Abstract
The strength of γ-aminobutyric acid (GABA)-mediated inhibitory synaptic input is a principle determinant of neuronal activity. However, because of differences in the number of GABA afferent inputs and the sites of synapses, it is difficult to directly assay for altered GABA transmission between specific cells. The present study tested the hypothesis that the level of mRNA for the GABA synthetic enzyme glutamate decarboxylase (GAD) can provide a reliable proxy for GABA release. This was tested in a mouse hypothalamic circuit important in the regulation of energy balance. Fluorescent in situ hybridization results show that the expression of Gad1 mRNA (encoding the GAD67 enzyme) was increased in hypothalamic neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons after an overnight fast, consistent with the ability of GABA from these neurons to stimulate food intake. Optogenetic studies confirmed that the observed increase in Gad1 mRNA correlated with an increase in the probability of GABA release from NPY/AgRP neurons onto downstream proopiomelanocortin neurons. Likewise, there was an increase in the readily releasable pool of GABA in NPY/AgRP neurons. Selective inhibition of GAD activity in NPY/AgRP neurons decreased GABA release, indicating that GAD67 activity, which is largely dictated by expression level, is a key determinant of GABA release. Altogether, it appears that Gad expression may be a reliable proxy of altered GABAergic transmission. Examining changes in Gad mRNA as a proxy for GABA release may be particularly helpful when the downstream targets are not known or when limited tools exist for detecting GABA release at a particular synapse.
Keywords: AgRP, in situ hybridization, mouse, proopiomelanocortin, readily releasable pool
Introduction
The amino acid transmitter γ-aminobutyric acid (GABA) has long been recognized as a critical mediator of neuronal inhibition, yet the role of GABA in several circuits is just beginning to be explored in detail. For example, despite the fact that GABA was first detected in neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons of the hypothalamus nearly two decades ago (Horvath et al., 1997), it has only recently been discovered that synaptic GABA released from these neurons acutely stimulates food intake independent of peptide release (Tong et al., 2008; Wu et al., 2008; Krashes et al., 2013). The dynamic regulation of GABA release from this population of cells, particularly in response to altered energy state, has not yet been explored. Several factors, such as sparse and spatially restricted afferent inputs and a potential lack of tools to detect transmitter release, hinder the ability to detect altered GABAergic transmission in various neuronal circuits. Therefore, the present study aimed to determine if the expression of GABA neuron-specific mRNA could serve as a proxy for altered GABAergic transmission.
Several proteins are only found in neurons that can package and release GABA, including the vesicular GABA transporter (vGAT), the plasma membrane GABA transporters (GATs), and GABA synthetic enzymes glutamate decarboxylase (GAD) 65 and 67. While vGAT is generally necessary for vesicular packaging and release of GABA, several lines of evidence suggest that modest changes in vGAT expression will not affect GABA release (Edwards, 2007; Apostolides & Trussell, 2013). Indeed, heterozygous mice with a significant reduction in vGAT expression show no apparent changes in synaptic GABA release (Yamada et al., 2012). By contrast, the levels of cytosolic GABA, which are derived primarily from glutamate uptake (Mathews & Diamond, 2003), greatly affect vesicular GABA content and synaptic strength (Apostolides & Trussell, 2013; Ishibashi et al., 2013; Wang et al., 2013). Cytosolic GABA content is largely controlled at the level of glutamate decarboxylation by the GAD enzymes, with GAD67 being responsible for > 90% of brain GABA content and essential for synaptic GABA release and survival (Asada et al., 1997; Chattopadhyaya et al., 2007; Obata et al., 2008; Lazarus et al., 2013). The necessary nature of GAD67, and the fact that GAD67 protein and its mRNA (Gad1) levels are often more sensitive to a number of experimental conditions compared with GAD65 (Rimvall & Martin, 1992, 1994; McCarthy, 1995; Bowers et al., 1998; Mason et al., 2001), led to the current hypothesis that changes in Gad1 expression could provide a means to assess overall changes in GABA transmission from a specific neuron type in response to a physiological challenge. The results show that changes in energy state are sufficient to selectively increase or decrease Gad1 mRNA in NPY/AgRP neurons and cause a concomitant change in synaptic GABA transmission from these neurons. Using Gad1 mRNA as a proxy for altered GABA release has the advantages of examining the whole population of neurons at once and does not require recording inhibitory postsynaptic currents (IPSCs) from the postsynaptic neuron.
Materials and methods
Animals
Proopiomelanocortin (POMC)-enhanced green fluorescent protein (EGFP) [C57BL/6J-Tg(Pomc-EGFP)1Low/J, stock 009593], NPY-hrGFP [B6.FVB-Tg(Npy-hrGFP)1Lowl, stock 006417] and AgRP-Cre mice [AgRPtm1(cre)Lowl, stock 012899] were obtained from The Jackson Laboratory. POMC-DsRed animals (Hentges et al., 2009) were originally obtained from Dr Malcolm Low. In the arcuate nucleus, NPY expression is restricted to cells that also express AgRP with overlap greater than 90% (Hahn et al., 1998), thus the transgenically expressed NPY-hrGFP specifically labels cells also expressing AgRP in this region. All transgenic animals were maintained on the C57BL/6J background. Both male and female mice (8–12 weeks old) were used for experiments and were distributed evenly over treatment conditions. Mice were maintained on a 12-h light/dark cycle, and were given ad libitum access to water and standard rodent chow unless noted otherwise. All experimental protocols were reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee, and were in accordance with the United States Public Health Service guidelines for animal use.
In situ hybridization
NPY-hrGFP mice were deeply anaesthetized with sodium pentobarbital and transcardially perfused with a 10% sucrose solution, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were post-fixed overnight at 4 °C in 4% paraformaldehyde solution in PBS. Fifty-micrometre sections containing the arcuate nucleus were cut on a vibratome, collected in cold PBS and processed for in situ hybridization to detect Gad1 or Gad2 mRNA as detailed previously (Jarvie & Hentges, 2012). The fluorescent signal of the hrGFP was quenched through the in situ hybridization procedure. Therefore, after completion of the in situ hybridization protocol, immunofluorescence was used to detect hrGFP by addition of a polyclonal antibody against hrGFP (1: 1000; Agilent Technologies, Santa Clara, CA, USA) overnight at 4 °C and detection with goat anti-rabbit conjugated to Alexa 568 (1: 400, 1 h room temperature). Confocal images were collected using a Zeiss 510-Meta confocal microscope. Z-stacks were initially constructed with 5–9 images 3 μm apart in depth for all tissue sections containing cells labelled for hrGFP. These stacks were pared down to four sequential images for analysis. Cell counts were made using a modification of the 3D counting method described by Williams and Rakic to limit oversampling (Williams & Rakic, 1988). Only gfp-positive cells with a clear nucleus and completely contained in a 300 × 300 × 12-μm counting box on the x–y–z plane were counted, and the presence or absence of Gad1 signal was determined for each gfp-positive cell. Average fluorescence intensity had to be greater than 10% above background for a cell to be considered positive for either signal and for Gad1, the signal had to be constrained within the somatic region of a gfp-positive cell for that gfp cell to be considered as expressing the Gad1 signal. All images were analysed using NIH ImageJ by an experimenter blinded to the treatment groups. NPY-hrGFP-positive cells were identified in a minimum of seven slices per animal. For each hrGFP-positive cell, the assessor determined whether or not the cell also contained the Gad label, while examining individual images from the image stack and label intensity was automatically determined for each cell.
In vivo gene delivery via adeno-associated virus (AAV)
AAV (3.56E + 13 GC/mL) containing a double-floxed sequence for ChR2 with an mCherry tag [AAV2/9.EF1.dflox.hChR2(H134R)–mCherry.WPRE.hGH; obtained from the Penn Vector Core at the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA] was delivered bilaterally into the arcuate nucleus as previously described (Dicken et al., 2012), and recordings were made 2–3 weeks post-injection.
Brain slice collection and electrophysiology
Sagittal brain slices (240 μm) containing the arcuate nucleus were prepared as previously described (Pennock & Hentges, 2011). Optogenetic stimulation and electrophysiological recordings were performed as previously described (Dicken et al., 2012). In brief, slices were maintained at 37 °C in artificial cerebrospinal fluid (aCSF) containing the following (in mm): NaCl, 126; KCl, 2.5; MgCl2, 1.2; CaCl2, 2.4; NaH2PO4, 1.2; NaHCO3, 21.4; glucose, 11.1 (saturated with 95% O2 and 5% CO2). Immediately after collection, slices were maintained in aCSF containing the N-methyl-d-aspartate (NMDA) receptor blocker MK-801 (15 μm) for at least 45 min before being transferred to the recording chamber. Whole-cell voltage-clamp recordings were made with an internal recording solution containing the following (in mm): KCl, 57.5; K-methyl sulphate, 57.5; NaCl, 20; MgCl2, 1.5; Hepes, 5; EGTA, 0.1; ATP, 2; GTP, 0.5; phosphocreatine, 10; pH 7.3. Recording pipettes had a tip resistance of 1.5–2.2 MΩ when filled with internal solution. For cell-attached recordings, the recording electrode contained aCSF. AgRP cells were identified by the fluorescence of the mCherry tag fused to ChR2. POMC cells were identified by EGFP fluorescence. Cells selected for patching were in the region containing mCherry-positive fibres or cell bodies. Recordings were excluded from analysis if access resistance changed significantly during the recording or if access resistance increased above 18 MΩ. IPSCs were evoked either with paired 2-ms 470-nm light pulses 100 ms apart for paired-pulse ratio (PPR), or by 40 2-ms light pulses 100 ms apart for the depletion protocol through the use of an LED/LEDD1B driver (Thorlabs, Newton, NJ, USA) connected to a TTL output on the ITC-18 data acquisition board (InstruTech, Longmont, CO, USA). For PPR acquisition, sweeps were 20 s apart. A 40-s break between sweeps was used in experiments with depletion protocol. In all light-evoked-release studies, the light intensity was adjusted to the minimum level that would evoke consistent currents from ChR2-expressing cells, and currents were evoked for data collection no sooner than 4 min after break-in. Recordings were collected at 10 kHz, digitally filtered at 1 kHz, and at least three consecutive sweeps were averaged for presentation and analysis. To verify that evoked currents were mediated by GABAA receptors, bicuculline methiodide (BMI; 10 μm; R&D Systems, Minneapolis, MN, USA) was bath-applied to the slice after experiments and always abolished the evoked currents. When recording from recurrent synapses in culture, GABA was evoked using a 2-ms depolarization to 0 mV (action potential artefacts are blanked in averaged traces).
Primary hypothalamic tissue culture
Tissue culture was performed as previously described (Hentges et al., 2004) from hypothalami of young (P2–P7) NPY-hrGFP mice with minor modifications. In brief, hypothalami were collected into ice-cold Hibernate-A medium (Life Technologies). Tissue was minced, and cells were dissociated after exposure to papain (20 U/mL; Worthington) by passing through glass pipettes with fire-polished tips. Cells were plated onto glass coverslips pre-coated with poly-l-lysine in Neurobasal-A medium (Fisher Scientific) supplemented with B27 (Fisher Scientific), 0.4 mm l-glutamine and 1% foetal calf serum. The media were replenished every 3–5 days. Recordings from these cells occurred between 8 and 16 days of culture with either chelidonic acid alone (1 mm; Sigma) or with GABA (10 mm) included in the normal internal recording solution.
Recombinant mouse intraperitoneal (i.p.) leptin injections
Purified leptin (National Hormone & Peptide Program) was rehydrated by gentle mixing in 15 mm sterile HCl, and the pH was adjusted to neutral. Leptin was brought to a final concentration of 1000 μg/mL with sterile saline. Mice received either a single i.p. saline or leptin injection (6.0 μg/g body weight) 2 h prior to death.
Data analysis
All data are presented as mean ± SEM. Comparisons between two groups were evaluated using either t-tests or, in the case of the intensity distribution data, a Kolmogorov–Smirnov test. One-way anova analyses followed by Tukey’s HSD were used for comparisons between three groups. Repeated-measures anova with Dunnett post hoc tests were used in the autapse experiments. For all experiments P < 0.05 was considered significant. Readily releasable pool (RRP) estimates were performed as previously reported (Schneggenburger et al., 1999; Thanawala & Regehr, 2013).
Results
Fasting increases Gad1 mRNA in NPY/AgRP neurons
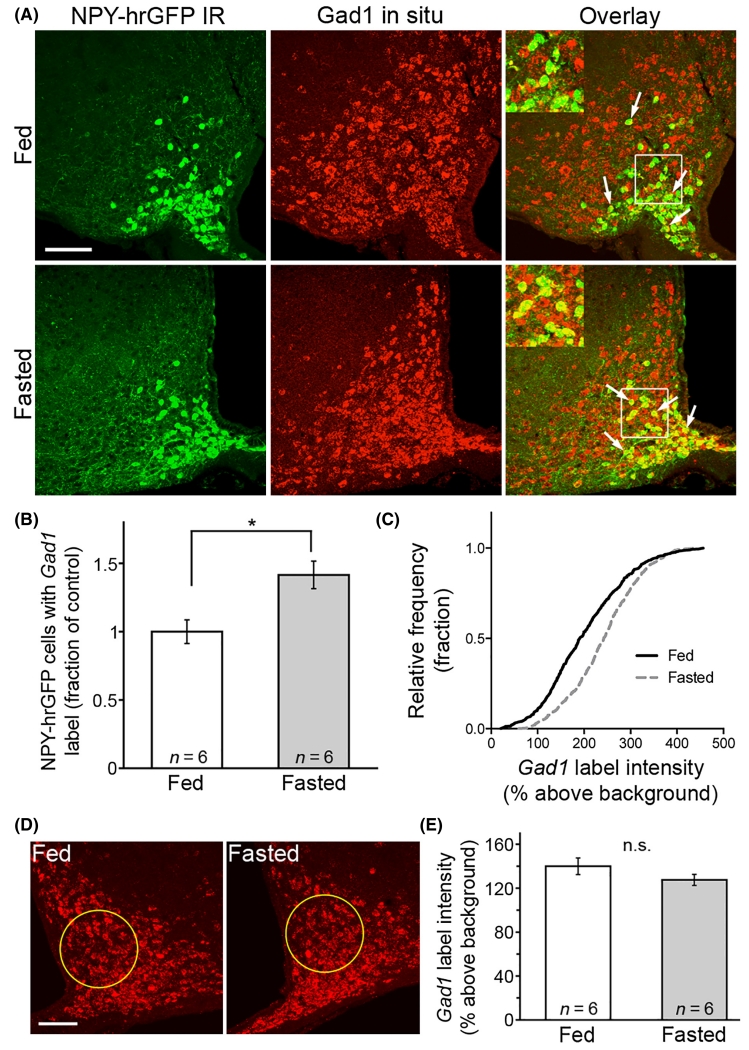
Because NPY/AgRP neurons are GABAergic, are activated by fasting (Takahashi & Cone, 2005; Yang et al., 2011) and may release more GABA in response to fasting (Vong et al., 2011), this study sought to determine whether Gad1 mRNA also increased in these cells in response to fasting as a putative indicator of enhanced GABA transmission. In situ hybridization for Gad1 was performed in tissue from NPY-hrGFP mice under fed and fasted (17 h) conditions. Food restriction caused a significant increase in the number of NPY-hrGFP-immunolabelled cells that were also labelled with the probe for Gad1 mRNA (fasted 1.41 ± 0.10 compared with normalized control values, n = 6 mice, P = 0.01 by unpaired t-test; Fig. 1A and B). Additionally, the proportion of NPY/AgRP neurons with strong Gad1 signal was increased in fasted mice (Kolmogorov–Smirnov test, P = 7.3 × 10−21, n = 608 cells in fed, 882 cells in fasted from six mice per group; Fig. 1C). The total number of hrGFP cells counted was the same for both groups (fed = 300 ± 16 cells/animal, fasted = 314 ± 20 cells/animal, P = 0.58 by unpaired t-test). To determine if Gad1 label intensity was broadly elevated in a majority of arcuate nucleus neurons, a large region of interest was drawn that included a small number of hr-GFP-positive cells and a large number of Gad1-labelled cells that were not hrGFP-positive (Fig. 1D, region indicated by yellow circles). The average intensity above background was not significantly different between groups (139.9 ± 7.6% in fed, 127.5 ± 5.0% in fasted, n = 6, P = 0.20 by unpaired t-test; Fig. 1E). Therefore, it appears that the fasting-induced increase in Gad1 is relatively restricted to AgRP neurons in the arcuate nucleus, consistent with a previous report that also showed no overall difference in Gad1 in the hypothalamic arcuate nucleus in response to fasting (Schwartz et al., 1993).
Fig. 1.
Gad1 increases in neuropeptide Y (NPY)/agouti-related peptide (AgRP) cells after an overnight fast. NPY-hrGFP neurons were identified by immunoreactivity to hrGFP (A, left column, green), and Gad1 was detected using a cRNA probe (A, middle column, red). Insets show an enlarged view of the area within the white box. White arrows point to some NPY-hrGFP cells containing Gad1. Fasting resulted in a significant increase in the percentage of NPY cells expressing detectable levels of Gad1 (B), and an increase in the proportion of cells with high levels of Gad1 label intensity (C). The overall intensity of Gad1 label in the area adjacent to the hrGFP-labelled cells (circled in D) was not changed by energy state (E). Scale bar: 100 μm. Data are plotted as mean ± SEM. *P < 0.05.
A separate in situ hybridization experiment to detect Gad2 mRNA (encoding GAD65) was also performed. While the proportion of NPY/AgRP neurons with strong Gad2 signal was increased in fasted mice (Kolmogorov–Smirnov test, P = 4.4 × 10−18, n = 1299 cells in fed, 1430 cells in fasted from four mice per group), fasting did not significantly increase the number of Gad2-labelled hrGFP-positive cells (fasted 1.02 ± 0.03 compared with normalized controls, n = 4, P = 0.41 by unpaired t-test). Thus, it appears that Gad1 expression is more sensitive to changes in energy balance compared with Gad2.
Fasting increases IPSCs in POMC neurons
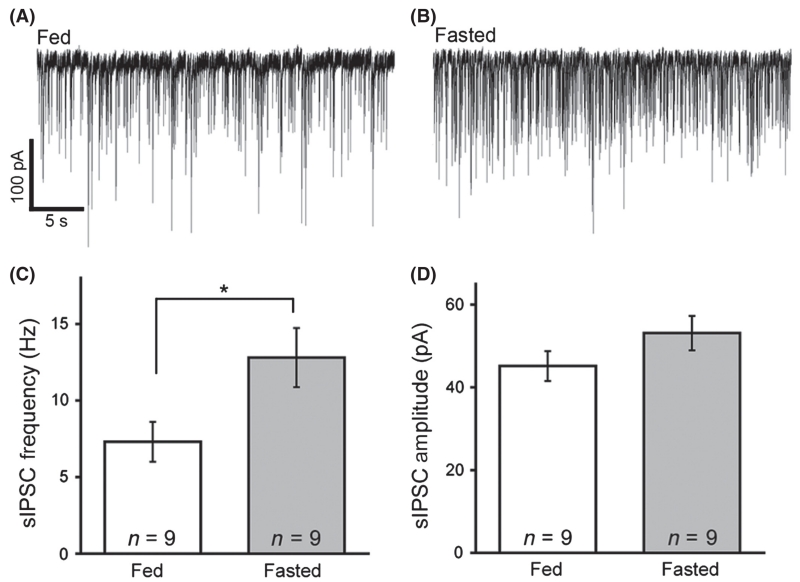
Next, the aim was to determine whether the increase in Gad1 expression after fasting might translate into increased synaptic GABA release from AgRP/NPY neurons. Thus, spontaneous IPSCs (sIPSCs) were recorded in hypothalamic POMC neurons, as POMC neurons are heavily innervated by AgRP/NPY cells (Cowley et al., 2001; Atasoy et al., 2012; Newton et al., 2013). IPSCs were recorded using a pipette solution containing a high concentration of chloride causing GABA-mediated currents to be inward. POMC neurons from slices prepared from fasted animals (17 h) had a higher frequency of GABA-mediated IPSCs compared with the frequency in control mice with ad libitum access to food (7.3 ± 1.3 Hz in fed, 12.8 ± 1.9 Hz in fasted, n = 9 cells from four fed and five fasted animals, P = 0.02 by unpaired t-test; Fig. 2A–C), consistent with a previous report (Vong et al., 2011). There was no significant difference in sIPSC amplitude between food-restricted and control mice (45.2 ± 3.6 pA in fed, 53.1 ± 4.2 pA in fasted, n = 9 cells from four fed and five fasted animals, P = 0.17 by unpaired t-test; Fig. 2D); however, the sample size may have precluded detecting an increase as other investigators have shown a significant increase in sIPSC amplitude in POMC neurons after fasting (Vong et al., 2011).
Fig. 2.
Spontaneous γ-aminobutyric acid (GABA) release onto proopiomelanocortin (POMC) cells is increased in fasted animals. Representative traces of spontaneous GABA-mediated inhibitory postsynaptic currents (IPSCs) in POMC neurons in tissue from fed (A) and fasted (B) mice. Compiled results are shown in the graph (C). Current amplitudes were not significantly different between feeding states (D). Data are plotted as mean ± SEM. *P < 0.05.
AgRP neuron activation is sufficient to acutely inhibit POMC neurons
Although POMC neurons are known to be postsynaptic to NPY/AgRP cells, it has been suggested that NPY/AgRP terminals do not account for a significant portion of the GABA inputs to POMC neurons (Tong et al., 2008). Therefore, the selectivity of functional coupling between AgRP and POMC neurons was examined. This was accomplished using specific activation of NPY/AgRP neurons with ChR2 expressed in NPY/AgRP cells. Strong expression of ChR2-mCherry in NPY/AgRP neurons was induced following injection of an AAV containing a Cre recombinase-dependent sequence for ChR2-mCherry into the arcuate nucleus of AgRP-Cre mice. To estimate the percentage of NPY/AgRP neurons expressing ChR2, NPY-hrGFP; AgRP-Cre double-transgenic mice were injected with the AAV and the tissue was processed for cell counting. Approximately 63% of the NPY-hrGFP cells expressed visible levels of ChR2-mCherry, whereas ChR2-mCherry was not expressed in NPY-hrGFP-negative cells. Thus, the AgRP-Cre line provides reliable expression of ChR2-mCherry in AgRP neurons.
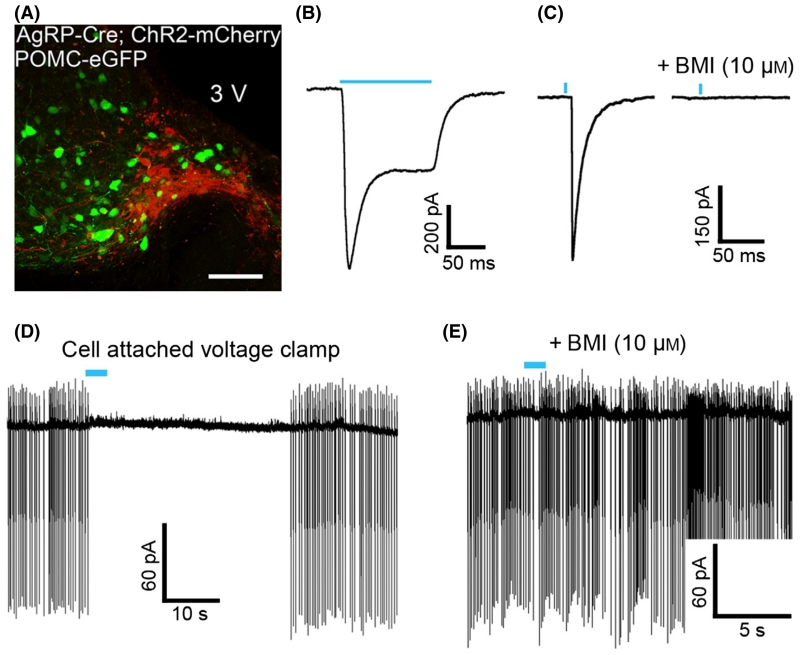
Next, AgRP-Cre mice were crossed to POMC-eGFP transgenic mice and double-transgenic offspring received injections of the virus containing floxed ChR2-mCherry. The mCherry tag on the ChR2 was used to detect NPY/AgRP cells, while POMC neurons were identified by the presence of eGFP (Fig. 3A). Light-stimulation of ChR2-expressing neurons caused an inward current (Fig. 3B) and caused evoked IPSCs (latency to onset of 4.6 ± 0.2 ms) in POMC neurons (81 of 91 cells tested; Fig. 3C, left). The GABAA antagonist BMI completely blocked the evoked IPSC in all cells tested (18/18 cells; Fig. 3C, right). To determine whether the connection between AgRP and POMC neurons was specific or could also result from other groups of arcuate nucleus neurons, recordings were made in non-POMC neurons (cells lacking eGFP). Only one of 16 non-POMC neurons displayed an IPSC in response to AgRP neuron stimulation. Thus, AgRP neurons preferentially innervate POMC neurons in the arcuate nucleus.
Fig. 3.
Agouti-related peptide (AgRP) cells expressing ChR2 reliably release γ-aminobutyric acid (GABA) onto proopiomelanocortin (POMC) cells when stimulated. (A) In double transgenic AgRP-Cre;POMC-eGFP mice injected with the ChR2 construct, the POMC cells are visualized using the GFP tag (green) and AgRP neurons expressing ChR2 are visualized using the mCherry tag (red). A brief flash of blue light (indicated by the blue lines) causes a depolarization of ChR2-expressing cells and a stereotypical photocurrent in the AgRP cells (B), and evokes inhibitory postsynaptic currents (IPSCs) in POMC cells (C, left) that are completely blocked by the GABAA antagonist bicuculline methiodide (BMI; C, right). In cell-attached voltage-clamp recording with external solution in the pipette, light-evoked GABA release inhibits POMC cell firing and action currents are lost (D). The addition of BMI to the bath prevents the light-evoked inhibition of cell firing (E). Scale bar: 100 μm (A).
To determine whether changing GABA release only from AgRP neurons is sufficient to alter the activity of POMC neurons, AgRP neurons were stimulated while measuring the firing of POMC neurons. Cell-attached recordings were made with external solution in the recording pipette such that the internal Cl− concentration was maintained at physiological levels. Activation of AgRP neurons decreased spontaneous action potentials and action currents in POMC neurons (0.006 ± 0.006 and 0.031 ± 0.031 of baseline firing, n = 3 cells from two animals, P = 0.01 and 0.04, respectively; Fig. 3D). Application of BMI blocked the inhibition (Fig. 3E). The results indicate that GABA release from AgRP neurons causes a profound inhibition of POMC neurons consistent with a previous report (Atasoy et al., 2012).
Fasting increases GABA release from NPY/AgRP to POMC neurons
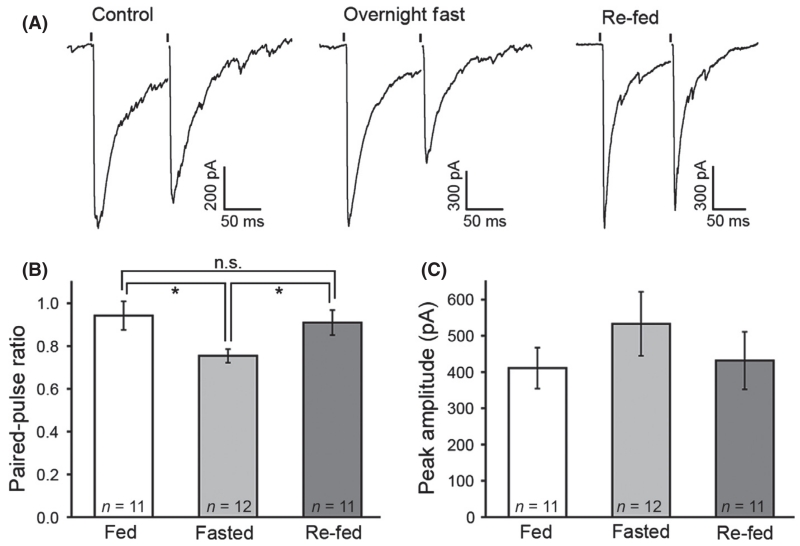
To determine whether fasting increased the strength of the functional coupling specifically from NPY/AgRP to POMC neurons, optogenetic activation of NPY/AgRP neurons was examined in slices from fasted animals. The probability of GABA release determined by a change in the PPR was examined in slices from fed and fasted animals. Consistent with a higher probability of release, the PPR decreased in slices from fasted animals (0.94 ± 0.07 in fed, 0.75 ± 0.03 in fasted, 0.91 ± 0.06 in re-fed, n = 11 fed, 12 fasted, 11 re-fed cells from six, five and six animals, respectively, one-way anova, significant with Tukey’s HSD; Fig. 4A and B). Although slightly higher after fasting, the overall amplitude of the first evoked current was not significantly different between groups (410.8 ± 56.5 pA in fed, 533.0 ± 88.4 pA in fasted, 431.7 ± 79.2 pA in re-fed, n = 11 fed, 12 fasted, 11 re-fed cells from six, five and six animals, respectively, one-way anova, not significant with Tukey’s HSD; Fig. 4C). There was a weak negative correlation between PPR and initial IPSC amplitude (r = −0.36, r2 = 0.15, P = 0.02).
Fig. 4.
Fasting increases the probability of release from neuropeptide Y (NPY)/agouti-related peptide (AgRP) cell terminals presynaptic to proopiomelanocortin (POMC) neurons. Representative traces (average of three consecutive sweeps) of γ-aminobutyric acid (GABA)-mediated inhibitory postsynaptic currents (IPCSs) light-evoked from NPY/AgRP neuron terminals in ad libitum fed (control, left), overnight fasted (middle) and re-fed (right) conditions are shown in (A). The paired-pulse ratio (PPR) in mice allowed a 3-h re-feeding period after fasting is comparable to controls (B). There was no significant difference in peak GABA current amplitude between groups (C). Data are plotted as mean ± SEM. *P < 0.05.
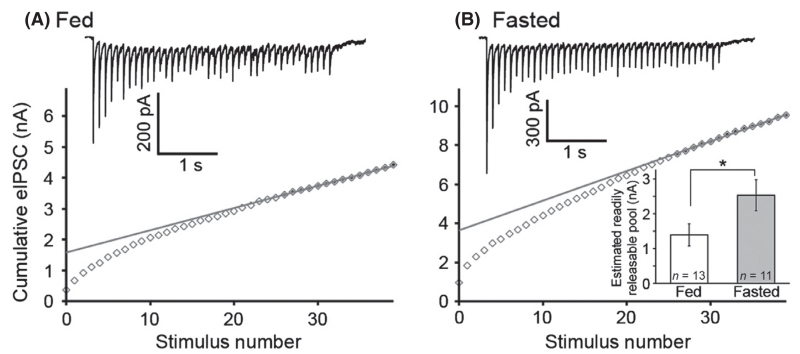
Next, the RRP of GABA was examined in slices from fed and fasted animals using a depletion protocol (Schneggenburger et al., 1999). Repetitive pulses of light (40 at 10 Hz) were applied to evoke GABA release while recording from POMC neurons (Fig. 5A and B, raw traces). The IPSC amplitudes were plotted, and a line was extrapolated from the linear portion of the curve and the y-intercept was used to determine the RRP (Fig. 5A and B, plotted data). A significantly higher RRP was observed in slices from fasted animals (1.4 ± 0.32 nA in fed, 2.5 ± 0.45 nA in fasted, n = 13 fed, 11 fasted cells from six fed and five fasted animals, P = 0.04 by unpaired t-test; Fig. 5B, inset). Additionally, when the initial current amplitudes were normalized to the last evoked current in the train, it became apparent that the peak (1st) current was relatively larger after fasting (fed = 7.7 ± 0.98; fasting = 12.4 ± 1.71, P = 0.02 by unpaired t-test). Importantly, the refilling rates (steady-state currents at the end of the train) are not significantly different between the groups (fed = 97.9 ± 28.4 pA, fasted = 81.5 ± 13.1, P = 0.63 by unpaired t-test). Thus, by multiple estimates, amplitudes, PPR and RRP size, it appears that fasting increased GABA release from AgRP neurons onto POMC neurons, although the possibility of postsynaptic contributions cannot be fully excluded.
Fig. 5.
Fasting increases the readily releasable pool (RRP) of γ-aminobutyric acid (GABA) in neuropeptide Y (NPY)/agouti-related peptide (AgRP) cell terminals. Trains of light stimuli (40 stimulations at 10 Hz) were applied to brain slices prepared from fed (A) and fasted (B) mice. Cumulative current plots were made, and the RRP was estimated by calculating the y-intercept from an extrapolated line drawn through the final 14 points of the plot. Estimated RRP is significantly increased in fasted animals (inset in B). Data are plotted as mean ± SEM. *P < 0.05.
GAD activity is necessary for maintaining GABA release from NPY/AgRP neurons
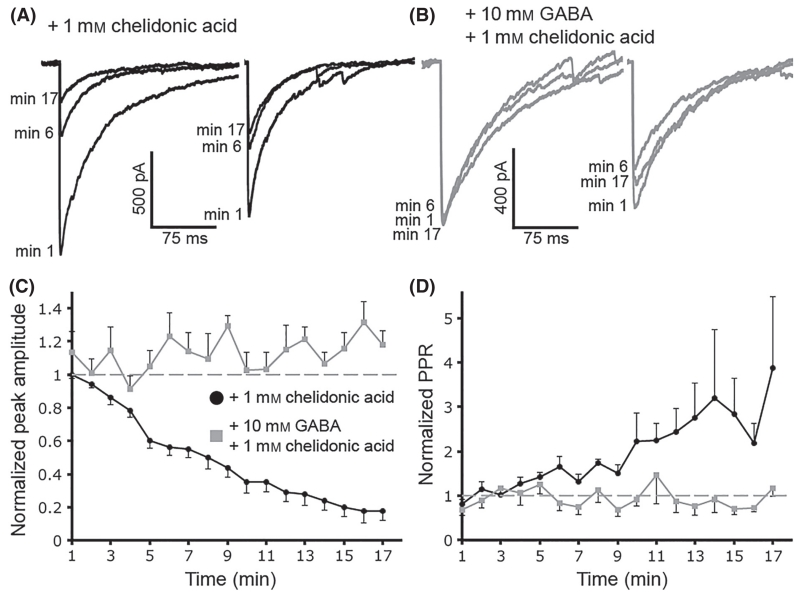
It was next asked whether the activity of GAD could be directly responsible for the increase in GABA release from AgRP/NPY neurons. This was examined in primary hypothalamic neuron cultures made from NPY-hrGFP transgenic mice and recordings were made in cells possessing recurrent synapse (autapses). When the conformationally restricted glutamate analogue chelidonic acid (1 mm), a competitive, potent and fast-acting GAD inhibitor (Porter & Martin, 1985), was included in the pipette solution there was a significant inhibition of evoked autaptic GABA currents (17.5 ± 5.5% of baseline after 17 min, n = 5, P < 0.0001 by repeated-measures anova, minutes 5–17 significantly lower than baseline using Dunnett’s multiple comparisons test; Fig. 6A and C). Thus, GAD activity is a pivotal determinant of GABA release in NPY/AgRP neurons consistent with previous reports at other GABAergic synapses (Apostolides & Trussell, 2013).
Fig. 6.
Inhibiting the glutamate decarboxylase (GAD) enzyme reduces autaptic γ-aminobutyric acid (GABA) release from neuropeptide Y (NPY)/agouti-related peptide (AgRP) cells in culture. Whole-cell recordings were made in NPY/AgRP cells that had formed autapses in culture. Addition of chelidonic acid (1 mm) in the pipette solution caused a significant inhibition of evoked GABA release over time (A). Adding GABA into the pipette along with the chelidonic acid prevented the decrease in eIPSC amplitude (B). Average amplitudes for each condition are plotted in (C) and average paired-pulse ratios (PPRs) are plotted in (D). Data are plotted as mean ± SEM.
To verify that chelidonic acid was not reducing GABA release independent of its actions on GAD activity, GABA (10 mm) was included in the pipette along with the chelidonic acid in some recordings. The inclusion of GABA prevented the chelidonic acid-induced inhibition of the autaptic currents (117.8 ± 8.4% of baseline after 17 min, n = 5, P = 0.38 by repeated-measures anova, no minute significantly different than baseline using Dunnett’s multiple comparisons test; Fig. 6B and C), indicating that chelidonic acid inhibited GABA release by directly reducing GABA levels in the cytoplasm. The chelidonic acid-induced reduction in the evoked IPSC correlated with increased PPR over time consistent with decreased GABA release, and this effect was absent when GABA was included in the pipette (n = 5, P = 0.02 by repeated-measures anova over time, minutes 14, 15 and 17 significantly different between groups using Sidak’s multiple comparisons test; Fig. 6D).
Decreased Gad1 expression correlates with reduced probability of GABA release
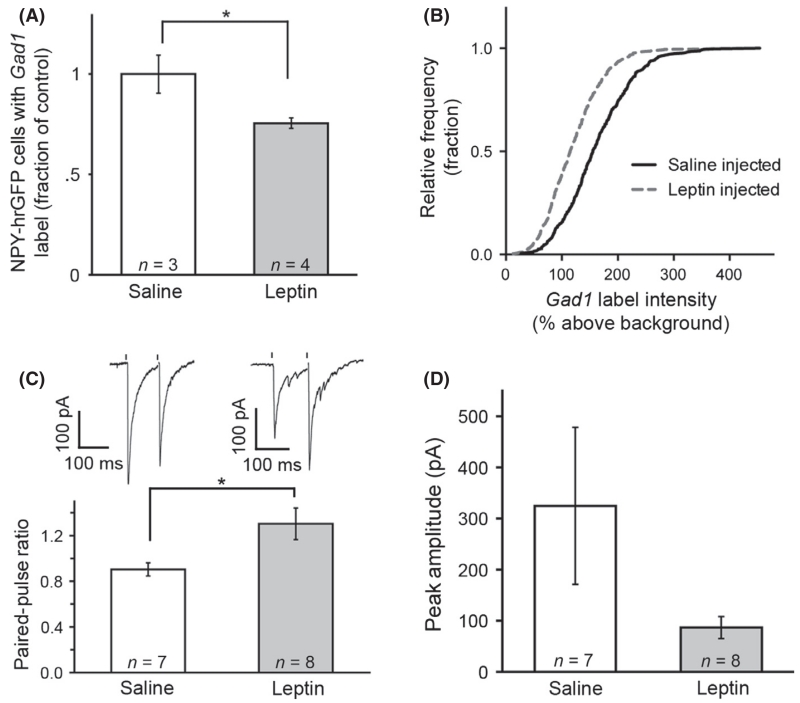
After observing the correlation between increased Gad1 expression and enhanced functional GABA release, the hypothesis that decreased Gad expression would reflect decreased GABA release from this synapse was tested. Leptin injections (i.p.) were used to approximate a satiated state. Compared with saline-injected controls, leptin caused a significant decrease in the number of NPY-hrGFP-immunolabelled cells that were also labelled with the probe for Gad1 mRNA (0.75 ± 0.03 for leptin normalized to control, n = 3, four mice, P = 0.035 by unpaired t-test; Fig. 7A). Correspondingly, the proportion of NPY/AgRP neurons with strong Gad1 signal was decreased in leptin-injected mice (Kolmogorov–Smirnov test, P = 4.0012 × 10−18, n = 390 saline, 565 leptin cells; Fig. 7B).
Fig. 7.
Leptin injection decreases Gad1 in neuropeptide Y (NPY)/agouti-related peptide (AgRP) cells and decreases probability of γ-aminobutyric acid (GABA) release. A single injection (i.p.) of leptin 2 h prior to tissue collection caused a significant decrease in the percentage of NPY cells expressing detectable levels of Gad1 (A), as well as a decrease in the proportion of cells with high levels of Gad1 label intensity (B). Whole-cell recordings in proopiome-lanocortin (POMC) neurons in slice preparations show that leptin injection caused a significant increase in paired-pulse ratio (PPR) when GABA release was light-evoked from NPY/AgRP terminals (C, averaged representative traces above respective bars). Peak amplitudes were not significantly different after leptin injection (D). Data are plotted as mean ± SEM. *P < 0.05.
Optogenetic activation of NPY/AgRP neurons was again used to observe changes in probability of release. Leptin treatment caused a significant increase in PPR (0.90 ± 0.06 for saline, 1.30 ± 0.14 for leptin, n = 7 saline, 8 leptin from three saline-injected and three leptin-injected animals, P = 0.025 by unpaired t-test; Fig. 7C), indicating a decrease in the probability of release. The peak amplitude of the first pulse in leptin-treated animals appeared to be less than in control tissue, but this was not statistically significant due to the high variance (324.63 ± 153.55 pA for saline, 86.71 ± 21.55 pA for leptin, n = 7 saline, 8 leptin from three saline-injected and three leptin-injected animals, P = 0.12 by unpaired t-test; Fig. 7D). Together with the fasting results, the leptin-induced reduction in Gad1 and corresponding decrease in the probability of GABA release indicates that Gad1 is a dynamic indicator of both increases and decreases in GABA release.
Discussion
The objective of the present study was to determine if a physiological perturbation could cause a change in GABA release that is reflected in the overall expression of a GABAergic marker in situ. Focusing on the NPY/AgRP population of neurons with a known GABAergic phenotype whose activity is sensitive to energy balance, fasting was found to increase the expression of Gad1 mRNA in these neurons. Further, the enhanced mRNA expression correlated with an increase in GABA transmission. It is likely that GABA release was increased at all terminals of NPY/AgRP neurons, and that other conditions would also change Gad expression and GABA release from these neurons as indicated by the decrease in Gad expression and GABA release observed after leptin treatment. The expression of Gad1 mRNA may prove to be a useful proxy for GABA release in other systems.
NPY/AgRP neurons and GABA release
The present study focused on GABA release from AgRP/NPY neurons. These neurons were chosen because of the availability of tools to examine and control them, the ability to identify and record from a postsynaptic target neuron, and because of the important role these neurons play in the regulation of energy balance (Parker & Bloom, 2012). Although the current work focused on NPY/AgRP/GABA to POMC neuron connections, it seems most likely that the fasting-induced increase and leptin-induced decrease in GABA release observed would be consistent for other terminals from these neurons throughout the brain. Previous studies have indicated that NPY/AgRP terminals in the paraventricular hypothalamus (Atasoy et al., 2012) or the parabrachial nucleus (Carter et al., 2013) account for the ability of NPY/AgRP neuron-derived GABA to increase food intake, depending on the conditions studied. While NPY/AgRP neuron-derived GABA can inhibit POMC neurons (Fig. 3; Atasoy et al., 2012), this inhibition is reportedly not essential for the acute increase in feeding that results from experimental activation of NPY/AgRP neurons (Aponte et al., 2011; Atasoy et al., 2012). However, the possibility exists that under different conditions and/or timescales, changes in GABA input to POMC neurons may be an important factor in tilting energy balance in one direction or the other. What is clear at present is that GABA release from NPY/AgRP neurons is able to induce food intake, and that both Gad1 mRNA and GABA release in these cells is dynamically regulated in an energy-state-dependent manner.
Correlating Gad67 mRNA and GABA tone
Numerous studies indicate that Gad mRNA, particularly Gad1 mRNA, is sensitive to a variety of factors, including steroids, stressors, glucose, insulin, seizure, lesions, cellular activity and GABA itself (Rimvall & Martin, 1992, 1994; McCarthy, 1995; Schwarzer & Sperk, 1995; Bowers et al., 1998; Mason et al., 2001; Pedersen et al., 2001; Patz et al., 2003). Separate studies have shown overall changes in regional GABA tone in response to many of these factors. However, the authors are aware of only one study showing a direct correlation between GABA release and Gad1 expression, and that study used a Gad1 reporter system in cultured hippocampal slices as well as an olfactory bulb preparation (Lau & Murthy, 2012). Additional studies have shown that GAD activity or expression and corresponding changes in cytosolic GABA levels dictate the strength of GABAergic transmission in interneurons of the dorsal cochlear nucleus (Apostolides & Trussell, 2013) and in the pre-frontal cortex (Lazarus et al., 2013). Together with the present results in the hypothalamus using the physiological stimulus of fasting and leptin injection, it appears that changes in Gad mRNA and corresponding changes in protein levels for GAD may closely reflect altered GABAergic transmission in many brain regions and cell types. Thus, at various synapses Gad may be a reasonable indicator of synaptic GABA levels.
It is important to note that, in addition to transcriptional regulation, the GAD enzymes are regulated by post-translational modifications, including coenzyme pyridoxal 5′-phosphate (PLP) binding and phosphorylation (Wei & Wu, 2008). While it cannot be ruled out that these additional levels of regulation may be important determinants of cytosolic GABA and GABA release, transcription appears to be a key point of regulation, particularly for Gad1. In many systems, Gad1 mRNA levels are more sensitive to perturbations than Gad2 (McCarthy, 1995; Bowers et al., 1998; Mason et al., 2001; Patz et al., 2003). This differential dependence on transcriptional regulation likely reflects the observation that the majority of the 65-kD form of GAD (transcribed from Gad2) is maintained in the inactive apoenzyme form and thus is dependent on post-translational activation by PLP, whereas GAD67 is maintained primarily in the active holoenzyme state (Wei & Wu, 2008) and is therefore more dependent on the level of expression rather than post-translational modification.
In addition to GAD activity, cytosolic GABA levels may also be affected via GABA uptake through the plasma membrane-bound GABA transporters. While the autaptic studies presented here suggest that GAD activity is a key determinant of GABA release from NPY/AgRP cells, a possible contribution from altered GABA uptake cannot be ruled out. The promotion of self-synapses necessitates that the neurons be grown under relatively sparse conditions, which may preclude the presence of substantial extracellular GABA concentrations. It is difficult to know if GABA uptake plays an important role in the intact system, although previous studies in intact circuits found that chemical inhibition of GAD was sufficient to reduce GABA release (Apostolides & Trussell, 2013) and the majority of GABA for release comes from glutamate decarboxylation (Mathews & Diamond, 2003), suggesting a limited role for GABA uptake in synaptic release. Consistent with the importance of GABA content as a primary driver of vesicular uptake and release are the present results showing an increase in PPR and decrease in evoked GABA currents as GAD activity is interrupted by chelidonic acid. While vesicle filling does not necessarily correlate with release probability (Zhou et al., 2000), there is evidence that vesicular content can affect probability of release (Herman et al., 2014) as suggested in the present study. Additionally, the correlated changes in release probability and RRP observed here are consistent with a previous report at the calyx of Held synapse in mice (Thanawala & Regehr, 2013), although the mechanism for coordinated increases in RRP and probability of release is unclear.
Broad potential for Gad mRNA as a proxy for GABAergic synaptic transmission
There are many conditions in which it may be desirable to detect changes in Gad mRNA as a proxy for plasticity in GABAergic transmission. For example, the use of single- or multi-label in situ hybridization as used here allows for entire populations of cells to be examined in relatively intact tissue, which may be particularly useful in heterogeneous tissues or cell types. Further, semi-quantitative mRNA analysis of Gad could allow for an approximation of GABA tone when direct detection of GABA release is not technically feasible, such as when transgenic tools do not exist or when the postsynaptic target cannot be identified for paired recordings. While further studies will be needed to determine how generalizable the use of Gad mRNA will be as an indicator of GABA release from other cell types, the present studies together with past work indicate the potential for broad utility.
Acknowledgements
This work was funded by NIH grant R01DK078749 and an award from the Monfort Family Foundation to S.T.H. The authors thank Ms Connie King for her excellent technical assistance, and Dr John T. Williams for critical review of this manuscript and helpful suggestions.
Abbreviations
- AAV
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- AgRP
agouti-related peptide
- BMI
bicuculline methiodide
- EGFP
enhanced green fluorescent protein
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase
- i.p.
intraperitoneal
- IPSC
inhibitory postsynaptic current
- NPY
neuropeptide Y
- PBS
phosphate-buffered saline
- PLP
pyridoxal 5′-phosphate
- POMC
proopiomelanocortin
- PPR
paired-pulse ratio
- RRP
readily releasable pool
- sIPSC
spontaneous inhibitory postsynaptic current
- vGAT
vesicular GABA transporter
References
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Rapid, activity-independent turn-over of vesicular transmitter content at a mixed glycine/GABA synapse. J. Neurosci. 2013;33:4768–4781. doi: 10.1523/JNEUROSCI.5555-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J. Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J. Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J. Neurosci. 2004;24:1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J. Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Ackermann F, Trimbuch T, Rosenmund C. Vesicular glutamate transporter expression level affects synaptic vesicle release probability at hippocampal synapses in culture. J. Neurosci. 2014;34:11781–11791. doi: 10.1523/JNEUROSCI.1444-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Yamaguchi J, Nakahata Y, Nabekura J. Dynamic regulation of glycine-GABA co-transmission at spinal inhibitory synapses by neuronal glutamate transporter. J. Physiol. 2013;591:3821–3832. doi: 10.1113/jphysiol.2012.250647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J. Comp. Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J. Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Krishnan K, Huang ZJ. GAD67 Deficiency in Parvalbumin Interneurons Produces Deficits in Inhibitory Transmission and Network Disinhibition in Mouse Prefrontal Cortex. Cereb. Cortex. 2013;25:1290–1296. doi: 10.1093/cercor/bht322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J. Neurosci. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Frank A. Beach Award. Functional significance of steroid modulation of GABAergic neurotransmission: analysis at the behavioral, cellular, and molecular levels. Horm. Behav. 1995;29:131–140. doi: 10.1006/hbeh.1995.1010. [DOI] [PubMed] [Google Scholar]
- Newton AJ, Hess S, Paeger L, Vogt MC, Fleming Lascano J, Nillni EA, Bruning JC, Kloppenburg P, Xu AW. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology. 2013;154:172–183. doi: 10.1210/en.2012-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Hirono M, Kume N, Kawaguchi Y, Itohara S, Yanagawa Y. GABA and synaptic inhibition of mouse cerebellum lacking glutamate decarboxylase 67. Biochem. Bioph. Res. Co. 2008;370:429–433. doi: 10.1016/j.bbrc.2008.03.110. [DOI] [PubMed] [Google Scholar]
- Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Patz S, Wirth MJ, Gorba T, Klostermann O, Wahle P. Neuronal activity and neurotrophic factors regulate GAD-65/67 mRNA and protein expression in organotypic cultures of rat visual cortex. Eur. J. Neuorsci. 2003;18:1–12. doi: 10.1046/j.1460-9568.2003.02702.x. [DOI] [PubMed] [Google Scholar]
- Pedersen AA, Videbaek N, Skak K, Petersen HV, Michelsen BK. Characterization of the rat GAD67 gene promoter reveals elements important for basal transcription and glucose responsiveness. DNA Sequence. 2001;11:485–499. doi: 10.3109/10425170109041332. [DOI] [PubMed] [Google Scholar]
- Pennock RL, Hentges ST. Differential expression and sensitivity of presynaptic and postsynaptic opioid receptors regulating hypothalamic proopiomelanocortin neurons. J. Neurosci. 2011;31:281–288. doi: 10.1523/JNEUROSCI.4654-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter TG, Martin DL. Chelidonic acid and other conformationally restricted substrate analogues as inhibitors of rat brain glutamate decarboxylase. Biochem. Pharmacol. 1985;34:4145–4150. doi: 10.1016/0006-2952(85)90207-2. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. Increased intracellular gamma-aminobutyric acid selectively lowers the level of the larger of two glutamate decarboxylase proteins in cultured GABAergic neurons from rat cerebral cortex. J. Neurochem. 1992;58:158–166. doi: 10.1111/j.1471-4159.1992.tb09291.x. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J. Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Sipols AJ, Grubin CE, Baskin DG. Differential effect of fasting on hypothalamic expression of genes encoding neuropeptide Y, galanin, and glutamic acid decarboxylase. Brain Res. Bull. 1993;31:361–367. doi: 10.1016/0361-9230(93)90228-4. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–709. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Thanawala MS, Regehr WG. Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool. J. Neurosci. 2013;33:4625–4633. doi: 10.1523/JNEUROSCI.4031-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tu P, Bonet L, Aubrey KR, Supplisson S. Cytosolic transmitter concentration regulates vesicle cycling at hippocampal GABAergic terminals. Neuron. 2013;80:143–158. doi: 10.1016/j.neuron.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Wei J, Wu JY. Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem. Res. 2008;33:1459–1465. doi: 10.1007/s11064-008-9600-5. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J. Comp. Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl. Acad. Sci. USA. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MH, Nishikawa K, Kubo K, Yanagawa Y, Saito S. Impaired glycinergic synaptic transmission and enhanced inflammatory pain in mice with reduced expression of vesicular GABA transporter (VGAT) Mol. Pharmacol. 2012;81:610–619. doi: 10.1124/mol.111.076083. [DOI] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Petersen CC, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J. Physiol. 2000;525(Pt 1):195–206. doi: 10.1111/j.1469-7793.2000.t01-1-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]