Graphical abstract
Nitrogen Mustard Reloaded: Over 60 years after nitrogen mustards (NMs) were the first agents used to treat tumors by chemotherapy, we provide a method to generate the main DNA adduct formed by NMs and validate them using molecular dynamics simulations. We are able to provide amounts permitting extensive structural and biological studies.

Keywords: DNA Interstrand crosslink, Cancer Chemotherapy, Nitrogen Mustard, Oligonucleotide synthesis, Molecular Dynamics Simulations
DNA interstrand cross-links (ICLs) are formed by bifunctional agents with the ability to covalently link two strands of duplex DNA. ICLs are extremely cytotoxic since they block essential processes such as DNA replication and transcription. Based on these properties, agents such as nitrogen mustards (NM) or cisplatin are widely used in cancer chemotherapy.[1] ICLs induce a number of biological responses, which counteract the therapeutic effects of crosslinking agents. However, the elucidation of the mechanism by which these responses cause resistance in tumor cells has been hampered by the lack of efficient methods to generate ICLs formed by antitumor agents. Current approaches toward the synthesis of site-specific ICLs have yielded mostly models of the clinically relevant lesions or ICLs formed by endogenous agents.[2] A number of recent studies have shown that ICLs with different structures are processed in distinct ways (see [3] for some recent reviews), emphasizing the need to generate substrates containing the ICLs formed by the clinically important drugs.
Here we report the synthesis of a stable NM ICL isostere (2) and use atomic detail simulations to show that it recaptures the structural and dynamic properties of the native NM ICL 1 (Figure 1). To obtain the NM ICL isostere we used a strategy that we recently developed for the synthesis of major groove ICLs[4].
Figure 1.

Structure of a NM ICL (1) and the stable analog 2.
This approach involves a double reductive amination reaction of two acetaldehyde functionalities (5) linked to the 7-position of G residues on complementary strands of dsDNA. dG was substituted with 7-deaza-2′-deoxyguanosine to counteract the inherent lability of the glycosidic bond in N7-alkylated guanines.[5] This approach allowed the generation of a six atom ICL by coupling two acetaldehyde groups with hydrazine. However, we were unsuccessful in generating ICL 3 with the 5-atom bridge found in NM ICLs[6] (Scheme 1), possibly because the reductive amination with ammonia was not powerful enough to introduce the strain in the DNA caused by the ~7.5Å bridge of the NM.[4, 7]
Scheme 1.

Formation of NM ICLs by reductive amination was not successful using two acetaldehyde precursors 5 and NH4Cl, prompting us to explore the generation of isostere 2 by linking precursors 4 and 5 with hydrazine.
We reasoned that an ICL isosteric to those formed by NM might be formed by reaction of hydrazine with an acetaldehyde (5) and a formyl aldehyde derivative (4) of deazaguanine, exploiting the higher reactivity of hydrazine over ammonia (Scheme 1). [4]
We synthesized formyl aldehyde 4 in an analogous fashion to the previously reported synthesis of acetaldehyde 5, masking the aldehyde as a protected diol during solid-phase DNA synthesis.[4, 8] The synthesis started with vinylation of a protected 7-iodo-7-deazaguanine derivative (6) using a Stille coupling reaction (Scheme 2). Oxidation of the allyl group to the diol, protection and functionalization yielded phosphoramidite 9. The aldehyde precursors were incorporated into complimentary 20-mer oligonucleotides in a 5′-d(GNC) sequence (the preferred sequence context for NM ICL formation[6]) by solid phase synthesis, and the oligonucleotides were deprotected and annealed.
Scheme 2.

Synthesis of the formyl aldehyde 4: a. vinyl-Sn(Bu)3, Pd[P(Ph)3]4, toluene, 90°C, 43h, 70%; b. pyridine-2-carboxaldoxime, N,N,N′,N′-tetra-methylguanidine, dioxane, DMF, rt, 42h, 88%; c. NaOMe, THF, rt, 5h, 97%; d. TBDMS-Cl, imidazole, DMF, rt, 16h, 93%; e. OsO4, NMMO, THF, 0°C, 3.5h, 63%; f. Ac2O, pyridine, rt, 1h, 82%; g. TBAF, AcOH, THF, rt, 20h, 79%; h. DMTr-Cl, pyridine, rt, 1h, 74%; i. iPr2NP(Cl)OC2H4CN, DIEA, CH2Cl2, rt, 1h, 72%; j. oligonucleotide synthesis; k. 33% NH4OH, 50°C, 12h; l. annealing; m. NaIO4, rt, 12h.
Following oxidation of the diols using periodate, the two aldehydes were coupled by double reductive amination with hydrazine and NaBH3CN.[4]
The reaction along with appropriate controls was analyzed by denaturing PAGE.
As already discussed,[4] ICL formation from two acetaldehyde precursors was successful with hydrazine, but not with ammonium acetate (Figure 2, lane 2 and 3). However, reaction of a duplex containing 4 and 5 with hydrazine led to formation of the desired 5-atom ICL 2, evidenced by a band with the same mobility as the previously analyzed crosslink (Figure 2, lanes 3 and 4). Although the yield of the 5 atom ICL was lower than that of the 6-atom ICL (~25% vs ~75%), we were able to isolate the product by gel purification and electroelution in amounts exceeding 100 nmol. For simplicity of analysis we also synthesized ICL 2 in an 11-mer duplex and unambiguously identified it as the desired product by ESI-MS (m/z calc. 6741.23, found 6741.6, Figure S1). If either aldehyde precursor was present only on one strand of the duplex, no significant amounts of the slower migrating species were observed, further demonstrating the specificity of ICL formation (Figure 2, lanes 1 and 5).
Figure 2.

Analysis of ICL formation by denaturing PAGE with methylene blue staining. The duplexes, amine used and position of single-stranded or ICL-containing DNA are indicated. The sequences used were 5′-d(GTCACTGGTAXACAGCATTG) and 5′-d(CAATGCTXTCTACCAGTGAC) where X represents the modified G. The small amount of bands running as duplexes in lanes 2 and 5 are likely due to residual amount of duplex that was not denaturated during electrophoresis.
Molecular modeling was used to validate ICL 2 as a model for NM 1 and to compare the structural consequences of the two ICLs with the uncrosslinked control (C) in identical 11-mer sequences. We used the Amber simulation package with atomic detail and explicit water (See Supporting Information) to validate that 2 gives similar amounts of distortion when compared to the NM ICL 1 and consistent differences when both are compared to C.[9] Two independent simulations of 50ns were run for each of the 1, 2 and C systems. The duplex was stable throughout all six simulations.
The N7 to N7 distances of the NM ICL 1 overlapped well with the C7 to C7 distances of 2; both are restricted as compared to C, which samples a broader range (Figure 3A).
Figure 3.
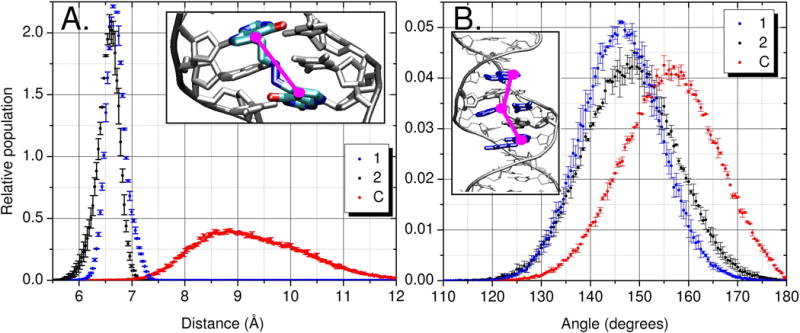
Data from MD simulations of 2 (black), 1 (blue) and C (red). A) Distance measurement marked in pink, C/N7 to C/N7 atoms between residues 5 and 16. B) Center of mass angle measurement, where each point is defined by the heavy atoms of the base pair (highlighted in blue in inset picture): point 1 represents 4A and 19T, point 2 represents 6T and 17A and point 3 represents 8A and 15T.[10]
The decreased distance between the two crosslinked bases has a direct effect on the local distortion around the ICL but is also influenced by the sequence (Figure 3B and Table S1). One measure of distortion is to examine the buckling and propeller twist[11] of the two crosslinked base pairs (Table S1, Figure S2 for sequence). Since molecular mechanics force fields have limited accuracy, it is more important to note the trends rather than the specific values. It is well accepted that differences are more reliable in simulations than absolute values, due to cancellation of systematic error. The crosslinked guanine 16 that neighbors the large purine ring of adenine (residue 17) accommodates the crosslink by buckling of the 7:16 base pair (C: −3.5° ±0.2°, 1: −13.2° ±0.1° and 2: −21.8° ±0.6°). The crosslinked guanine 5 that neighbors the small pyrimidine ring of thymine 6 has more room to move and accommodates the ICL by an increased propeller twist of the 5:18 base pair (C: −13.2° ±0.1°, 1: −22.3° ±0.2° and 2: −26.9° ±0.8°). The addition of a covalent bridge between both strands of DNA nearly doubles residue to residue correlation in the central region of the duplex. Residues 5 and 16 have a correlation coefficient (r) of 0.29 ±0.04, 0.62 ±0.01 and 0.58 ±0.02 for C, 1 and 2 respectively (Figure S2). 1 and 2 have a substantial decrease in flexibility (Figure 3A) when compared to the uncrosslinked reference CNT, resulting in the formation of a slight kink in the duplex of both 1 and 2 to accommodate the ICL (Figure 3B).
To test for local duplex bending at the crosslink site we measured an angle that would encompass the tightening of the 7 to 7 position of residues 5 and 16 (Figure 3A). The smaller angles sampled by 1 and 2 suggest that the analog slightly bends the duplex outside the crosslink in the same manner that the NM ICL simulations do (Figure 3B). The slight bending of the central region of the ICL simulations of 1 and 2 compared to C is qualitatively similar to past experimental work.[7]
Circular dichroism (CD) spectra were recorded to gain experimental insight into to what extent the NM-ICLs containing oligonucleotide 2 deviates from B-form DNA. The CD spectra of 2 and a unmodified duplex of the same sequence displayed the characteristic features of B-form DNA (Figure S4), consistent with our molecular dynamics simulations indicating the NM ICL induces only a minor bend in the DNA. It has been shown that only more dramatic distortions, such as the ones induced by cisplatin ICL result in significant changes in CD spectra.[12]
We describe the synthesis of the stable NM ICL analog 2 by post-synthetic double reductive amination. Although this ICL has three atoms substituted compared to the NM ICL 1 (the two N at the 7 position of dG by C and one C by N in the hydrazine-formed bridge), our molecular dynamics simulations show that the two ICLs affect DNA structure and motion in equivalent ways. The availability of stable, site-specific NM ICLs will enable studies of the structural consequences and biological responses induced by NM ICLs,[13] more than sixty years after NMs were the first agents to be used in cancer chemotherapy.[14] Such studies will provide new insights into how tumors become resistant to treatment by crosslinking agents.
Experimental Section
Crosslink formation: A solution of the single strand oligonucleotides (25 nmol) in 125 μl 10 mM NaCl was heated to 95°C and allowed to cool to room temperature over a period of 4 hrs to allow for duplex formation. After addition of 15 μl 1M sodium phosphate buffer (pH 5.4) and 10 μl 50 mM NaIO4 the reaction mixture was kept in the dark overnight at 4°C. Excess NaIO4 was removed by centrifugation through Microcon columns with a 3K cutoff (Millipore). Then 10 μl 5 mM aqueous hydrazine and 10 μl 0.5 M NaCNBH3 were added and the reaction mixture was left overnight at room temperature in the dark. ICL formation was assessed by electrophoresis on a denaturing 20% polyacrylamide gel. The band containing the crosslinked oligonucleotide was excised from the gel and the DNA was extracted by electroelution using D-Tube™Dialyzer (Novagen) or the Elutrap™(Schleicher & Schuell) device.
Full experimental details and computational methods are available as supplementary material.
Supplementary Material
Acknowledgments
We are grateful to Robert Rieger and the Proteomic Center of Stony Brook University for MS spectral analyses supported by grant NIH/NCRR1 S10 RR023680-1. Support for this project was provided by NSF 0549370 (AJC), NIH GM6167803 (CS), the New York State Office of Science and Technology and Academic Research NYSTAR, C040069 (ODS), the Swiss Cancer League OCS-01413-080-2003 (ODS) and supercomputer resources through the NSF Teragrid MCA02N028 (CS), DOE (DE-AC02-98CH10886), and the State of New York (CS).
Footnotes
Dedicated to Prof. Francis Johnson on the occasion of his 80th birthday
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
Contributor Information
Prof. Carlos Simmerling, Email: carlos.simmerling@gmail.com.
Prof. Orlando D. Schärer, Email: orlando@pharm.stonybrook.edu.
References
- 1.Schärer OD. Chembiochem. 2005;6:27. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 2.a) Ferentz A, Keating T, Verdine G. J Am Chem Soc. 1993;115:9006. [Google Scholar]; b) Erlanson DA, Chen L, Verdine GL. J Am Chem Soc. 1993;115:12583. [Google Scholar]; c) Harwood E, Sigurdsson S, Edfeldt N, Reid B, Hopkins P. J Am Chem Soc. 1999;121:5081. [Google Scholar]; d) Kobertz W, Essigmann JM. J Am Chem Soc. 1997;119:5960. [Google Scholar]; e) Dooley PA, Tsarouhtsis D, Korbel GA, Nechev LV, Shearer J, Zegar IS, Harris CM, Stone MP, Harris TM. J Am Chem Soc. 2001;123:1730. doi: 10.1021/ja003163w. [DOI] [PubMed] [Google Scholar]; f) Noll DM, Noronha AM, Miller PS. J Am Chem Soc. 2001;123:3405. doi: 10.1021/ja003340t. [DOI] [PubMed] [Google Scholar]; g) Wilds CJ, Noronha AM, Robidoux S, Miller PS. J Am Chem l Soc. 2004;126:9257. doi: 10.1021/ja0498540. [DOI] [PubMed] [Google Scholar]; h) Hong IS, Greenberg MM. J Am Chem Soc. 2005;127:3692. doi: 10.1021/ja042434q. [DOI] [PubMed] [Google Scholar]; i) Alzeer J, Schärer OD. Nucleic Acids Res. 2006 doi: 10.1093/nar/gkl587. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Wilds CJ, Xu F, Noronha AM. Chem Res Toxicol. 2008;21:686. doi: 10.1021/tx700422h. [DOI] [PubMed] [Google Scholar]
- 3.a) Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM. Crit Rev Biochem Mol Biol. 2010;45:23. doi: 10.3109/10409230903501819. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ho TV, Schärer OD. Environ Mol Mutagen. 2010. [DOI] [PubMed] [Google Scholar]; c) Hlavin EM, Smeaton MB, Miller PS. Env Mol Mutagen. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelov T, Guainazzi A, Schärer O. Org Lett. 2009;11:661. doi: 10.1021/ol802719a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Bowman BR, Ueno Y, Wang S, Verdine GL. J Am Chem Soc. 2008;130:11570. doi: 10.1021/ja8025328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Ojwang JO, Grueneberg DA, Loechler EL. Cancer Res. 1989;49:6529. [PubMed] [Google Scholar]; b) Millard JT, Raucher S, Hopkins PB. J Am Chem Soc. 1990;112:2459. [Google Scholar]; c) Rink SM, Solomon MS, Taylor MJ, Rajur SB, Mclaughlin LW, Hopkins PB. J Am Chem Soc. 1993;115:2551. [Google Scholar]
- 7.a) Rink SM, Hopkins PB. Biochemistry. 1995;34:1439. doi: 10.1021/bi00004a039. [DOI] [PubMed] [Google Scholar]; b) Dong Q, Barskt D, Colvin ME, Melius CF, Ludeman SM, Moravek JF, Colvin OM, Bigner DD, Modrich P, Friedman HS. Proc Natl Acad Sci USA. 1995;92:12170. doi: 10.1073/pnas.92.26.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khullar S, Varaprasad CV, Johnson F. J Med Chem. 1999;42:947. doi: 10.1021/jm980605u. [DOI] [PubMed] [Google Scholar]
- 9.Case DA, Cheatham TE, 3rd, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. J Comput Chem. 2005;26:1668. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 11.Lavery R, Sklenar H. J Biomol Struct Dyn. 1989;6:655. doi: 10.1080/07391102.1989.10507728. [DOI] [PubMed] [Google Scholar]
- 12.a) Hofr C, Brabec V. J Biol Chem. 2001;276:9655. doi: 10.1074/jbc.M010205200. [DOI] [PubMed] [Google Scholar]; b) Wilds CJ, Xu F, Noronha AM. Chem Res Tox. 2008;21:686. doi: 10.1021/tx700422h. [DOI] [PubMed] [Google Scholar]
- 13.a) Räschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC. Cell. 2008;134:969. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Knipscheer P, Räschle M, Smogorzewska A, Enoiu M, Ho TV, Schärer OD, Elledge SJ, Walter JC. Science. 2009;326:1698. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman L, Wintrobe M, Dameshek W, Goodman M, Gilman A. JAMA - J Am Med Assoc. 1946;132:126. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


