Abstract
The immune system has been recognized as one of the most important regulators of bone turnover and its deregulation is implicated in several bone diseases such as postmenopausal osteoporosis and inflammatory bone loss; recently it has been suggested that the gut microbiota may influence bone turnover by modulation of the immune system. The study of the relationship between the immune system and bone metabolism is generally indicated under the term ‘osteoimmunology'. The vast majority of these studies have been performed in animal models; however, several data have been confirmed in humans as well: this review summarizes recent data on the relationship between the immune system and bone with particular regard to the data confirmed in humans.
Introduction
Bone is an active tissue that undergoes continuous remodeling. The increase in bone resorption with unbalanced bone formation leads to bone diseases characterized by bone loss. Bone turnover is due to the combined action of bone resorbing cells, osteoclasts (OCs), and bone forming cells, osteoblasts (OBs). The regulation of these two cell types is due to multiple systemic and local factors such as hormones, cytokines, and mechanical load. The immune system has been recognized as one of the important regulators of bone turnover and its deregulation is implicated in several bone diseases.
The interaction between the immune system and bone has been studied in depth in conditions such as inflammatory diseases and postmenopausal osteoporosis.1 Recently it was suggested that gut microbiota (GM) may influence bone loss through modulation of the immune system.2 Several data on the interaction between the immune system and bone have been generated in animal models, whereas human data are scarce. The aim of this paper is to review the current knowledge on the role of the immune system in the control of bone metabolism, pointing out data confirmed in humans with particular regard to inflammatory diseases, postmenopausal osteoporosis and the role of the GM.
Inflammatory diseases, immune system, and bone
Rheumatoid arthritis (RA) is the paradigmatic disease linking immune alteration and bone metabolism. RA is a chronic disease characterized by immune deregulation associated with articular bone erosions, regional and systemic bone loss. Bone loss in RA is multifactorial. However, at least during the early disease phases, it is due to the activation of T cells that produce pro-osteoclastogenic cytokines.
The imbalance of T helper (Th) cell subtypes in RA may be the first driver of increased osteoclastogenesis in these patients. In RA, different alterations of the Th subset have been described. In humans conflicting results have been obtained, depending on different study designs. Some studies have analyzed T cells in peripheral blood, whereas others have evaluated these cells in the synovial fluid.3 The increased Th1 cells may have an important role in the pathogenesis of RA. These cells are increased in the synovium.3 Th1 produces several cytokines that have been related to the control of bone turnover, such as IFNγ, TNFα, and RANKL: IFNγ has a controversial effect on OC formation and activity; it was found both to inhibit and to activate OCs.1 More recently it has been suggested that IFNγ is responsible for the ability of OCs to act as antigen presenting cells (APCs) and to modulate T-cell proliferation.4
TNFα and RANKL are well known pro-osteoclastogenic cytokines.5 TNF-α induces OC formation in the presence of adequate levels of RANKL,5 upregulates stromal cell production of RANKL and increases the responsiveness of OC precursors to RANKL. RANKL has an essential role in bone physiology by upregulating OC activity and formation. Its role is antagonized by its decoy receptor osteoprotegerin (OPG): the RANKL–OPG ratio is the main regulator of OC formation and activity. In RA patients, RANKL levels predict the therapeutic response to anti-TNF therapy,6 and denosumab, which is an anti-RANKL monoclonal antibody, blocks the effect of increased RANKL on bone loss in RA patients by reducing bone resorption.7
Th17 are increased in RA and have a crucial pathogenetic role in the appearance of bone erosions in mice.3,8 In humans Th17 are recruited within the synovium, where they exert pro-inflammatory and pro-osteoclastogenic effects.9 Th17 cells produce IL-17. This cytokine is increased in RA10 and has a crucial role in inflammation and in the development of the disease; however, its mechanism of action in the development of bone loss, especially in relation to other known key cytokines, such as IL-1, TNF-α, and RANKL, remains unclear. Recently, IL-17 has been suggested to be involved in the upregulation of OC formation in inflammation by increasing the release of RANKL, which may synergize with IL-1 and TNF-α.11
T regulatory cells (Tregs) are known to be crucial in maintaining the peripheral tolerance mechanism and in preventing autoimmunity. Hence, a decreased number of Treg or a reduced function of these cells are both possible factors involved in the chronic inflammation observed in RA joints. However, studies on Tregs phenotype and function in RA patients have yielded conflicting results: some studies found a decrease in Tregs in peripheral blood from RA patients;12,13 whereas others did not.14,15
In experimental models of arthritis, Tregs have been shown to inhibit OC formation and activity.16 These data have been confirmed by our group in humans as well, during periprosthetic osteolysis.17
Non-conventional T cells such as γδ T cells have been suggested to have a pathogenetic role in a murine model of RA through the production of IL-17.18 However, this role has not been confirmed in humans.19,20 In RA patients an increased number of γδ T cells have been found in the synovia. However, Th17- and not IL-17-positive γδ T cells seem to drive bone erosions in humans.19 A cross-talk between γδ T cells and OCs has also been investigated in vitro. In particular, it has been suggested that OCs are able to recruit and activate γδ T cells through the production of TNFα.21 In contrast, activated γδ T cells inhibit OC formation and activity through IFNγ production in co-cultures.22 The conflicting results in animal models, humans and in vitro suggest that a clear role for γδ T cells in the control of bone remodeling is not clearly established yet.
Taken together these data suggest that an increase in both Th1 and Th17 may be responsible for increased OC formation and bone erosions in RA, whereas a role for Tregs and γδ T cells is more controversial.
T cells interact with OCs not only through the production of cytokines but also through a cell-to-cell interaction. OCs behave like APCs; they share a common precursor with dendritic cells and macrophages. OCs express major histocompatibility complex molecules,23 and costimulatory molecules involved in the regulation of T cells answer to immune stimuli, such as CD80 and CD86. CD80 and CD86 are expressed on professional APCs as well as on OCs and bind CD28 expressed on activated T cells. T-cell activation is tightly regulated by molecules that interrupt CD80/86 and CD28 signal, such as CTLA-4, which is expressed on the activated T-cell surface. Hence, CTLA-4 modulates APC function. These molecules have an essential role in OC–T-cell interactions as it has been brilliantly demonstrated that CD80/86-deficient mice display increased OC differentiation as CD80/86-deficient OCs are not inhibited by CTLA-4 or Tregs.24 In humans, the use of abatacept that targets CD80/86 reduces OC formation, whereas the use of ipilimumab that blocks CTLA-4 increases OC formation. The interaction between T cells and OCs may be a fundamental mechanism of increased osteoclastogenesis in RA.
On the basis of a possible function of OCs as APCs, a role for autoantibodies in bone destruction in RA patients has been postulated. It has been demonstrated that OC function is influenced by antibodies, Fc receptors, and related molecules. In particular, in RA patients a direct effect of anti-citrullinated protein antibody in the control of osteoclastogenesis has been demonstrated; it is known that in more than 70% of patients with RA anti-citrullinated protein antibodys are detectable. Positivity for anti-citrullinated protein antibodys is a strong predictor of bone erosions in RA, and anti-citrullinated protein antibodys bind to OCs and stimulate OC-mediated bone resorption by increasing the production of TNF-α, IL-8, and RANKL.25,26,27
Clinically the activation of the immune system and increased inflammation in RA correlate with joint erosions, bone mineral density, and vertebral fractures.28
In RA the inflammatory milieu increases OC formation and activity both locally and systemically. Several pro-inflammatory cytokines increased in RA are responsible for increased OC formation and activity: IL-1 acts by increasing RANKL expression by bone marrow stromal cells and directly targets OC precursors, promoting OC differentiation in the presence of permissive levels of RANKL. The effect of TNF-α on osteoclastogenesis is upregulated by IL-1.6 In humans, it has been demonstrated that anti-TNF and anti-IL-1 monoclonal antibodies reduce bone resorption.29
IL-6 is increased in RA and even though it is not essential for bone resorption it may contribute to the increased bone resorption in RA.30
In RA and in other chronic inflammatory conditions, not only bone resorption but also bone formation is affected. The increase in TNF-α during inflammation induces an increase in dickkopf-1 (DKK-1), which is a negative regulator of the Wnt pathway. Wnts are a family of secreted lipid-modified proteins that bind to a receptor complex comprising frizzled and the low-density lipoprotein receptor-related proteins 5 or 6 (LRP5 or LRP6). Activation of this receptor leads to induction of bone formation by OBs. DKK-1 and sclerostin (SOST) bind to and inactivate signaling from LRP5/LRP6,31 inhibiting bone formation. In RA an increase in DKK-1 and SOST has been shown, which blunts OB formation and activity and, together with the increase in bone resorption, is responsible for reduced bone mass32 and for the formation of articular bone erosions. In fact, DKK-1 levels are associated with an increase in the number of articular erosions independently of age, baseline radiological features, C-reactive protein, or disease activity.33
Systemic bone loss has been correlated with inflammation as well with aging and other inflammatory diseases; the mechanisms are very similar to the ones demonstrated in RA.1
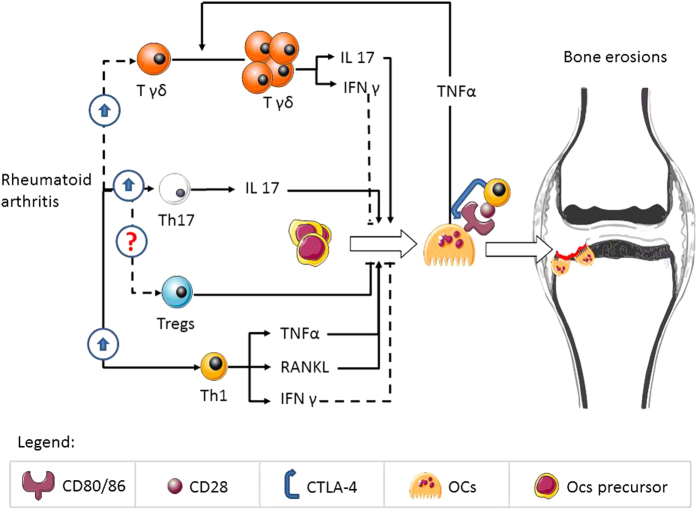
Figure 1 summarizes the role of T cells in the regulation of osteoclastogenesis in RA.
Figure 1.
The figure represents an interaction between T cells and osteoclastogenesis in rheumatoid arthritis; continuous lines represent pathways demonstrated in humans, whereas dotted lines represent pathways not clearly demonstrated in humans.
Estrogen loss, immune system, and bone
Postmenopausal osteoporosis is the most frequent metabolic skeletal disease. It is characterized by reduced bone mineral density and microarchitectural deterioration of bone with increased fracture risk. In postmenopausal osteoporosis, the uncoupling between OB-mediated bone formation and OC-mediated bone resorption results in bone loss. Estrogen deficiency is the main driver of postmenopausal bone loss: during estrogen depletion OC formation and activity are increased. This increase is partially mediated through the effect of estrogen deficiency on the immune system.
Data on animals and humans demonstrated that both cellular and humoral immune responses are enhanced by estrogens.1 During estrogen deficiency the immune response is altered and, in particular, T cells become more active and able to produce inflammatory and pro-osteoclastogenic cytokines such as TNFα and RANKL. Despite some inverse reports,34,35 the main body of literature firmly supports the essential role of activated T cells in regulating bone loss induced by estrogen deficiency,5,36,37,38 both in animal models and in humans.
My laboratory and others demonstrated that in postmenopausal osteoporosis osteoclastogenesis occurs only in the presence of T cells and that T cells of osteoporotic patients produce more RANKL and TNF-α compared with controls.5 RANKL directly correlates with increase in bone resorption markers and inversely with serum estrogen levels.37 Confirmation of the effect of estrogen on cytokine production is noteworthy as hormone-replacement therapy decreases the production of pro-osteoclastogenic cytokines in postmenopausal women.39
One of the key mechanisms through which estrogen deficiency induces proliferation and increases the life span of bone marrow T cells is the increase in antigen presentation by macrophages and dendritic cells,36 thanks to a greater expression of Class II TransActivator (CIITA), a transcriptional co-activator acting on the major histocompatibility complex class II promoter, with the final effect of upregulation of major histocompatibility complex class II on macrophages.23,36 These data have been generated in mouse models. However, in humans a role for low-grade inflammation and major histocompatibility complex class II expression in bone loss has been suggested.40 T-cell activation increases after OVX thanks to the upregulation of the CD40 ligand (CD40L) expression. The CD40/CD40L system is crucial for T-cell activation and for several functions of the immune system. It promotes macrophage activation and differentiation, antibody isotype switching, and the adequate organization of immunological memory in B cells. The increase in the number of activated CD40L-expressing T cells after OVX promotes the expression of M-CSF and RANKL by stromal cells and downregulates the production of OPG. This results in a significant increase in osteoclastogenesis.38 This mechanism has been demonstrated in mice but has not been confirmed in humans. Nevertheless, some papers confirm the role of estrogen in the regulation of immune function in humans in healthy or different disease states.41,42 In humans the effect of estrogens on the immune function has been demonstrated in the ability to modulate T-cell cytokine production,42,43 to answer to immune stimulation,44 whereas OVX increases T-cell activation.5,43
Estrogen deficiency increases the number of T cells by increasing their thymic output. It has been demonstrated in humans as well as in mice that after ovariectomy (OVX) the size of the thymus increases, and that T-cell activation is increased after OVX.43
In mouse models, Th17 cells have been implicated in OVX-induced bone loss. These cells increased after OVX because of the upregulation of STAT3, ROR-ct and ROR-a and downregulation of Foxp3, which antagonizes Th17-cell differentiation.9 In humans, a recent paper found no effect of estradiol in the secretion of IL-17 by T cells in patients affected by multiple sclerosis,42 whereas another suggests that estradiol inhibited Th17 differentiation through downregulation of Rorγt mRNA and protein expression.45 Nevertheless, in these studies, the effect of estradiol was evaluated in vitro on cells from patients not in menopause and in men. Hence, the results cannot be extended to women in menopause. We recently demonstrated both in mice and in humans a fundamental role for Th17 in bone loss induced by primary hyperparathyroidism: we showed that IL-17 is upregulated by primary hyperparathyroidism in humans and by continuous PTH treatment in mice and that parathyroidectomy normalizes IL-17 production.46
Also B cells have recently been directly implicated in the regulation of bone resorption. These cells are able to produce OPG47 and, under certain conditions, could produce RANKL. B-cell knockout mice are osteoporotic with enhanced osteoclastic bone resorption due to decreased OPG level.47 B-cell OPG production is upregulated by the activation of CD40;38 thus, T-cell signaling to B cells, through CD40L and CD40, has an important role in regulating basal OC formation and in regulating bone homeostasis. During estrogen deficiency the upregulation of CD40 enhances the cross-talk between T and B cells.
Activated B cells express RANKL, contributing to bone resorption.48 The number of RANKL-expressing B lymphocytes in the bone marrow increases after OVX.49 Recently, Onal et al.50 demonstrated that RANKL produced by B cells and not by T cells have a role in OVX-induced bone loss in mice. This paper disagrees with previous reports showing a fundamental role of RANKL produced by T cells in OVX-induced bone loss.1,5,17,36,47 In humans there are no convincing data on the role of B cells in postmenopausal osteoporosis. Nevertheless, changes in several B lymphocyte populations in patients affected by postmenopausal osteoporosis regardless of their estrogen status have been shown.51 The role of B cells in the control of bone turnover has been studied in other diseases that affect bone, such as RA48,52 and periodontal inflammation.53 These studies suggest that B cells may be involved in the control of bone turnover in humans as well, mainly by the production of cytokines such as OPG and RANKL.
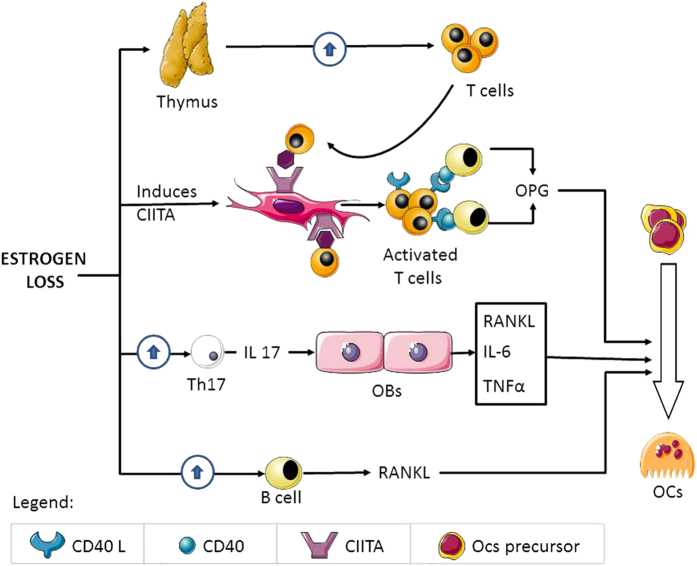
Figure 2 summarizes the role of the immune system in the regulation of osteoclastogenesis in postmenopausal osteoporosis.
Figure 2.
The figure represents the interaction between estrogen deficiency, the immune system and bone cells.
Gut microbiota, immune system and bone
The GM is the whole of the commensal bacteria living in our intestine. These are acquired at birth and can be considered a multicellular organ that communicates with and affects its host in numerous ways. The GM composition tends to stabilize after the first 3 years of birth. In this period, GM offers several antigens for the host's immune system, and during this first 3 years the neonatal immune system reaches maturity under the influence of the GM and its relation with environmental factors as diet, infections, antibiotics, and breastfeeding. The GM composition is extremely variable between individuals. Its symbiotic relationship with the human host is useful in food digestion and in fighting pathogens.
Environmental factors such as diet, antibiotic treatments, and infections can change the GM composition. Recently it was suggested that the altered gut colonization patterns, associated with decreasing microbial diversity, have a central role in human health and disease and are being increasingly implicated in the physiological, immunological, and metabolic deregulation seen in several chronic noncommunicable human diseases (NCDs). Immune homeostasis disruption is the causal mechanism of noncommunicable human diseases, such as allergy, asthma, some autoimmune diseases, cardiovascular disease, metabolic disease, and neurodegenerative disorders. These disorders are characterized by a low grade of inflammation. Although inflammation and the pathways to disease are multifactorial, GM disbiosis may have a central role in the pathogenesis of these diseases.54
Recent studies suggest that the GM may be involved in OVX-induced bone loss through the modulation of the immune system and, in particular, T-cell activation and pro-osteoclastogenic cytokine production. The most robust data have been produced in mouse models. In particular, it has been shown that mice grown up in a bacteria-free environment—that is, mice with a sterile bowel, called ‘germ-free' mice—demonstrate alteration of the immune system and are protected by OVX-induced bone loss. Germ-free mice have an immature gut mucosal immune system and a reduced number of T helper cells in the spleen, and in peripheral blood this suggests that the GM is responsible for the correct development of systemic immunity.54
Bone mass and density are increased in germ-free mice because of decreased bone resorption, without alteration in bone formation.2 In these animals OC formation is decreased thanks to a reduced number of T cells and pro-inflammatory and pro-osteoclastogenic cytokines such as IL-6 and TNFα. These observations suggested that the effect of GM on bone turnover is mediated through the modulation of the immune system by GM.2 Fecal transplantation in germ-free animals with GM from mice raised in a conventional environment leads to a normalization of bone mass, T helper cells and OC number.
Animal data also suggested that the use of some strain of probiotics can prevent OVX-induced bone loss55 and increase bone formation by the upregulation of genes involved in OB formation and activity, such as Sparc and BMP-2.56 Also the effects of prebiotics have been evaluated in animal models showing a positive effect on GM composition and subsequently on bone mass.57
According to the data obtained in mice one may speculate that an unfavorable GM composition may increase inflammation and hence favors postmenopausal bone loss. However, data obtained in humans are scarce. There are no data linking a microbiome profile to the risk of postmenopausal bone loss, whereas data on the possible effect of modifying GM through the use of probiotics and prebiotics are scarce.
Another intriguing hypothesis on the effect of GM on bone is based on the direct ability of GM to influence calcium absorption. A recent post hoc analysis on the use of Lactobacillus reuteri showed an increased level of serum 25OH vitamin D in healthy subjects treated with this probiotic.58 As regards the use of prebiotics, a recent study showed that supplementation with galacto-oligosaccharide in adolescent girls increased the presence of bifidobacteria in the GM, as previously shown in rats, and improved calcium absorption.59 A previous study demonstrated that a mixture of short- and long-chain inulin-type fructans, given as prebiotics during adolescence as oral supplements for a 1-year period, increases bone mineralization and calcium absorption.60 A possible explanation of the effect of dietary or prebiotic fiber on bone metabolism is the effect of the fiber on calcium absorption; the microbiota ferments the fiber to short-chain fatty acids (SCFAs), reducing the gut pH, thus reducing the formation of calcium phosphates and increasing the calcium absorption favoring bone. In the studies on the effect of prebiotics calcium absorption was investigated, whereas immune phenotype and inflammation were not. The effect of SCFAs may be more complex that the simple effect on gut pH and, indirectly, on calcium absorption. In fact, some studies suggest that SCFAs increased calcium transport, whereas reducing gut pH with HCl did not; thus, SCFAs may be useful to improve the gut function and health. It has also been suggested that SCFAs influence calcium absorption through signaling pathway modulation. In fact, butyrate modulates calcium absorption by non-gut cells.61
These seminal studies in mice, and less clearly in humans, suggest that alteration of GM composition influences bone metabolism through different mechanisms. The most attractive hypothesis, as suggested by the findings from germ-free mice, is that the GM influences bone turnover and mass by modulating the host's immune system. However other mechanisms, such as influence on calcium absorption and on vitamin D synthesis, may also be involved.
Conclusions
The interactions between the immune system and bone are complex and have a significant role in both health and disease. Nevertheless, not all pathways discovered in animal models have been fully demonstrated in humans, and several challenging questions remain unsolved.
Footnotes
The authors declare no conflict of interest.
References
- Mori G, D'Amelio P, Faccio R, Brunetti G. The interplay between the bone and the immune system. Clin Dev Immunol 2013; 2013: 720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res 2012; 27: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Takayanagi H. Arthritogenic T cells in autoimmune arthritis. Int J Biochem Cell Biol 2015; 58: 92–96. [DOI] [PubMed] [Google Scholar]
- Li H, Lu Y, Qian J, Zheng Y, Zhang M, Bi E et al. Human osteoclasts are inducible immunosuppressive cells in response to T cell-derived IFN-γ and CD40 ligand in vitro. J Bone Miner Res 2014; 29: 2666–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008; 43: 92–100. [DOI] [PubMed] [Google Scholar]
- Sakthiswary R, Das S. The effects of TNFα antagonist therapy on bone metabolism in rheumatoid arthritis: a systematic review.. Curr Drug Targets 2013; 14: 1552–1557. [DOI] [PubMed] [Google Scholar]
- Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 2010; 69: 872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA et al. Treatment with a neutralizing anti-murine interleukin-17 anti-body after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 2004; 50: 650–659. [DOI] [PubMed] [Google Scholar]
- Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE 2012; 7: e44552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 2015; 74: 101–107. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther 2005; 7: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere-Ortells JM, Pérez-Garcìa V, Marìn-Alberca G, Peris-Pertusa A, Benito JM, Marco FM et al. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28. Autoimmunity 2009; 42: 636–645. [DOI] [PubMed] [Google Scholar]
- Zare HR, Habibagahi M, Vahdati A, Habibagahi Z. Patients with active rheumatoid arthritis have lower frequency of nTregs in peripheral blood. Iran J Immunol 2015; 12: 166–175. [PubMed] [Google Scholar]
- Basdeo SA, Moran B, Cluxton D, Canavan M, McCormick J, Connolly M et al. Polyfunctional, pathogenic CD161+ Th17 lineage cells are resistant to regulatory T cell-mediated suppression in the context of autoimmunity. J Immunol 2015; 195: 528–540. [DOI] [PubMed] [Google Scholar]
- Walter GJ, Fleskens V, Frederiksen KS, Rajasekhar M, Menon B, Gerwien JG et al. Phenotypic, functional, and gene expression profiling of peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127(low) Treg cells in patients with chronic rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald ZS, Kiesel JR, DiPaolo R, Pagadala MS, Aurora R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLoS ONE 2012; 7: e38199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I, Caldo D, D'Amico L, D'Amelio P, Godio L, Patanè S et al. Osteoclastogenesis in peripheral blood mononuclear cell cultures of periprosthetic osteolysis patients and the phenotype of T cells localized in periprosthetic tissues. Biomaterials 2010; 31: 7519–7525. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol 2007; 179: 5576–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol 2011; 186: 2602–2612. [DOI] [PubMed] [Google Scholar]
- Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheumatol 2009; 60: 2294–2303. [DOI] [PubMed] [Google Scholar]
- Pappalardo A, Thompson K. Novel immunostimulatory effects of osteoclasts and macrophages on human γδ T cells. Bone 2015; 71: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo A, Thompson K. Activated γδ T cells inhibit osteoclast differentiation and resorptive activity in vitro. Clin Exp Immunol 2013; 174: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasciutti E, Mariani E, Oliva L, Scolari M, Perilli E, Barras E et al. MHC class II transactivator is an in vivo regulator of osteoclast differentiation and bone homeostasis co-opted from adaptive immunity. J Bone Miner Res 2014; 29: 290–303. [DOI] [PubMed] [Google Scholar]
- Bozec A, Zaiss MM, Kagwiria R, Voll R, Rauh M, Chen Z et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med 2014; 6: 235ra60. [DOI] [PubMed] [Google Scholar]
- Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 2012; 122: 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis 2016; 75: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensvold AH, Joshua V, Li W, Larkin M, Qureshi F, Israelsson L et al. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther 2015; 17: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugeberg G, Lodder MC, Lems WF, Uhlig T, Ørstavik RE, Dijkmans BA et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50-70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis 2004; 63: 1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res 2007; 22: 724–729. [DOI] [PubMed] [Google Scholar]
- Klimek E, Skalska A, Kwaśny-Krochin B, Surdacki A, Sulicka J, Korkosz M et al. Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short duration. Mediators Inflamm 2014; 2014: 681635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. Wnt signaling and osteoporosis. Maturitas 2014; 78: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Liu YY, Ye H, Guo JP, Li R, Liu X et al. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol 2011; 38: 821–827. [DOI] [PubMed] [Google Scholar]
- Garnero P, Tabassi NC, Voorzanger-Rousselot N. Circulating dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J Rheumatol 2008; 35: 2313–2315. [DOI] [PubMed] [Google Scholar]
- Anginot A, Dacquin R, Mazzorana M, Jurdic P. Lymphocytes and the Dap12 adaptor are key regulators of osteoclast activation associated with gonadal failure. PLoS ONE 2007; 2: e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kadono Y, Okada F, Jacquin C, Koczon-Jaremko B, Gronowicz G et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res 2006; 21: 1704–1712. [DOI] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA 2003; 100: 10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 2003; 111: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci USA 2011; 108: 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone 2001; 29: 30–34. [DOI] [PubMed] [Google Scholar]
- Swanberg M, McGuigan FE, Ivaska KK, Gerdhem P, Åkesson K. Polymorphisms in the inflammatory genes CIITA, CLEC16A and IFNG influence BMD, bone loss and fracture in elderly women. PLoS ONE 2012; 7: e47964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka HP, Sharma U, Gopinath S, Sharma V, Hima L, ThyagaRajan S. Menstrual cycle and reproductive aging alters immune reactivity, NGF expression, antioxidant enzyme activities, and intracellular signaling pathways in the peripheral blood mononuclear cells of healthy women. Brain Behav Immun 2013; 32: 131–143. [DOI] [PubMed] [Google Scholar]
- Javadian A, Salehi E, Bidad K, Sahraian MA, Izad M. Effect of estrogen on Th1, Th2 and Th17 cytokines production by proteolipid protein and PHA activated peripheral blood mononuclear cells isolated from multiple sclerosis patients. Arch Med Res 2014; 45: 177–182. [DOI] [PubMed] [Google Scholar]
- Adeel S, Singh K, Vydareny KH, Kumari M, Shah E, Weitzmann MN et al. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J Investig Med 2013; 61: 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C et al. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS ONE 2013; 8: e62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RY, Fan YM, Zhang Q, Liu S, Li Q, Ke GL et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter. J Immunol 2015; 194: 4019–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, D'Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T et al. IL-17 A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab 2015; 22: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li A, Yang X, Weitzmann MN. Ovariectomy-induced bone loss occurs independently of B cells. J Cell Biochem 2007; 100: 1370–1375. [DOI] [PubMed] [Google Scholar]
- Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis 2011; 70: 2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y. Prostaglandin E2 induces expression of receptor activator of nuclear factor-kappa B ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res 2000; 15: 1321–1329. [DOI] [PubMed] [Google Scholar]
- Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC et al. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem 2012; 287: 29851–29860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil V, Ticchioni M, Testa J, Roux CH, Ferrari P, Breittmayer JP et al. Immune changes in post-menopausal osteoporosis: the Immunos study. Osteoporos Int 2010; 21: 805–814. [DOI] [PubMed] [Google Scholar]
- Wheater G, Hogan VE, Teng YK, Tekstra J, Lafeber FP, Huizinga TW et al. Suppression of bone turnover by B-cell depletion in patients with rheumatoid arthritis. Osteoporos Int 2011; 22: 3067–3072. [DOI] [PubMed] [Google Scholar]
- Abe T, AlSarhan M, Benakanakere MR, Maekawa T, Kinane DF, Cancro MP et al. The B cell-stimulatory cytokines BLyS and APRIL are elevated in human periodontitis and are required for B cell-dependent bone loss in experimental murine periodontitis. J Immunol 2015; 195: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 2015; 179: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014; 9: e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015; 2015: 897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem 2011; 59: 6501–6510. [DOI] [PubMed] [Google Scholar]
- Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab 2013; 98: 2944–2951. [DOI] [PubMed] [Google Scholar]
- Whisner CM, Martin BR, Schoterman MH, Nakatsu CH, McCabe LD, McCabe GP et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr 2013; 110: 1292–1303. [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr 2005; 82: 471–476. [DOI] [PubMed] [Google Scholar]
- Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep 2015; 13: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]