Abstract
In this article we propose a new approach to pricing for patent-protected (on-patent) pharmaceuticals. We describe and define limit pricing as a method for drug companies to maximize revenue for their investment by offering budget-neutral pricing to encourage early adoption by payers. Under this approach, payers are incentivized to adopt innovative but expensive drugs more quickly if drug companies provide detailed analyses of the net impact of the new pharmaceutical upon total health budgets. For payers to adopt use of a new pharmaceutical, they would require objective third-party evaluation and pharmaceutical manufacturer accountability for projected outcomes efficacy of their treatments on population health. The pay for outcomes underpinning of this approach falls within the wider aspirations of health reform.
Keywords: drug industry, drug innovation, financing models, healthcare payers, high-cost drugs, paying for outcomes, pharmaceutical innovation, specialty pharmaceuticals, value-based purchasing
THERE IS AN inherent tension between granting patent protection for innovative drug treatments and patient access. Pharmaceutical companies are awarded exclusive market protections during the on-patent period, granting them the ability to set prices so as to generate maximum sales profit whereas patients in need of therapies may be excluded from life-enhancing treatments because of their expense. The regulatory conundrum is therefore to set sufficient reward for innovation while not impeding necessary use.
Time affects the purchasing and selling decisions in the on-patent period differently. Pharmaceutical companies face reduced rewards from patent protection over time. This can be the result of moving to off-patent and generic manufacture, the cost of pay for delay to keep generic rivals out of the market, or the introduction of rival therapeutics within the same disease space to provide price competition. As patent protection begins to expire, generic manufacturers gain access to the intellectual property in preparation for “off-patent” competition as stipulated under the Hatch-Waxman Act (Drug Price Competition and Patent Term Restoration Act, P.L 1984). The transition to off-patent is itself the subject of much discussion such as the evolving “pay for delay” environment (Choi et al., 2013), but all paths lead to reduced profitability. Price restraint resulting from the introduction of rival therapeutics and “me too” drugs is particularly important Congressional Budget Office (1998) in helping competition constrain prices. Taken together, it can be seen that speedier dissemination offers manufacturers greater reward. The opposing incentive faces payers, here defined broadly to include insurers and pharmacy benefit managers. New patent-protected drugs and treatments are expensive to adopt, but their price will fall over time for the reasons previously outlined. This means that lengthy evaluation processes or restrictive access policies will be recognized as a saving to payers, albeit at the expense of patients.
In our article we build upon the premise that, absent more detailed knowledge, purchasers yield benefit through delay and sellers gain benefit through rapid adoption. We propose a novel approach in which pharmaceutical companies and regulators combine to provide effectiveness analysis with warranted outcomes, thereby creating mutual benefit to speed adoption. We develop what we term a limit-pricing calculation on the basis of health outcomes. Limit pricing creates a range of optimal prices within which health budgets are kept cost neutral while permitting ongoing innovation and minimizing the need for overt regulatory intervention such as direct price control.
ESTABLISHING VALUE FOR PHARMACEUTICALS
We start by asserting our belief that, even in the pharmaceutical market, competition across sellers can maximize societal welfare and establish optimal prices, prices at which the right amount of a product is delivered relative to purchasers' preferences. When innovators are afforded patent protection to reward their unique contribution (and costs), we restrict competition and accept their right to set prices unilaterally. The implications of this can be understood by use of an example for which we use the experience of Sovaldi (Sofobuvir).
Sovaldi has a production cost at approximately $140 Hill et al. (2013) per treatment course but carried an asking price of $84 000, a large premium on the value of the innovation. Sovaldi was cited as having the potential to completely change the treatment process and outcomes of one common disease, hepatitis C. After being granted patent protection, the manufacturer set a price level similar to those commanded for orphan drugs. Orphan drugs are aimed at far smaller patient populations and hence carry far higher price tags to offset their development and production costs. In setting the price of Sovaldi, the manufacturer bridged the gap between what are termed “blockbuster drugs,” those with widespread demand and “niche-buster drugs,” those like orphan drugs with more limited demand and high price (Dolgin, 2010). The combination of high price plus large volume, in conjunction with the disproportionate number of poorer end users covered by Medicaid programs, resulted in significant budgetary pressure for states and an ongoing debate over the ethics of drug pricing. California allocated $200 million for 2015 to 2016 to fund access to the drug on behalf of Medicaid enrollees while restricting access for some of those that might benefit (Siders, 2015). Restrictions on use protect budgets that are not unlimited. The increased cost of a single drug for a specific disease will either lead to offsetting reductions elsewhere National Association of Medicaid Directors (2014) or a further expansion of health care's share of the economy. Partial or full restrictions on use are one response to high prices. Another option is for governments to have the power to set lower prices administratively.
There is a plethora of ways to establish administrative prices through regulation. However, each solution requires rewards for research investment (not just paying for tangible production costs) and the cross-subsidization of product failures from successes. Administratively determined prices also need to target a fixed return to be recouped from initial investment, which requires projection not only of price but of volume. For example, a high price for niche drugs may be warranted because the ratio of users to research costs means that a high unit price is necessary to retain investment in rarer more complex disease treatments. The history of drug adoption has routinely been one of use expansion away from the target population associated with initial approval to new and novel uses (ie, off-label use) complicating projections of volume. At the end of the spectrum of governmental price setting is for government to assume direct ownership of innovations by assuming the burden of research funding (Stein & Valery, 2015). In this way the government would set its own prices but have the onerous task of deciding where to innovate and how much to fund. Outside of government setting prices or use restrictions, other models to make drugs more affordable are being touted. One of these is subscription pricing.
Subscription pricing has been established in industries where a product or service is paid for in anticipation of future repeated delivery (such as a newspaper subscription), with the seller providing a use agreement under prearranged terms. This may cover a single service or product within a fixed period or varying bundles of products (such as with cable television subscription). Drug subscription models can be developed for individuals; individuals grouped together by disease; or even a total enrolled population. The subscribed population is identified and provided access for future use of the drug. The benefit of subscription pricing models to payers is that they can achieve discounts on “must have” drugs by agreeing to purchase large quantities or by deferring cost across a wider array of drugs within a portfolio. In these arrangements, care must be taken to avoid reducing potential competition by inadvertently limiting market access to rival drugs. As with any advance purchase, fixing prices at a point in time may also result in paying a higher premium over the duration of the subscription period. In contrast to existing models that require use of restrictions/delays, government action to determine prices or the dilution of acquisition cost across time or other drugs, we propose a far more modest partial solution for drugs that can be established as truly cost saving. We term this solution “limit pricing” as health care budget neutrality provides the pharmaceutical pricing limit. The “limit” is a difference between the acquisition price of the drug and the outcome benefit of the new medication translated into dollars. In this approach we divide drugs into those for which limit prices encourage rapid adoption and those that will require additional analysis of their pricing relative to coverage (ie, those that will be delayed).
LIMIT PRICING
A limit price provides a threshold that, if exceeded, represents a net increase in health spending. In some ways this resembles the approach taken by the UK's National Institute for Health and Care Excellence (NICE). In the NICE framework, a complex assessment of incremental cost-effectiveness ratios (ICERs) is used to determine whether a manufacturer's requested price offers sufficient value to warrant coverage. No formal decision is taken to purchase as a result of the ICER analysis rather it marks the beginning of price negotiation. The general NICE process is outlined in the recent review of Kadcyla (NICE, 2014).
Our limit pricing proposal is similar in that it establishes a threshold price at which the acquisition of a drug is viable without requiring additional funding, not the price that should be paid. As with the NICE framework, final prices are subject to negotiation. As described below, limit prices offer a rapid evaluation of whether a drug price request will add to the net budget.
The focus upon net budget draws a distinction between a limit price and typical ICER analysis. Whereas ICER analysis takes the viewpoint of an individual and the benefit they may receive from a particular drug, limit prices look at the impact of drug expenditure on health budgets. The reality of health budgets, such as those highlighted as facing duress in California as a result of Sovaldi, is that when exceeded, other services, both existing and proposed, are cut. Without knowing how the additional expenditure will be funded, cost-effectiveness analysis for an individual omits reductions absorbed by others.
Limit prices are established equal to the reduced utilization of related health care services. Reduced utilization can result from the elimination of redundant services (such as drugs, tests, or procedures no longer required) or by improving outcomes that result in avoided expenditures (such as reducing hospitalizations). Changes in utilization can be measured by comparing current expenditure patterns for the range of health services associated with the target population and the projected change associated with the adoption of the drug. It should be emphasized that payers can pursue a similar approach to value any pharmaceutical, both new and established, with the limit price quantifying when specific treatments reduce population health cost.
HOW LIMIT PRICING WORKS—DETERMINING VALUE FROM REDUCED UTILIZATION AND IMPROVED OUTCOMES
Model 1: Limit Pricing for a Pharmaceutical Incorporating Improved Outcomes
The target population for a novel therapeutic drug is identified by an applicant drug manufacturer. The population is thereafter assigned by a classification system, such as Hierarchical Condition Categories or Clinical Risk Groups (Hughes et al., 2004), capable of stratifying patient populations through the interaction of chronic disease and severity of illness. A reference data set is used from which to estimate treatment costs per patient within the classification group. Utilization for different categories of health expense (hospitalizations, drugs, physician services) is broken out within the patient categories. Thus, for each class of patient, a current pattern of health cost and utilization is created.
The applicant for reference pricing attests to which expenditures will be averted and provides the time horizon over which this will occur. Using the reference data, the reduction in patient utilization can be quantified so as to derive the net financial impact upon the health system of utilizing the drug. The net benefit is the basis for the limit price—the total cost averted by using the drug as a mixture of averted service utilization through better outcomes and direct displacement of alternative treatments. Payers can compare the limit price to the price being asked, and if the net system benefit is positive, the price is not inflationary to health budgets.
The quantification of the limit price would also contain recommendations as to the timing of benefits relative to the timing of costs. For example in the case of Sovaldi, the significant initial costs of acquiring the drug may be offset by averting future utilization. Changes in related health services that result from improved health outcomes, once quantified, improve estimates of value as it becomes possible to incorporate long-term treatment gains within the pricing model. Thus, claims made on behalf of Sovaldi that health care costs from transplant or avoidance of treatment costs associated with cirrhosis of the liver can be included as offsets to the initial asking price.
To avoid having the estimate of future savings being extrapolated from limited clinical trials, payers would continue to monitor the impact of the new pharmaceutical on their budget. In this process payers can establish milestones of outcome benefit against expense. When targets are not attained, payers would apply penalties such as additional rebates. In short, the process is one of establishing clear expectations of whether an acquisition price is a net drag on health budgets. Payers will mark for rapid adoption drugs that have a positive outcome. Conversely, payers will delay adoption of pharmaceuticals with prices set above the limit price or net outcome losses.
Nothing in establishing limit prices directly regulates the asking price for a drug or precludes alternative drugs entering the market and setting a lower price.
Model 2: Limit Pricing for Pharmaceuticals Within Provider-Directed Episodes
The approach outlined in Model 1 summarizes the types of arrangement that can be constructed between payers and pharmaceutical companies that a central agency trusted to provide limit pricing information directs or coordinates. Another class of arrangement can be envisioned whereby payers incentivize providers using an episode of illness approach to improve clinical outcomes, and in so doing are transformed into value-conscious purchasers of drugs.
All-inclusive episode pricing allows treatment to vary by provider and patient discretion without being linked to the use of a single drug or treatment approach. If payers pay providers the quality-adjusted payment amount for the average patient, providers assume the risk/reward for superior outcomes and are incentivized to select the most cost-effective treatments. However, the ability to select across alternatives is only possible if their comparative cost and value is known.
Take for example episodes of illness for cancer stages. An episode can either include both surgical and nonsurgical treatment options (eg, medication vs radiation therapy) or include separate treatment episodes for different types of treatment. In an episode payment, the limit price for the drug is included in the episode payment.
For other illnesses, paying for a years' treatment for an individual with, for example, rheumatoid arthritis may be most appropriate. Paying for an individual a capitated or total cost of care rate takes into account other illnesses the patient may have. Such a payment approach for rheumatoid arthritis can include a variety of alternative medications including cheaper generics and brand name and/or generic biologics that are typically much more expensive. Given the decentralized nature of such arrangements, a single payer may incentivize different providers using different episodes. However, the time horizon for attaining comparative drug value for these types of episode will likely be shorter. In our example of Sovaldi, an episode duration of 5 years might be necessary to capture the value of fewer transplants. In this situation, a payer might adjust upward the short-term episode payment made to providers to account for the higher limit price of a fair, yet longer term, episode. That said, pharmaceutical manufacturers desiring such an upward episode payment adjustment from payers would need to produce data on the favorable long-term impact of a pharmaceutical on outcomes in advance.
Benefit of Limit Prices
A cornerstone of determining limit prices is the explicit warranting of the manufacturer's claims of outcome quality gains. These assurances can be converted into performance milestones that, if not met, can be converted into price reductions or nonpayment of quality-related bonuses. Similar approaches have been undertaken as risk-sharing arrangements (managed entry agreements) to varying degrees across European countries. For example, one of many approaches used in Italy is to pay a reduced amount until a short-term treatment goal is met. Those that continue on treatment become the responsibility of the payer at the full price (Jommi, 2009). As currently formulated, such approaches incur significant administrative overhead costs coupled with significant variation in the terms of what is assessed as constituting success (Aggarwal, 2014). Defining success by changes in health costs can avert many of these issues and reduce administrative verification by relying on standard analysis of claims data.
Functionally, limit prices are intended to serve as tools to help facilitate market competition and rational choices. In most industries, quality gains are as likely to be reflected by gains in market share (sales volume) as price but, given extensive patent protections and the absence of detailed comparative cost and outcomes information, this is not as keenly felt in pharmaceutical pricing. Limit pricing permits improvement in outcome quality to be introduced to pricing decisions while remaining open to both new entrants and the sensitivity of global health budgets. The information provided by limit prices is intended to help both payers and manufacturers understand the impact of their pricing decisions and to adjust their actions accordingly. Simply stated, high prices for drugs that generate net savings of health dollars are far more likely to be covered rapidly than those that inflate budgets. Conversely, high drug prices that create budgetary issues will take longer to obtain coverage and reduce sales in the on-patent period where manufacturers might expect to generate their largest returns.
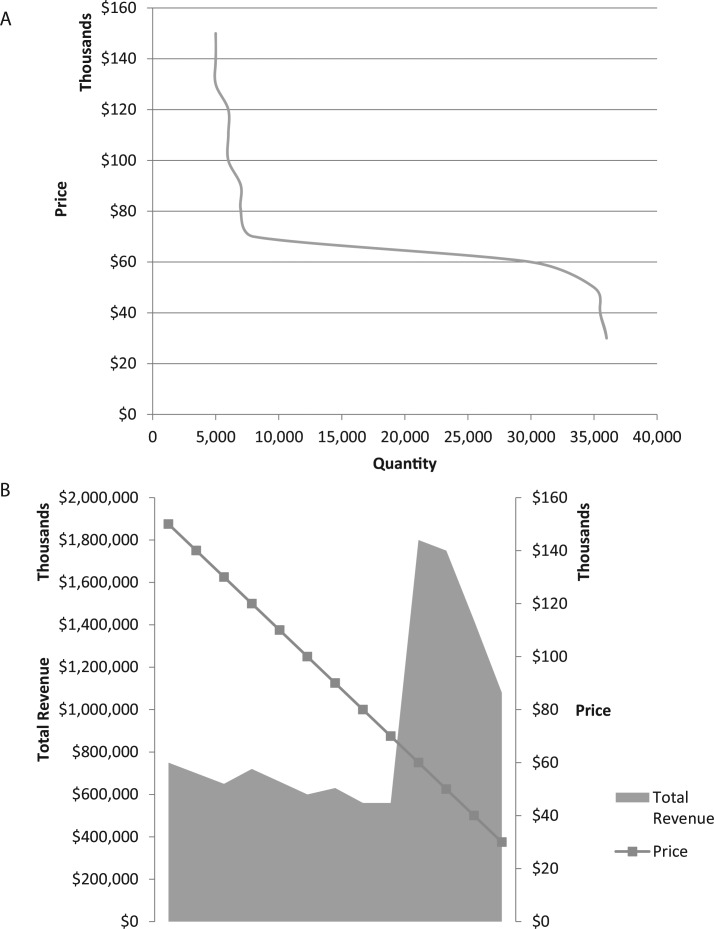
This can be shown more clearly in Figure 1 portraying a hypothetical example of limit pricing. In Figure 1a, we display the volume of drug sales during the on-patent window as a function of price. When price is high, there is significant delay in payer drug coverage and increased use restrictions. With each year of delay, potential sales are lost. With lower prices, there is a greater likelihood of coverage thereby increasing lifetime sales. The effect on total revenue is shown in Figure 1b. In Figure 1b, we show the effect of creating a limit pricing value of $60 000. The surge in volume at the limit pricing level reflects the expectation that the credible promise of net gains to total health system spending will stimulate coverage. Moreover, the rapid uptake and more reasonable pricing will also serve to deter “me too” pharmaceuticals within the patent protection window as returns are tied to sales volume. The range above the limit price reflects the current situation where there exists no coordinated response (ie, greater demand) to price. In this range there is little gain from setting prices marginally higher or lower as lifetime revenue will likely remain constant.
Figure 1.
(A) Pricing reduction stimulates increased quantity within the on-patent window. (B) Revenue response to price reduction.
To maintain credibility in limit prices, manufacturers will need to warrant outcomes. To accomplish this, pharmaceutical companies will want to actively work with providers to generate better outcomes. Pharmaceutical companies will want to work with providers who carefully track which groups of individuals benefit from a drug rather than extending use to off-label populations with negligible patient gains.
While appealing, this approach is not applicable to all situations, such as when financial gains from utilization reductions are insufficient to be reflected as positive pricing offsets despite unquantified gains such as reduced pain or extending life by 6 months being generated. These gains would remain the subject of the ongoing debate over ways in which to best measure value and cost and how such gains should be financed.
The net effect of limit pricing complete with an outcomes warranty is a clearer path for drug companies to establish prices and obtain profit through their drug sales coupled with greater engagement with the health sector that they serve to reduce health system cost and waste. These sentiments are in keeping with the wider goals of health system reform.
SUMMARY
In response to increasing budgetary duress resulting from skyrocketing prices, we have outlined a proposal to improve payment of on-patent pharmaceuticals. Addressing this challenge, we suggest an approach that includes in the calculation of the value of pharmaceuticals existing payment for outcomes such as preventable hospitalizations. In addition, the public will appropriately demand that a pay for outcomes approach also include outcomes that are not as easily translated into dollars such as decreased mortality/increased length of life. Payers can utilize limit pricing for either an individual pharmaceutical or as part of an episode of illness payment in an effort to support market forces to reward innovation while maintaining patient choice and preserving new entrant access to markets. Underlying this approach is a manufacturer guarantee and continuing engagement in improved clinical and financial outcomes and a focus upon drug innovation that improves outcomes that translate into dollar savings.
Footnotes
The authors are employed by 3M Health Information Systems in the design of clinical classification models.
REFERENCES
- Aggarwal R. (2014). Risk-sharing agreements: Country experiences and challenges written. Retrieved from http://centres.insead.edu/healthcare-management-initiative/thought-pieces/documents/RSA-ExperiencesandChallenges-April2014_000.pdf
- Choi W., Den Uyl B., Hughes M. (2013). Pay-for-delay practices in the pharmaceutical sector: Lundbeck, Actavis, and others. Journal of European Competition Law and Practice, 5(1), 44–52. 10.1093/jeclap/lpt071 [Google Scholar]
- Congressional Budget Office. (1998). How increased competition from generic drugs has affected prices and returns in the pharmaceutical industry. Retrieved from www.cbo.gov/sites/default/files/105th-congress-1997-1998/reports/pharm.pdf
- Drug Price Competition and Patent Term Restoration Act, P.L. (1984). Public Law 98–417.
- Dolgin E. (2010). Big pharma moves from “blockbusters” to “niche busters.” Nature Medicine, 16(8), 837. [DOI] [PubMed] [Google Scholar]
- Hill A., Khoo S., Simmons B., Ford N. (2013). Minimum costs to produce Hepatitis C Direct Acting Antivirals. In: 64th Annual Meeting of AASLD Washington, DC; Poster 1097. Retrieved from http://freepdfhosting.com/d4a7e2bba6.pdf [Google Scholar]
- Hughes J. S., Averill R. F., Eisenhandler J., Goldfield N. I., Muldoon J., Neff J. M. (2004). Clinical Risk Groups (CRGs): A classification system for risk-adjusted capitation-based payment and health care management. Medical Care, 42(1):81–90. 10.1097/01.mlr.0000102367.93252.70 [DOI] [PubMed] [Google Scholar]
- Jommi C. (2009). Conditional pricing/reimbursement in Italy. Retrieved from http://www.emaud.org/Doc/Jommi_1.pdf
- National Association of Medicaid Directors. (2014). Challenges posed to Medicaid agencies by new and emerging treatments. Retrieved from http://medicaiddirectors.org/sites/medicaiddirectors.org/files/public/namd_sovaldi_letter_to_congress_10-28-14.pdf
- NICE. (2014). Breast cancer her2 positive unresectable trastuzumab emtansine after trastuzumab taxane appeal decision 2. Retrieved January 1, 2015, from https://www.nice.org.uk/guidance/gid-tag350/documents/breast-cancer-her2-positive-unresectable-trastuzumab-emtansine-after-trastuzumab-taxane-evaluation-report2
- Siders D. (2015). Hepatitis C drug's high cost hits California budget. The Sacramento Bee. Retrieved from http://www.sacbee.com/news/politics-government/article7058828.html
- Stein P., Valery E. (2015). Competition: An antidote to the high price of prescription drugs. Health Affairs (Millwood), 23(4), 151–158. [DOI] [PubMed] [Google Scholar]