory effects of HLA-G expressed on gliomas.
Wiendl et al. first reported that HLA-G expression by gliomas provided a means for immune escape.35 They found HLA-G to be expressed in four of five glioma biopsies and in several glioma cell lines, and that just a few HLA-G–positive tumor cells can inhibit anti-tumor responses. Subsequent studies confirmed and extended this work to show that HLA-G is frequently expressed by primary GBM biopsies (65 out of 108 in one study).36,37 These and other studies suggest that HLA-G expression by tumors is a mechanism for immune evasion.
Emerging evidence muddies the waters, however. For example, neuroimaging analyses of patients with low grade gliomas show that high HLA-G expression correlates with large size and blurred boundaries, characteristics consistent with tumor invasiveness.38 Together these studies confirm aberrant HLA-G expression by low- and high-grade gliomas, but do not clarify whether HLA-G expression contributes to tumor growth by immune suppression or remodeling of the tumor micro-environment.
Despite the ability of tumors to escape NK cell functions, many therapeutic trials for a variety of malignancies are exploiting NK cells for their functional responses.39,40 Several approaches have been used for NK cell–based immunotherapy, including in vivo cytokine-mediated expansion of NK cells and adoptive transfer of autologous or allogeneic NK cells or of some NK cell lines such as NK-92.41,42 Moreover, genetically modified NK cells expressing chimeric Ag receptors (CARs) are being investigated for clinical therapeutic use based on their cytotoxic function.42,43
III. NATURAL KILLER T CELLS (NKT)
There is another population of lymphocytes, natural killer T cells (NKTs), that are differentiated from NK cells. NKT cells are heterogeneous lymphoid cells that exhibit characteristics of both the innate and adaptive arms of the immune system. Similar to NK cells, these lymphocytes react quickly to stimuli that modulate the immune response.44,45 NKT cells respond in an Ag-specific manner through an unconventional T cell receptor (TCR), which can react to multiple self and foreign Ags46,47 through CD1b presentation.45,48 Unlike traditional lymphocytes, NKT cells have the ability to simultaneously secrete helper T cell 1(Th1)/ pro-inflammatory (e.g., IFN-γ, TNF-α) and Th2/anti-inflammatory (e.g., IL-4, IL-10, IL-13) cytokines49,50 that activate other NK cells as well as T and B cells.45
Because of the heterogeneity of TCR rearrangements, NKT cells are separated into two categories, type I and type II. Type I NKT cells are usually associated with the promotion of tumor immunity, whereas type II NKT cells appear to suppress tumor immunity.51,52 A combination of activation variables dictates type I NKT cell function: the affinity of the Ag presented to the NKT TCR, the presence of co-stimulatory molecules, and the tissue environment in which the interaction takes place.53 Type I NKT cells employ several mechanisms to promote cytolytic activity. For instance, both murine and human NKT cells can directly lyse tumor cells by a perforin-dependent mechanism,54 and cell killing can be potentiated by intracellular granzyme B expression.55 In vitro experiments have demonstrated that tumor cells expressing CD1d may be especially susceptible to direct NKT cell lysis.56 This pattern has been observed in vivo in patients with B-cell lymphoma.57 There is also evidence that high CD1d expression levels correlate with lower metastasis rates in a murine breast cancer model.58
Type I NKT cells are capable of mediating direct tumor lysis that is dependent on the activation of innate and adaptive immune cells.59,60 The recruitment of anti-tumor cytolytic cell populations primarily involves the initiation of Th1 cytokine cascades. The first NKT cell ligand identified was α-GalCer, a potent activator of type I NKT cells. The clinical therapeutic potential of α-GalCer was demonstrated when application of a synthetic form of this ligand, KRN7000, increased survival in B16 melanoma–bearing mice.56,61
Type I NKT cells recognize microbial glycolipids and self Ags.62,63 As mentioned, α-GalCer is a potent activator of all type I NKT cells, causing them to produce copious amounts of IFN-γ, which facilitates the activation of CD8+ T cells and Ag-presenting cells (APCs).64 NKT cells specifically stimulate DCs through CD1d-TCR complexes and CD40-CD40L interactions, which induce DC maturation and IL-12 secretion.65,66 IL-12 stimulates both NK and NKT cells, as well as other T cells, to produce more IFN-γ, and together these cytokines significantly impact the activation of downstream effector populations, such as NK cells, CD8+ T cells, and γδ T cells.67
CD1d–restricted NKT cells that do not express the semi-invariant TCR are classified as type II. This NKT cell subset recognizes glycolipid Ags distinct from those recognized by type I NKT cells and is not as well characterized as its type I counterpart. In contrast to their role in enhancing an immune response to tumors, NKT cells, especially type II, have demonstrated suppressive activity in cancer immunology. Type II NKT cells were shown to be sufficient for down-regulating tumor immune surveillance in several studies using different tumor models.57,68 CD4+ type II NKT cells were shown to produce higher levels of IL-13 and IL-4 compared to type I NKT cells, and NKT cell–dependent IL-13 was found to be necessary for tumor recurrence in a growth-regression-recurrence-pattern 15-12RM fibrosarcoma tumor model.69 The immunosuppressive effect appeared to be mediated by the sulfatide-reactive subset of type II NKT cells.68
Tumor immune surveillance is also blocked by increased production of TGF-β by a CD11b+Gr1+ population known as MDSCs.70 The increase in TGF-β production is stimulated by IL-13–initiated signaling through the IL-4R-STAT6 pathway and TNF-α.69,71
IV. GAMMA DELTA T CELLS
B cells, alpha beta (αβ) T cells, and gamma delta (γδ) T cells are the three main lineages of lymphocytes in vertebrates that use genetically recombined receptors to survey their environment and mediate host defenses against disease.72 Gamma delta (γδ) T cells have emerged as an evolutionarily conserved immune cell population,73 with various percentages among species ranging 60–80% in cattle, pigs, and sheep, and 10–60% in humans depending on immune challenge.74,75 Alpha beta (αβ) TCRs express either CD4 or CD8 co-receptors and interact with classical MHC molecules presenting peptides; they are used in adaptive immune responses. In contrast to αβ T cells, γδ T cells respond to Ags in a non-MHC manner through receptors including CD1, and they play an important role in innate immune surveillance as well as in adaptive immune responses.76,77
Because γδ T cells have multiple TCRs, they can recognize a wide range of Ags; however, in most human peripheral blood, they express a Vg9 Vd2 T cell TCR that recognizes cellular stress in a MHC-independent manner.77 This population has three functionally distinct subsets: naïve (CD45RA+CD27+), central memory (CD45RA− CD27+), and effector memory (CD45RA− CD27− ).77 Vg9 Vd2 TCRs recognize nonpeptidic prenyl pyrophosphate metabolites, generically known as phosphoantigens.78,79 Moreover, in response to antigenic stimulation, naive and central memory Vd2 cells will proliferate and secrete cytokines including high levels of IFN-γ and TNF-α.80,81
A. Gamma Delta T Cells and Cancer
Gamma delta T cells (γδ T cells) play an important role in immune surveillance and immune defense against tumors, including melanoma,82 leukemia, lymphoma, neuroblastoma, and other types of carcinoma.83,84 The antitumor activity of γδ T cells has been confirmed by ex vivo expansion followed by infusion to cancer patients.85,86 Recently, in vitro activated γδ T cells have been shown to target a small number of colon cancer stem cells, which had been demonstrated to be responsible for the failure of conventional therapies. In addition, γδ T cells can kill chemotherapy (imatinib)–resistant chronic myelogenous leukemia lines. Due to the lack of appropriate animal models, there is no direct evidence to suggest that human Vd2 cells eradicate or reduce tumor burden in vivo. However, a number of studies imply that Vd2 cells may contribute to anti-tumor immunity, and are thus a promising target for cancer immunotherapy.
In vitro experiments, although limited in their extrapolation to physiological systems, have demonstrated that Vd2 cells are capable of recognizing and killing tumor cells through multiple pathways, including granule exocytosis, Fas/Fas-ligand (CD95/ CD178)-induced apoptosis, antibody-dependent cell-mediated cytotoxicity and TNF-related apoptosis inducing ligand.87,88
Moreover, γδ T cells rapidly produce large amounts of IFN-γ and TNF-α80,81 while having strong cytotoxic activity as a result of perforin, granzymes, and death receptor ligands.76 In response to tumors, NK cells and γδ T cells share similar properties of both innate and adaptive effector activity. NK and γδ T cells recognize similar ligands that are expressed by tumor cells.89,90 Most hematopoietic cancer cells express various stress-induced molecules, acting as ligands for activation receptors such as NKG2D, CD94/NKG2A, CD94/NKG2C, DNAM-1, Ig-like transcript 2, CD161, KIR2DL1–3, NKp30, and NKp44, which all present on both NK and γδ T cells to regulate their activities.91,92
Although the cytokine-induced anti-tumor activities of γδ T cells appear more prominent against hematological cancers than other types of malignancies,93,94 γδ T cells have demonstrated efficacy against solid tumors through the recruitment of immune cells including small peritoneal macrophages that respond directly against cancer immunosurveillance.95 In clinical studies, γδ T cells have been reported to infiltrate into various solid of tumors including lung carcinoma,96 renal cell carcinoma,97 and breast carcinoma.98 In patients, both positive and negative correlations have been made between clinical responses and tumor-infiltrating Vd2 cells. γδ T cells, consisting of both Vd1+ and Vd2+ cells, were predominant tumor-infiltrating lymphocytes in melanoma lesions, and that low numbers of tumor infiltrating γδ T cells correlated with advanced disease.82
V . TUMOR-REACTIVE B CELLS AND ANTIBODIES
B cells are lymphocytes that express a unique membrane-anchored antibody B cell receptor (BCR).99 Unlike CD8 T cells, B cells do not require interaction with APCs to engage Ag to their BCR. However similar to CD8 T cells, B cells require costimulatory and cytokine signals to achieve full activation. Costimulation occurs when a CD4 T helper cell recognizes its cognate peptide-MHC II complex on the surface of the B cell.100 Costimulation is followed by CD40L-CD40 interactions with T cells that activate an antibody response.101,102 Activated B cells then undergo an immunoglobulin class switch by DNA recombination to produce specific isotypes of membrane-bound antibody/BCR (IgM to IgG, IgA, or IgE) and differentiation into short-lived plasma cells for antibody production,103 long-lived plasma cells or memory B cells,104 or long-lived memory B cells.105
Vaccine-based immunotherapy involves tumor cell vaccinations that stimulate production of tumor-specific antibodies that circulate and bind to tumor Ags in the blood or at the tumor site. Antibody binding to tumor-specific Ag induces opsonization of the Ag, which in turn facilitates its uptake by APCs. Antibody binding to Ag on the surface of live tumor cells can trigger a multitude of responses including neutralization of the target protein function,106 tumor clearance by phagocytosis and/or adaptive immunity,107 complement-dependent cytotoxicity,108,109 chemoattraction of other leukocytes, or antibody-dependent cell-mediated cytotoxicity.109,110
VI. CD4+ T CELL ANTI-TUMOR ACTIVITY
A. CD4+ T Cell Subsets
Naïve CD4+ T cells can differentiate into one of several functionally distinct subsets that directly mediate, or indirectly stimulate or suppress, tumor-specific immunity.111,112 Here we focus on conventional TCR α/β CD4+ T cell subsets that contribute to anti-tumor immunity via Ag recognition of peptides presented by MHC class II molecules, and not on subsets that recognize other types of Ags (e.g., glycolipids presented by CD1d to NKT cells) or suppress immunity (e.g., inducible and natural CD4+ T regulatory cells).
The choice of which immune stimulatory pathway a naïve CD4+ T cell takes occurs in the periphery when its TCR first engages its cognate peptide/ MHC class II Ag. The pathway choice is dictated during activation by Ag concentration, the type of APC engaged by the CD4+ T cell, the costimulatory molecules the APC presents, and most importantly, on the cytokine milieu of the microenvironment.113,114 Together these factors lead to epigenetic changes and the corresponding expression of key transcription factors, the balance of which determine the gene expression and cytokine secretion profiles that define subsets of activated CD4+ Th cells.115–121 The cytokines secreted by CD4+ Th cells then shape ensuing immune responses by signaling through cytokine receptors on other immune effector cells. Table 2 summarizes the CD4+ Th subsets known in mice and humans, their cytokine and effector molecule secretion profiles, and their dominant, defining transcription factors. Note that, in the T follicular helper subset family, specialized T follicular helper cells secrete the various cytokines.
TABLE 2.
Subsets of CD4+ Th cells and their known effector molecules and defning transcription factors
| Th Subset | Secreted effector molecules | Transcription factors |
|---|---|---|
| Th1 | IL-2, IL-10, IFN-γ, TNF-α, TNF-β/LT-α | T-bet |
| Th2 | IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, IL-25, IL-31 | GATA3 |
| Th9 | IL-9, TNF-α, and IL-10 in mice) | PU.1/Spi-1 and IRF4 |
| Th17 | IL-17A, IL-17F, IL-17A/F, IL-21, IL-22 (and IL-26 in humans) | RORγt |
| Th22 | IL-13, IL-22, TNF-α | AHR |
| Thf | IL-4, IL-10, IL-12, IL-21, IFN-γ | Bcl-6 |
| ThCTL | IL-2, IFN-γ, TNF-α, granzyme/perforin | Runx3 |
Activated CD4+ Th cells also indirectly stimulate Ag-specific CD8+ T cells by “licensing” APCs such as DCs. Licensing results from engagement of CD40 on the DC with its ligand (CD154) on the activated CD4+ Th cell.122,123 CD40-mediated signaling stimulates the DC to provide all three signals necessary for the differentiation of Ag-specific naïve CD8+ T cells to effector cytotoxic T lymphocytes (CTLs): Ag, co-stimulation, and pro-inflammatory cytokines.124–126
CD40-mediated signaling promotes Ag cross-priming of CD8+ T cells to exogenous proteins taken up, processed, and cross-presented as selected peptides with MHC class I molecules by DCs.127–129 CD40-mediated signaling also enhances DC expression of the costimulatory molecules CD80 and CD86 while stimulating the secretion of pro-inflammatory cytokines, most notably IL-12.125 The effects on DCs mediated by Th cells are critically important in stimulating, amplifying, and directing effector and memory Ag–specific CD8+ T responses.130
In addition to helping CD8+ CTLs, Ag-specific CD4+ T cells may themselves directly lyse MHC class II positive cells. Mouse and human CD4+ Th cytotoxic (ThCTL) cell lines were first reported over three decades ago, but were discounted as artifacts because they were derived from long-term Ag stimulation in vitro.131–134 Now ThCTLs are considered a naturally occurring CD4+ T cell subset; they exist in many species and in healthy individuals (about 2% of peripheral blood CD4+ T cells).135,136 This subset is expanded in people seropositive for chronic viral infections such as human immunodeficiency virus 1, human cytomegalovirus, and hepatitis viruses, and in mice chronically infected with gamma-herpes and lymphocytic choriomeningitis viruses.136–140 In addition to chronic Ag exposure, acute Ag exposure expands ThCTLs as their frequencies are increased in mice within a week after infection with influenza virus A or the intracellular bacterial pathogen Brucella abortus.141,142 Increased numbers of ThCTLs are also detectable in mice and humans with various malignancies, as discussed next.
1. CD4+ Th1 Cells and Anti-Tumor Immunity
CD4+ Th1 cells are traditionally thought to be secondary to cytolytic effector cells in anti-tumor immunity. Ascribing “second class citizen” status to CD4+ Th1 cells is misleading, though, because they are usually necessary to eradicate tumors by licensing DCs and providing cytokine support to CD8+ CTLs and NK cells. Evidence for this comes from reports of limited anti-tumor effects of cytolytic effectors alone.143–145 Consequently, the paradigms of immunizing cancer patients with vaccines containing peptides only recognized by CD8+ T cells, or adoptively transferring ex vivo expanded autologous CD8+ lymphocytes alone, have shifted to strategies that include inducing or adoptively transferring tumor-specific CD4+ Th1 cells.
One such strategy is to immunize with long peptides containing both CD4+ and CD8+ T cell epitopes. Harao et al. used genome-wide microarray analysis to identify a novel cancer-testis Ag, cell division cycle–associated 1 (CDCA1), overexpressed in lung, head-and-neck, and other cancers.146,147 They reported CDCA1 contained immunogenic HLA-A2 (A*02:01) –restricted peptides that induce Ag-specific CD8+ CTLs from the peripheral blood mononuclear cells (PBMCs) of lung cancer patients.147 The investigators then employed a recently developed computer algorithm to find long CDCA1-derived peptides (24–26 amino acids) that include one of these defined HLA class I epitopes plus novel peptides predicted to bind HLA class II molecules.148 They identified two long peptides, CDCA139–64-LP and CDCA155–78-LP, that induce both CD4+ Th1 and CD8+ CTL responses from PBMCs isolated from head-and-neck cancer patients but not healthy controls. This approach also successfully defined long peptides derived from the kinesin family member 20A, a protein frequently overexpressed in many solid tumors, including bladder, breast, gastric, lung, and pancreatic cancers.149 Again, these long peptides induced both CD4+ Th1 and CD8+ CTL responses from PBMCs isolated from cancer patients but not healthy individuals. Together these findings should inform future vaccine-based or adoptive cell transfer clinical trials for patients with solid tumors.
The approach arguably having the most profound success in cell-based cancer immune therapy is the adoptive cell transfer (ACT) of autologous, ex vivo expanded, tumor-recognizing T cells. Recognition is conferred either by the endogenously expressed TCR on tumor-infiltrating lymphocytes (TILs) or by genetically engineered CARs expressed by transduced PBMCs.150,151 TIL-based ACT typically employs CD8+ T cells with some stunning positive results: ~50% objective clinical responses in patients with advanced melanoma refractory to standard therapies.152 However, many patients do not achieve objective tumor responses despite the persistence of adoptively transferred CD8+ TIL clones that retain in vivo Ag responsiveness.153 These data suggest that the specificity of the selected TILs is not optimal, that CD4+ Th cells are required, or both.154
CAR-based ACT uses transduced PBMCs that contain a mixture of CD4+ and CD8+ T cells; no direct clinical comparisons between pure CD4+ and CD8+ CAR-transduced cells are yet available. However, recent preclinical data suggest that early activation of CD4+ CAR cells is critical for potent and durable anti-tumor immunity in an orthotopic model of mesothelioma.155 These and other data are redirecting attention to the inclusion of CD4+ T cells in ACT trials for cancer.
Hunder et al. adoptively transferred ex vivo expanded autologous, PBMC-derived, CD4+ Th1 cell clones into a patient with melanoma refractory to conventional chemotherapy.156 The clones were specific for a peptide derived from the melanoma-associated Ag NY-ESO-1 presented by the HLA class II molecule HLA-DOB1*0401. The patient did not require exogenous cytokine therapy because the clones produced autocrine IL-2. The entire tumor regressed even though only 50–75% of the cells expressed NY-ESO-1, which suggests that responses against other epitopes were also elicited. Epitope spreading was confirmed because the patient had PBMCs specific for melanoma Ags MART-1 and MAGE-3 after therapy. Responses to these Ags were undetectable prior to ACT.
What this case report has in common with most clinical trials describing objective tumor responses following ACT of TILs or CAR-transduced T cells is that it targeted the “low-hanging fruits” on the cancer Ag tree. These include differentiation Ags overexpressed on malignant versus healthy tissues (e.g., MART-1), Ags shared by malignancies and nonessential healthy tissues (e.g., CD19), and cancer-testis Ags, which are shared tumor Ags with expression on healthy cells limited to male germ cells in the testis (e.g. NY-ESO-1 and MAGE-3). For ACT therapies to be maximally effective, they should spare healthy tissues by targeting truly tumor-specific Ags that arise by mutations, ideally by mutations that drive the malignant phenotype.154
Tran et al. used whole exomic sequencing to show that TILs isolated from a patient with meta-static cholangiocarcinoma contained CD4+ Th1 cells specific for a mutated peptide derived from ERBB2IP (ERBB2IPE850G), an ERBB2IP-interacting protein mutated in that patient’s cancer.157 About 25% of the polyclonal, ex vivo expanded CD4+ Th1 cell TILs transferred back into the patient recognized ERBB2IPE850G presented by HLA-DQB1*0601. Tumor regression lasted for approximately one year and after recurrence the patient was infused with a highly purified (>95%) population of ERBB2IPE850G/ HLA-DQB1*0601–specific Th1 cells. Tumor regression occurred more quickly than before (within one month) and remained stable for at least six months (the time of publication). These and other clinical data support expanding autologous CD4+ T cells specific for tumor-associated and, more important, tumor-specific Ags for ACT therapy.
2. CD4+ Th17 Cells and Anti-Tumor Immunity
CD4+ Th17 cells can infiltrate solid tumors, but their contribution to anti-tumor immunity is unclear.158 This is because the cytokines that Th17 cells secrete can be both proinflammatory and immune suppressive, and Th17 differentiation and function are affected by the tumor micro-environment. Melanoma is one tumor in which Th17 TILs appear to stimulate anti-tumor immune responses. Martin-Orozco et al. reported that poorly immunogenic B16/F10 melanoma cells colonized the lungs of IL-17–deficient mice in significantly higher numbers than the lungs of wild-type controls, and that Th17 ACT triggered a strong tumor-specific CTL response in wild-type mice.159 The transferred Th17 cells promoted DC infiltration of the tumors and subsequent Ag cross-presentation and cross-priming of naïve CD8+ T cells. The Th17 TILs also stimulated stromal cells to produce chemokines (e.g., CCL20) that recruited CTLs to the tumor. These findings are consistent with others showing that Th17 cells are more effective than Th1 cells in eradicating melanomas in ACT mouse models, most likely because Th17 cells are superior to Th1 cells in promoting CD8+ T cell cross-priming.160,161
3. ThCTLs and Anti-Tumor Immunity
Ag-specific CD4+ Th cells can differentiate into MHC class II–restricted cytotoxic lymphocytes (ThCTLs) that are comparable to CD8+ CTLs in potency.112,162,163 Like CD8+ CTLs, ThCTLs kill Ag-expressing target cells via the perforin/granzyme B pathway or CD95/ CD95L (Fas/FasL) engagement. CD4+ ThCTLs comprise TILs in solid tumors such as melanoma and hepatocellular carcinoma (HCC).164,165 In a study of 547 patients with hepatitis B virus (HBV) –related HCC, Fu et al. found significantly higher frequencies of circulating and liver-infiltrating ThCTLs in early stage HCC patients compared to healthy controls and patients chronically infected with HBV.165 Granzyme A and B and perforin expression in ThCTLs and CD107a mobilization (a lysosomal-associated membrane glycoprotein associated with the release of cytolytic granules) were also higher in HCC patients than in patients chronically infected with HBV. ThCTLs from HCC patients secreted IFN-γ and TNF-α, cytokines associated with the Th1 subset. Despite the overall elevated numbers of ThCTLs in HCC patients, the frequency of ThCTLs in the tumor itself was significantly lower than in normal liver, suggesting that the tumor micro-environment was immune suppressive. Finally, both ThCTL frequency and lytic function decreased as HCC progressed. The loss in ThCTL frequency correlated significantly with disease progression and high mortality. Because the levels and functions of circulating ThCTLs mirrored those of liver-infiltrating ThCTLs, the frequency of the former may prove to be a useful biomarker for HCC progression.
4. CD4+ Th Plasticity and Anti-Tumor Immunity
A hallmark of CD4+ T cell biology is subset plasticity.111 The expression of subset-defining activating and inhibitory transcription factors that drive subset differentiation is not terminal but dynamic, and it is the balance that dictates CD4+ Th phenotype and function. The result of this dynamic is plasticity: a Th1 cell may become a Th17 or a ThCTL as the balance of transcription factors shifts as a result of changes in the in vivo micro environment or in the ex vivo culture conditions. In a mouse model of late-stage melanoma, Quezada et al. transferred low numbers of naïve CD4+ T cells bearing a transgenic TCR specific for the melanoma-associated Ag Trp1 into radiation-induced lymphopenic mice bearing established B16 melanoma.166 The naïve CD4+ T cells proliferated and differentiated into effector ThCTLs in vivo and eradicated the tumors in a MHC class II–restricted manner, which was enhanced by CTLA-4 blockade.
These preclinical results were replicated clinically when Kitano et al. generated MHC class II-restricted, NY-ESO-1-specific CD4+ T cell clones from four advanced melanoma patients treated with Ipilumimab.164 The clones secreted Th1-associated cytokines (IL-2, IFN-γ, and TNF-α) but not Th2-associated cytokines. They also expressed the Th1-defining transcription factor T-bet as well as the transcription factor Eomesodermin (Eomes). T-bet and Eomes are members of the T-box family of transcription factors, which is closely associated with CD8+ T cell differentiation into cytolytic effectors. Correspondingly, the NY-ESO-1–specific clones expressed the degranulation markers perforin, granzyme B, and CD107a, and lysed NY-ESO-1–positive target cells in an MHC class II–restricted manner. For all intents and purposes, these clones are poly-functional CD4+ Th1/ThCTLs.
Polyfunctionality is a point on a continuum between one Th subset and another. Such subset plasticity results from transcriptional reprogramming of mature CD4+ Th in the periphery. Mucida et al. identified a population of CD4+CD8a+ ThCTLs in the gut that lack expression of Th-inducing POZ-Kruppel Factor (ThPOK); this is a transcription factor turned on during thymic development that helps flip the differentiation switch to the CD4+ lineage in double positive thymocytes.167 These ThCTLs arose from CD4+ Th cells that extinguished ThPOK expression during their migration from the thymus to the gut. Reis et al. showed ThPOK gene silencing begins with upregulation of Runx3, a transcription factor that orchestrates expression of specific genes by CD8+ CTLs.168 The nascent CD4+CD8a+ ThCTLs remain functionally dormant in the gut until activated by the proinflammatory cytokine IL-15. ThPOK and Runx3 negatively regulate each other, as ThPOK is required by mature CD4+ Th cells to repress Runx3.169
As our knowledge of CD4+ T cell biology grows, so does our ability to manipulate these cells for therapy. Further insights into the signals and transcription factors that drive differentiation of CD4+ Th into anti-tumor effector cells will no doubt lead to more effective in vitro culture conditions to drive functionally heterogeneous TILs into effector subsets, or will permit differentiation of PBMCs into long-lived T cells for CAR-based therapies.
VII. CONCLUSIONS
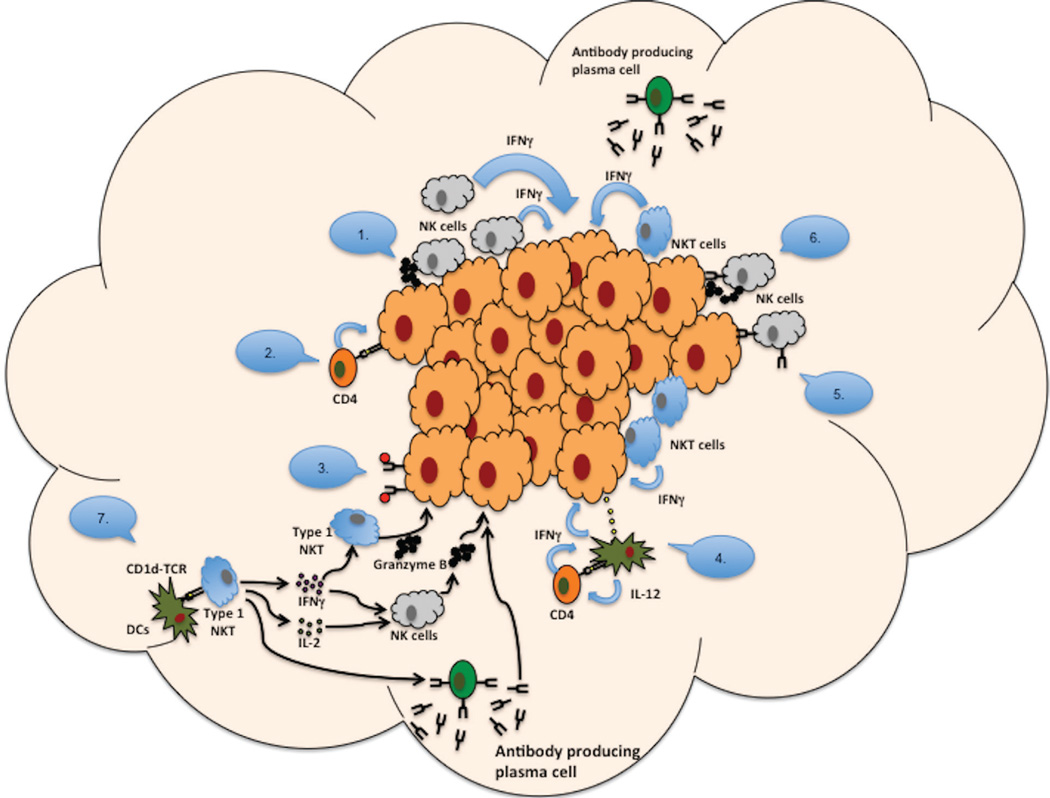
Despite increasing interest in using immunotherapeutic approaches in cancer treatment, the prognosis for patients diagnosed with malignant glioma remains poor. Advances in cancer immune therapy have been limited, and an inherent hurdle to progress is the lack of a basic understanding of the mechanisms needed for an effective immune-based anti-tumor response. Understanding the many facets of the immune system and examining the immune components involved in an effective anti-tumor response can provide insight into these mechanisms (Figure 1).
FIG. 1.
Mechanisms used by immune cells to attack tumors: (1) NK cells can release cytotoxic granules at sites of direct contact with Ag-bearing tumor cells. (2) Cytotoxic CD4+ T cells can kill tumor cells that express Ag on MHC II. (3) Antibodies bind to target Ags (e.g., CD20) and complement binds to antibodies, resulting in a complement cascade that lyses tumor cells. (4) Following Ag presentation, IFN-γ–activated macrophages can acquire a tumoricidal phenotype and up-regulate CTL markers, including granzyme A/B and NKG2D. (5) Fc receptors on NK cells can bind to tumor cells opsonized by plasma cell–secreted antibodies. (6) FcγRIII receptors on NK cells can bind to tumor cells by plasma cell–secreted antibodies, inducing ADCC; tumor cells are lysed by perforins and granzymes secreted by NK cells. (7) NKT cells can respond through CD1b presentation to DC and secrete a variety of cytokines (e.g., IFN-γ, IL-2) that activate other NKT cells, NK cells, and antibody-producing plasma cells.
Many cancer vaccines have been designed to induce a robust CD8+ T cell response. Although CD8+ T cell cytotoxicity may play a role in effecting an antitumor response, at least one study in a murine model of glioma demonstrated effective tumor control that was CD8+ cell–independent and showed that CD8+ cell depletion actually improved overall survival.110 Even in CD8+ T cell–mediated tumor clearance, recruitment of additional immune cells such as CD4+ Th1 cells that maintain the CD8+ T cell response is necessary. It is likely that a concerted effort of a variety of immune cells may be necessary to elicit and maintain an effective anti-tumor response.
In this article, we reviewed several potential mechanisms to stimulate an anti-tumor immune response other than CD8+ T cell cytotoxicity. CD4+ T cells are capable of cytolytic function through their direct and indirect killing in tumor models. CD4+ T cells activated through OX40 ligation or direct activation by OX40L may mediate cytokine secretion for the recruitment and activation of NK cells, NKT cells, and neutrophils. Other subsets of CD4+ T cells, such as CD4+ Th1 cells, may play an important and necessary role in tumor eradication by licensing APCs and providing cytokines that support CD8+ CTLs and NK cells.
The importance of B cells in a successful response to therapy has been largely overlooked. B cells can act as APCs for CD4+ T cell activity and can differentiate into plasma cells and secrete tumor-reactive antibodies or antibodies required for ADCC. Glioma-bearing mice generated tumor-reactive antibodies that were shown to bind to tumors after immunotherapy with tumor lysate vaccine and OX40L.110 The infiltration of innate immune cells in vaccinated animals points the possibility that NK cells, neutrophils, and other immune cells actively kill antibody-coated tumor cells. For this reason, it is likely that many different mechanisms are at play for successful tumor clearance.
A deeper understanding of immune-mediated mechanisms of tumor clearance should drive the design of new immunotherapy regimens. The interplay of many components is likely necessary to fully harness the power of the immune system to mediate lasting tumor clearance with memory.
ABBREVIATIONS
- α-GalCer
α-galactosylceramide
- Ag
antigen
- APC
antigen-presenting cell
- CAR
chimeric Ag receptor
- CD
cluster of differentiation
- CNS
central nervous system
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DLN
draining lymph node
- GBM
glioblastoma multiforme
- HBV
Hepatitis B virus
- HLA
human leukocyte antigen
- IFN-γ
interferon gamma
- IL
interleukin
- KIR
killer cell immunoglobulin-like receptor
- M2
type 2 macrophage
- MDSC
myeloid-derived suppressor cell
- MHC
major histocompatibility complex
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- PBMC
peripheral blood mononuclear cell
- PGE2
prostaglandin E2
- si-RNA
small interfering ribosomal nucleic acid
- TADC
tolerogenic tumor-associated dendritic cell
- TAM
tumor-associated macrophage
- TCR
T cell receptor
- TGF-β
transforming growth factor beta
- Th
helper T cell
- ThPOK
Th-inducing
- POZ
Kruppel Factor
- Treg
T regulatory cell
REFERENCES
- 1.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, Consortium NC. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omuro A, Deangelis LM. Glioblastoma and other malignant gliomas: a clinical review. J Am Med Assoc. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, Mcguire P, Cherlow JM, Tefft M, Turski PA, Wara WM, Edwards M, Sutton LN, Berger MS, Epstein F, Ayers G, Allen JC, Packer RJ for the Childrens Cancer Group. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, prednisone with the eight-drugs-in-1-day regimen Childrens Cancer Group. J Clin Oncol. 1995;13(1):112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DR, Ma DJ, Buckner JC, Hammack JE. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118(22):5608–5613. doi: 10.1002/cncr.27590. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. European Organisation for Research, Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature Rev. 2014;15(5):300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 7.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 8.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo . Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71(24):7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Mills C, Kincaid K, Alt J, Heilman M, Hill A. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 15.Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools N, Van Tendeloo V, Smits E, Lenjou M, Nijs G, Van Bockstaele D, Berneman Z. Immunosuppression induced by immature dendritic cells is mediated by TGF-/IL-10 double-positive CD4+ regulatory T cells. J Cell Mol Med. 2007;12:690–700. doi: 10.1111/j.1582-4934.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretta L, Ciccone E, Poggi A, Mingari MC, Moretta A. Ontogeny, specific functions and receptors of human natural killer cells. Immunol Lett. 1994;40(2):83–88. doi: 10.1016/0165-2478(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28(6):252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38(9):637–660. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 22.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 24.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23(1):96–103. doi: 10.1016/j.coi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, Olive D. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front Immunol. 2014;5:122. doi: 10.3389/fimmu.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 28.Münz C, Nickolaus P, Lammert E, Pascolo S, Stevanovic S, Rammensee HG. The role of peptide presentation in the physiological function of HLA-G. Semin Cancer Biol. 1999;9:47–54. doi: 10.1006/scbi.1998.0105. [DOI] [PubMed] [Google Scholar]
- 29.Pietra G, Romagnani C, Moretta L, Mingari MC. HLA-E and HLA-E-bound peptides: recognition by subsets of NK and T cells. Curr Pharm Des. 2009;15:3336–3344. doi: 10.2174/138161209789105207. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi P, Naik S, Agrawal S. Role of HLA-G, HLA-E and KIR2DL4 in pregnancy. Int J Hum Genet. 2007;7(3):219–233. [Google Scholar]
- 31.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94yNKG2A. Proc. Natl. Acad. Sci. USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Welle M. HLA-E protects glioma cells from NKG2D-mediated immune response in vitro: implications for immune escape in vivo . J Neuropathol Exp Neurol. 2006;64:523–528. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 33.Wolpert F, Roth P, Lamszus K, Tabatabai G, Weller M. HLA-E contributes to an immune-inhibitory phenotype of glioblastoma stem-like cells. J Neuroimmunol. 2012;250:27–34. doi: 10.1016/j.jneuroim.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopalan S, Long EO. KIR2DL4 (CD158d): an activation receptor for HLA-G. Frontiers Immunol. 2012;3(258):1–6. doi: 10.3389/fimmu.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiendl H, Mitsdoerffer M, Wischhusen J, Hofmeister V, Bornemann A, Meyermann R, Weiss EH, Weller M. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;268:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 36.Kren L, Slaby O, Muckova K, Lzicarova E, Sova M, Vybihal V, Svoboda T, Fadrus P, Lakomy R, Vanhara P, Krenova Z, Sterba J, Smrcka M, Michalek J. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathol. 2011;31:129–134. doi: 10.1111/j.1440-1789.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 37.Wastowski IJ, Simões RT, Yaghi L, Donadi EA, Pancoto JT, Poras I, Lechapt-Zalcman E, Bernaudin M, Valable S, Carlotti CG, Jr, Flajollet S, Jensen SS, Ferrone S, Carosella ED, Kristensen BW, Moreau P. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2’-deoxycytidine interferon-γ treatments Results from a multicentric study. Am J Pathol. 2013;182(2):540–552. doi: 10.1016/j.ajpath.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Fan X, Li H, Lin Z, Bao H, Li S, Wang L. Tumor border sharpness correlates with HLA-G expression in low-grade gliomas. J Neuroimmunol. 2015;282:1–6. doi: 10.1016/j.jneuroim.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24(4):487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Gil-Krzewska A, Peruzzi G, Borrego F. Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy. Clin Exp Immunol. 2013;173(1):131–139. doi: 10.1111/cei.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med. 2012;6(1):56–66. doi: 10.1007/s11684-012-0177-7. [DOI] [PubMed] [Google Scholar]
- 42.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192(5):741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d–dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhost S, Lofbom L, Rynmark BM, Pei B, Mansson JE, Teneberg S, Blomqvist M, Cardell SL. Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d–restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012;42(11):2851–2860. doi: 10.1002/eji.201142350. [DOI] [PubMed] [Google Scholar]
- 47.Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63(3):199–213. doi: 10.1007/s00262-013-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swann J, Crowe NY, Hayakawa Y, Godfrey DI, Smyth MJ. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol. 2004;82(3):323–331. doi: 10.1111/j.0818-9641.2004.01254.x. [DOI] [PubMed] [Google Scholar]
- 49.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180(6):3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 50.Parekh VV, Wu L, Olivares-Villagomez D, Wilson KT, Van Kaer L. Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells. J Immunol. 2013;190(5):1948–1960. doi: 10.4049/jimmunol.1201718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyth MJ, Godfrey DI. NKT cells and tumor immunity—a double-edged sword. Nat Immunol. 2000;1(6):459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 53.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59(20):5102–5105. [PubMed] [Google Scholar]
- 55.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 56.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated α NKT cells. Proc Natl. Acad. Sci. USA. 1998;95(10):5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111(12):5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hix LM, Shi YH, Brutkiewicz RR, Stein PL, Wang CR, Zhang M. CD1d–expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS One. 2011;6(6):e20702. doi: 10.1371/journal.pone.0020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99(4):1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 60.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(10–11):529–534. [PubMed] [Google Scholar]
- 62.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5(6):405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 63.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112(4):1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27(7):322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189(7):1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomura M, Yu WG, Ahn HJ, Yamashita M, Yang YF, Ono S, Hamaoka T, Kawano T, Taniguchi M, Koezuka Y, Fujiwara H. A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J Immunol. 1999;163(1):93–101. [PubMed] [Google Scholar]
- 67.Paget C, Chow MT, Duret H, Mattarollo SR, Smyth MJ. Role of gammadelta T cells in alpha-galactosylceramide-mediated immunity. J Immunol. 2012;188(8):3928–3939. doi: 10.4049/jimmunol.1103582. [DOI] [PubMed] [Google Scholar]
- 68.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179(8):5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 69.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 70.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68(9):3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, Cooper MD. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Girardi M, Hayday AC. Immunosurveillance by gamma delta T cells: focus on the murine system. Chem Immunol Allergy. 2005;86:136–150. doi: 10.1159/000086658. [DOI] [PubMed] [Google Scholar]
- 74.Baldwin CL, Telfer JC. The bovine model for elucidating the role of gamma delta T cells in controlling infectious diseases of importance to cattle and humans. Mol Immunol. 2015;66:35–47. doi: 10.1016/j.molimm.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 75.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human γδ T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 76.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 77.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 78.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 80.Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 81.Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of γδ T cells. J Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- 82.Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G, Todaro M, Cicero G, Zichichi L, Donni PL, Caccamo N, Stassi G, Dieli F, Moschella F. Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PLoS One. 2012;7:e49878. doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomes AQ, Correia DV, Grosso AR, Lança T, Ferreira C, Lacerda JF, Barata JT, Silva MG, Silva-Santos B. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood γδ T cells. Haematologica. 2010;95:1397–1404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chargui J, Combaret V, Scaglione V, Iacono I, Péri V, Valteau-Couanet D, Dubrel M, Angevin E, Puisieux A, Romagne F, Bergeron C. Bromohydrin pyrophosphate-stimulated γδ T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother. 2010;33:591–598. doi: 10.1097/CJI.0b013e3181dda207. [DOI] [PubMed] [Google Scholar]
- 85.Liu Z, Guo BL, Gehrs BC, Nan L, Lopez RD. Ex vivo expanded human γδ-T cells mediate innate antitumor activity against human prostate cancer cells in vitro . J Urol. 2005;173:1552–1556. doi: 10.1097/01.ju.0000154355.45816.0b. [DOI] [PubMed] [Google Scholar]
- 86.Saito A, Narita M, Yokoyama A, Watanabe N, Tochiki N, Satoh N, Takizawa J, Furukawa T, Toba K, Fuse I, Aizawa Y, Shinada S, Takahashi M. Enhancement of anti-tumor cytotoxicity of expanded γδ T Cells by stimulation with monocyte-derived dendritic cells. J Clin Exp Hematop. 2007;47:61–72. doi: 10.3960/jslrt.47.61. [DOI] [PubMed] [Google Scholar]
- 87.D’Asaro M, La C, Di Mendola Liberto D, Orlando V, Todaro M, Spina M, Guggino G, Meraviglia S, Caccamo N, Messina A, Salerno A, Di Raimondo F, Vigneri P, Stassi G, Fourniè JJ, Dieli F. γδ T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–3268. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 88.Nishio N, Fujita M, Tanaka Y, Maki H, Zhang R, Hirosawa T, Demachi-Okamura A, Uemura Y, Taguchi O, Takahashi Y, Kojima S, Kuzushima K. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells. J Immunother. 2012;35:598–606. doi: 10.1097/CJI.0b013e31826a745a. [DOI] [PubMed] [Google Scholar]
- 89.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 90.Castella B, Vitale C, Coscia M, Massaia M. γδ T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci. 2011;68:2419–2432. doi: 10.1007/s00018-011-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lanca T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, Ramalho JS, Barata JT, Moita LF, Gomes AQ, Silva-Santos B. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood. 2010;115:2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 92.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 93.Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, Becker J, Schmidt-Wolf IG, Einsele H, Wilhelm M. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- 94.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 95.Rei M, Goncalves-Sousa N, Lanca T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva-Santos B. Murine CD27(−) γ(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci USA. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrarini M, Pupa SM, Zocchi MR, Rugarli C, Menard S. Distinct pattern of HSP72 and monomeric laminin receptor expression in human lung cancers infiltrated by γδ T lymphocytes. Int J Cancer. 1994;57:486–490. doi: 10.1002/ijc.2910570408. [DOI] [PubMed] [Google Scholar]
- 97.Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent γδ tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–3940. [PubMed] [Google Scholar]
- 98.Dhar S, Chiplunkar SV. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by γδ T cells. Cancer Immun. 2010;10:10. [PMC free article] [PubMed] [Google Scholar]
- 99.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26(4):491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 101.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 102.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 103.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16(10):1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 104.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33(6):281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012;33(12):590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28(28):4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199(12):1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, Foley PL, Taylor RP. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101(3):1071–1079. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 109.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 110.Murphy KA, Erickson JR, Johnson CS, Seiler C, Bedi J, Hu P, Pluhar GE, Epstein AL, Ohlfest JR. CD8+ T cell independent tumor regression induced by Fc-OX40L and therapeutic vaccination in a mouse model of glioma. J Immunol. 2014;192:224–233. doi: 10.4049/jimmunol.1301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;397:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheroutre H, Husain MM. CD4 CTL: living up to the challenge. Semin Immunol. 2013;25(4):273–281. doi: 10.1016/j.smim.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159(12):5956–5963. [PubMed] [Google Scholar]
- 114.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Ann Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 115.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. Epub 2012 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Ann Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 117.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, Campbell L, Yawalkar N, Kupper TS, Clark RA. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6(219):219ra8. doi: 10.1126/scitranslmed.3007828. {AU: please check p. no.} [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5(276):1–13. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 120.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 121.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T Cells: lineage and location. Immunity. 2009;30(3):324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4 T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 124.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 125.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 126.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 127.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells, which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 129.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120(10):2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 130.Ahmed KA, Wang L, Munegowda MA, Mulligan SJ, Gordon JR, Griebel P, Xiang J. Direct in vivo evidence of CD4+ T cell requirement for CTL response and memory via pMHC-I targeting and CD40L signaling. J Leuk Biol. 2012;92:289–300. doi: 10.1189/jlb.1211631. [DOI] [PubMed] [Google Scholar]
- 131.Billings P, Burakoff S, Dorf ME, Benacerraf B. Cytotoxic T lymphocytes specific for I region determinants do not require interactions with H-2K or D gene products. J Exp Med. 1977;145:1387–1392. doi: 10.1084/jem.145.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krensky AM, Reiss CS, Mier JW, Strominger JL, Burakoff SJ. Long-term human cytolytic T-cell lines allospecific for HLA-DR6 antigen are OKT4. Proc. Natl. Acad. Sci. USA. 1982;79:2365–2369. doi: 10.1073/pnas.79.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984;308:365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- 134.Lukacher AE, Morrison LA, Braciale VL, Malissen B, Braciale TJ. Expression of specific cytolytic activity by H-2I region-restricted, influenza virus-specific T lymphocyte clones. J Exp Med. 1985;162:171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4+ CTLs ex vivo . J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 136.van Leeuwen EM, Remmerswaal EB, Vossen MT, Rowshani AT, Wertheim-van Dillen PM, van Lier RA, ten Berge IJ. Emergence of a CD4+CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 137.Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, Maino VC. CD4+CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31(8):2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 138.Aslan N, Yurdaydin C, Wiegand C, Greten T, Ciner A, Meyer MF, Heiken H, Kuhlmann B, Kaiser T, Bozkaya H, Tillman HL, Bozdayi AM, Manns MP, Wedemeyer H. Cytotoxic CD4+ T cells in viral hepatitis. J Vir Hepat. 2006;13(8):505–514. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 139.Stuller KA, Flaño E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83(9):4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jellison ER, Kim SK, Welsh RM. Cutting Edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174(2):614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 141.Martirosyan A, Von Bargen K, Arce Gorvel V, Zhao W, Hanniffy S, Bonnardel J, Méresse S, Gorvel J-P. In Vivo identification and characterization of CD4+ cytotoxic T cells induced by virulent Brucella abortus infection. PLoS One. 2013;8(12):e82508. doi: 10.1371/journal.pone.0082508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. Epub 2011 Nov 22. Review. PMID:22174559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peterson AC, Harlin H, Gajewski TF. Immunization with melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 144.Boon T, Coulie PG, Van Den Eynde BJ, Van Der Bruggen P. Human T cell responses against melanoma. Ann Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 145.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hayama S, Daigo Y, Kato T, Ishikawa N, Yamabuki T, Miyamoto M, Ito T, Tsuchiya E, Kondo S, Nakamura Y. Activation of CDCA1-KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res. 2006;66:10339–10348. doi: 10.1158/0008-5472.CAN-06-2137. [DOI] [PubMed] [Google Scholar]
- 147.Harao M, Hirata S, Irie A, Senju S, Nakatsura T, Komori H, Ikuta Y, Yokomine K, Imai K, Inoue M, Harada K, Mori T, Tsunoda T, Nakatsuru S, Daigo Y, Nomori H, Nakamura Y, Baba H, Nishimura Y. HLA-A2-restricted CTL epitopes of a novel lung cancer-associated cancer testis antigen, cell division cycle associated 1, can induce tumor-reactive CTL. Int J Cancer. 2008;123:2616–2625. doi: 10.1002/ijc.23823. [DOI] [PubMed] [Google Scholar]
- 148.Tomita Y, Yuno A, Tsukamoto H, Senju S, Yoshimura S, Osawa R, Kuroda Y, Hirayama M, Irie A, Hamada A, Jono H, Yoshida K, Tsunoda T, Kohrogi H, Yoshitake Y, Nakamura Y, Shinohara M, Nishimura Y. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int J Cancer. 2014;134:352–366. doi: 10.1002/ijc.28376. [DOI] [PubMed] [Google Scholar]
- 149.Tomita Y, Yuno A, Tsukamoto H, Senju S, Kuroda Y, Hirayama M, Irie A, Kawahara K, Yatsuda J, Hamada A, Jono H, Yoshida K, Tsunoda T, Kohrogi H, Yoshitake Y, Nakamura Y, Shinohara M, Nishimura Y. Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor. Clin Cancer Res. 2013;19(16):4508–4520. doi: 10.1158/1078-0432.CCR-13-0197. [DOI] [PubMed] [Google Scholar]
- 150.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Ann Rev Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chandran SS, Paria BC, Srivastava AK, Rothermel LD, Stephens DJ, Dudley ME, Somerville R, Wunderlich JR, Sherry RM, Yang JC, Rosenberg SA, Kammula US. Persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in humans. Clin. Cancer Res. 2014;21(3):534–543. doi: 10.1158/1078-0432.CCR-14-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosenberg SA. Finding suitable targets is the major obstacle to cancer gene therapy. Cancer Gene Ther. 2014;21:45–47. doi: 10.1038/cgt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor- specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventative and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60(10):1473–1484. doi: 10.1007/s00262-011-1054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hildemann SK, Eberlein J, Davenport B, Nguyen TT, Victorino F, Homann D. High efficiency of antiviral CD4+ killer T cells. PLoS One. 2013;8(4):e60420. doi: 10.1371/journal.pone.0060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lai YP, Jeng CJ, Chen SC. The Roles of CD4+ T Cells in Tumor Immunity ISRN Immunology. 2011;2011 Article ID 497397, 6 pages. http://dx.doi.org/10.5402/2011/497397. [Google Scholar]
- 164.Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, Allison JP, Gnjatic S, Yuan JD, Wolchok JD. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res. 2013;1(4):235–244. doi: 10.1158/2326-6066.CIR-13-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, Shi J, Fu B, Liu Z, Zhang JY, Jin L, Zhao Y, Lau GK, Zhao J, Wang FS. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58:139–149. doi: 10.1002/hep.26054. [DOI] [PubMed] [Google Scholar]
- 166.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M, Attinger A, Shui JW, Kim G, Lena CJ, Sakaguchi S, Miyamoto C, Wang P, Atarashi K, Park Y, Nakayama T, Honda K, Ellmeier W, Kronenberg M, Taniuchi I, Cheroutre H. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14(3):281–291. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol. 2013;14(3):271–281. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vacchio MS, Wang L, Bouladoux N, Carpenter AC, Xiong Y, Williams LC, Wohlfert E, Song KD, Belkaid Y, Love PE, Bosselut R. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat Immunol. 2014;15(10):947–957. doi: 10.1038/ni.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]