Abstract
Toxoplasmosis is a serious disease caused by Toxoplasma gondii, one of the most widespread parasites in the world. Lipid metabolism is important in the intracellular stage of T. gondii. Stearoyl-CoA desaturase (SCD), a key enzyme for the synthesis of unsaturated fatty acid is predicted to exist in T. gondii. Sterculic acid has been shown to specifically inhibit SCD activity. Here, we examined whether sterculic acid and its methyl ester analogues exhibit anti-T. gondii effects in vitro. T. gondii-infected Vero cells were disintegrated at 36 hr because of the propagation and egress of intracellular tachyzoites. All test compounds inhibited tachyzoite propagation and egress, reducing the number of ruptured Vero cells by the parasites. Sterculic acid and the methyl esters also inhibited replication of intracellular tachyzoites in HFF cells. Among the test compounds, sterculic acid showed the most potent activity against T. gondii, with an EC50 value of 36.2 μM, compared with EC50 values of 248-428 μM for the methyl esters. Our study demonstrated that sterculic acid and its analogues are effective in inhibition of T. gondii growth in vitro, suggesting that these compounds or analogues targeting SCD could be effective agents for the treatment of toxoplasmosis.
Keywords: Toxoplasma gondii, sterculic acid, anti-Toxoplasma gondii effect, stearoyl-CoA desaturase
INTRODUCTION
Toxoplasma gondii is a zoonotic pathogen and one of the most widespread parasites, and chronically infects approximately 30% of the global human population, as well as animal species [1]. T. gondii is the etiological agent of toxoplasmosis, causing severe neurological deficits in immunosuppressed individuals and causing hydrocephalus, chorioretinitis, and blindness in congenitally infected newborns [2]. The main effect of T. gondii infection in farm animals is abortion, which causes significant reproductive losses, and as a result, economic losses [3]. The most frequently used and successful chemotherapy of T. gondii infection is a combination of antifolates, such as pyrimethamine and sulfadiazine. However, other options are desirable because of the emergence of resistant strains and side effects caused by the available anti-parasitic agents [4].
Sterculic acid is naturally found in plants such as Sterculia foetida, which is used as an anti-parasite drug in traditional Chinese medicine. Sterculic acid specifically inhibits the conversion of stearic acid to oleic acid by Δ9-desaturation [5]. Methyl esters of sterculic acid and analogues were shown to have parasiticidal activities against the asexual blood stage of Plasmodium falciparum, probably by targeting stearoyl-CoA desaturase (SCD) [6]. We investigated the anti-parasitic activity of sterculic acid and methyl esters against T. gondii in vitro. Herein, we show that sterculic acid and its analogue methyl esters also inhibit the growth of T. gondii tachyzoites in vitro.
MATERIALS AND METHODS
Candidate compounds
Sterculic acid is a cyclopropene fatty acid. The analogues used in our experiments were methyl esters of cyclopropene fatty acids that had similar structures to sterculic acid (Fig. 1). INT15 is the corresponding methyl ester of sterculic acid while INT13 and INT14 has 1 less or 1 more carbon atom than INT15 in the chains, respectively. INT21 is composed of the backbone of INT15 and a methoxyl. The compounds were prepared as previously described [6-8]. DMSO was used to dissolve these compounds at 1 M as stock solutions.
Fig. 1.
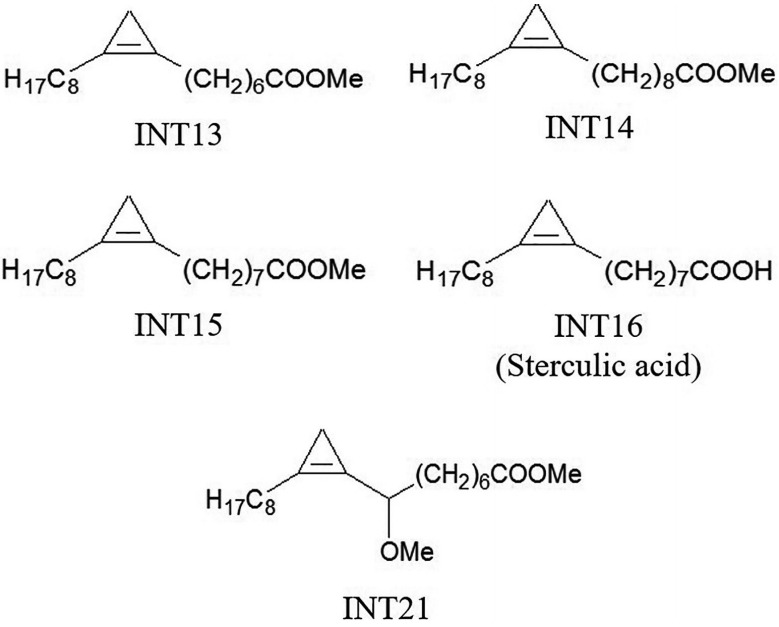
Molecular structures of test compounds. Sterculic acid was coded as INT16. The methyl esters INT13, INT14, and INT15 are different in the length of carbon chain. INT21 has the same length of carbon chain with sterculic acid but contains a methoxy moiety.
Host cells and T. gondii strain
Vero cells and human foreskin fibroblasts (HFF) were used to culture T. gondii. Vero cells replicate rapidly and undergo many cycles of division without becoming senescent, while the strong contact inhibition and slow growth of HFF make the cells of choice for monitoring parasite development under a microscope [9]. The cells were maintained in RPMI 1640 medium (Vero cells) or DMEM (HFF) supplemented with 10% FBS and penicillin (100 units/ml) and streptomycin (100 μg/ml), and cultured at 37˚C in a humidified 5% CO2 incubator. Tachyzoites of the RH strain of T. gondii were maintained in Vero cells. The medium was changed to RPMI 1640 with 1% FBS to avoid overgrowing after the cells were inoculated with T. gondii. Tachyzoites were harvested and passed through a 5 μm filter when most of the parasites were still intracellular, and then the parasites were counted in a hemocytometer.
Tachyzoite release assay
Tachyzoite release from host cells in the presence of test compounds was observed as previously described with minor modifications [4]. Vero cells were cultured in 12-well plates until confluency, and then infected with 5×104 tachyzoites/well. After incubation for 6 hr, extracellular tachyzoites were removed by washing with PBS. The cells were further cultured in DMEM with 2% FBS. To study the effect of the test compounds on intracellular T. gondii, 1 mM (final concentration) of each compound or 0.1% DMSO as a negative control was added to the culture. The culture was observed for host cell rupture and tachyzoites release under an inverted microscope (Olympus IX71, Tokyo, Japan) and images were captured (Olympus DP70 camera) at 0, 12, 24, and 36 hr after the addition of the test compounds.
Tachyzoite proliferation assay
The effect of the test compounds on tachyzoite proliferation was studied using HFF. Freshly released tachyzoites (1×104 tachyzoites/well) were inoculated in confluent HFF on 12-well plates for 30 min to allow cell invasion, followed by extensive washing and further incubation in DMEM with 2% FBS and 0.1 mM sterculic acid or 1 mM methyl esters for 18 hr. Subsequently, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100. The intracelullar parasites were incubated with anti-T. gondii rabbit sera (1:50) and then FITC-conjugated goat anti-rabbit IgG (1:50, Proteintech Group Inc., Chicago, Illinois, USA) for 1 hr at 37˚C for each antibody incubation step. Tachyzoites labelled with green fluorescence were observed under an inverted microscope (Olympus), and intracellular parasites at different proliferation stages (i.e. 1, 2, 4, or 8 tachyzoites) were counted from 100 parasitophorous vacuoles in 3 separate wells per experiment.
MTS assay
This experiment was performed as previously described with minor modifications [4]. Vero cells of exponential growth stage were digested and cultured in 96-well plates at 6×103 cells/well. The adherent cells were infected with T. gondii tachyzoites resuspended in RPMI 1640 containing 10% FBS (3×104 tachyzoites in 100 μl medium per well). The infected cells were washed 6 hr after inoculation, and RPMI 1640 medium without FBS was added. After 18 hr, the medium in each well was changed into RPMI 1640 supplemented with 2% FBS along with different concentrations of sterculic acid or the methyl esters (final concentrations 15.6-1,000 μM). After treatment for 24 hr, anti-T. gondii activity and host cell cytotoxicity of the test compounds were evaluated by the 3-(4,5-dimethylthiazolzyl) -5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl) -2H-tetrazoliuzolium, inner salt (MTS) assay using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega Biotech, Beijing, China) according to the manufacturer’s instruction. The OD value of each well was assayed at the wavelength of 450 nm on a microplate reader (Model 680, Bio-Rad, Richmond, California, USA). Cell viability was expressed as percentage of the control value. The 50% cytotoxicity concentration (CC50) was expressed as the concentration reducing Vero cell growth by 50%, and the 50% effective concentration (EC50) was the concentration inhibiting T. gondii-induced cytopathic effect by 50%. Anti-T. gondii activity was expressed as the selectivity index calculated from the CC50 and EC50 values (CC50/EC50).
Statistical analysis
All experiments and measurements were performed in triplicate. The number of extracellular tachyzoites was compared using the Student’s t-test. The proliferation results were statistically analyzed using the chi-square test (PASW Statistics 18). EC50 and CC50 were calculated in GraphPad Prism 5 using non-linear regression.
RESULTS
Inhibitory activity against tachyzoite egress from host cells
Normal growth and proliferation of intraceullar tachyzoites result in host cell rupture and thus release of tachyzoites from the host cell. Prevention of tachyzoite egress from Vero cells was used as a marker for antiparasitic activity of the test compounds. The high concentration of sterculic acid (1 mM) caused cell death within 12 hr (data not shown), while methyl ester analogues were not cytotoxic at 1 mM. Thus, concentrations of 0.1 mM sterculic acid and 1 mM methyl esters were tested for antiparasitic activity in the Vero cell assay. Vero cells were unaffected at 12 hr and were of the same appearance in the treated and control groups. In the control group, tachyzoites started to egress at 24 hr, and by 36 hr most of the tachyzoites were released from the host cells with only a small number of intact cells remaining. Most host cells of all the treated groups remained intact at 36 hr (Fig. 2A-E), with a smaller number of tachyzoites released (Fig. 2G). The lowest number of extracellular tachyzoites was seen in the INT21-treated group, and the intracellular tachyzoites seemed to cease developing at 36 hr.
Fig. 2.
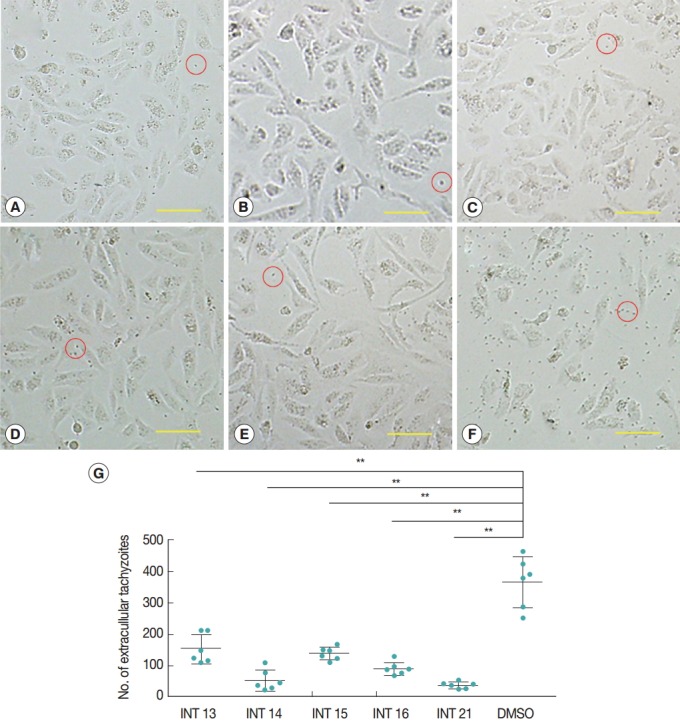
Sterculic acid and methyl esters inhibited the egress of tachyzoites from Vero cells. Representative pictures were presented to show that most of host cells remained intact after incubation with INT13 (A), INT14 (B), INT15 (C), INT16 (D), or INT21 (E) for 36 hr, with a small number of released tachyzoites, compared with numerous tachyzoites and a smaller number of intact host cells in the negative control group (F). Scale bar=100 μM. Extracellular tachyzoites are marked in red circles. (G) The number of released tachyzoites in the culture incubated with the test compounds was decreased relative to the DMSO control. Extracellular tachyzoites were counted in 6 random fields under the microscope per group. Data are presented as mean±SD. **P<0.01.
Inhibitory activity against tachyzoite proliferation
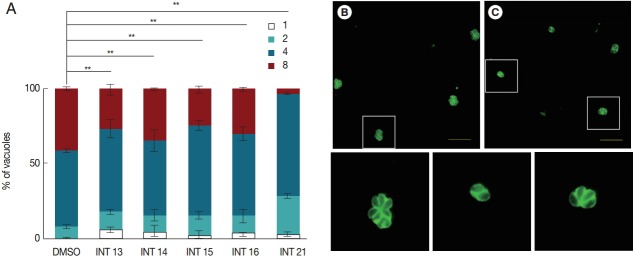
The decreased tachyzoite egress indicated that the test compounds inhibited the growth of T. gondii tachyzoites. We then examined the anti-proliferation activity of the test compounds using the indirect fluorescent antibody assay. The number of parasitophorous vacuoles containing 1-8 intracellular tachyzoites was counted after incubation in HFF for 18 hr. All test compounds inhibited tachyzoite proliferation. Significantly more parasitophorous vacuoles of all treated groups remained in the 1 or 2 tachyzoites per vacuole stage than the DMSO control (Fig. 3). Of the test compounds, INT21 was most effective, with over 90% parasitophorous vacuoles arresting at 2 or 4 tachyzoites stage and only 3% grown into 8 tachyzoites. In other treated groups, about 30% of parasitophorous vacuoles contained 8 tachyzoites. In comparison, approximately 50% parasites developed to 4 tachyzoites and 40% to 8 per vacuole in the DMSO culture.
Fig. 3.
Sterculic acid and all methyl ester analogues inhibit tachyzoite proliferation. Freshly released parasites were used to infect HFF cell monolayers. (A) Invaded parasites were allowed to replicate for 18 hr in the culture medium containing the test compounds, and the number of vacuoles containing 1, 2, 4, or 8 parasites was counted under a fluorescence microscope after anti-T. gondii staining. (B) Representative fluorescent microscopic images were presented to show that many vacuoles contained 8 tachyzoites in DMSO control groups, (C) while 4 or 2 tachyzoites were often observed in a vacuole in INT21-treated groups after culture for 18 hr. The magnified images of the areas marked with white boxes in (B) and (C) were presented at the bottom in order. Data are presented as mean±SD from 3 independent experiments each in triplicate. **P<0.01.
Anti-T. gondii activity by MTS assay
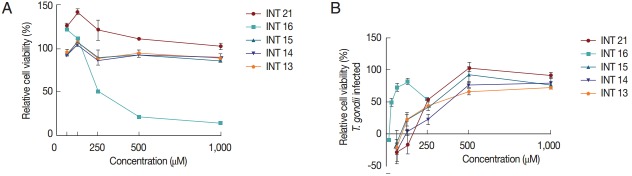
Anti-T. gondii activity was also tested using the MTS assay. Vero cells grew normally in culture medium with methyl esters (INT13, INT14, and INT15) no matter whether the test concentration was low or high. INT21 seemed to promote the cell growth, especially in the low concentration. In contrast, sterculic acid was cytotoxic to Vero cells (Fig. 4A), with a mean CC50 of 215 μM. The viability of Vero cells infected with T. gondii was increased with increasing compound concentrations, indicating anti-T. gondii activity of the test compounds (Fig. 4B). Sterculic acid had the lowest EC50 value (36.2 μM). The mean EC50 values for the methyl esters ranged from 248.0 μM (INT21) to 428.4 μM (INT14). All the test compounds have a selectivity index of >2 (Table 1). The antiparasitic activity in MTS assay was consistent with the release assay and proliferation assay results, with INT21 being the most effective moiety of the methyl esters.
Fig. 4.
Relative cell viability was calculated from the MTS assay. The cell viability of 0.1% DMSO was regarded as 100%. (A) Sterculic acid was cytotoxic to Vero cells at high concentrations while the methyl esters displayed no cytotoxicity. (B) All test compounds inhibited T. gondii growth as shown by increased viability of Vero cells infected with T. gondii.
Table 1.
In vitro anti-T. gondii activity of sterculic acid and its methyl ester analogues
| Compound code | ECa50 (μM) | CCb50 (μM) | SIc |
|---|---|---|---|
| Sterculic acid (INT16) | 36.2 ± 5.1 | 215.2 ± 15.8 | 5.9 |
| INT13 | 356.9 ± 14.1 | > 1,000 | > 2.8 |
| INT14 | 428.4 ± 15.7 | > 1,000 | > 2.3 |
| INT15 | 306.4 ± 18.8 | > 1,000 | > 3.3 |
| INT21 | 248.0 ± 17.9 | > 1,000 | > 4.0 |
Results are presented as mean±SD from 3 independent experiments with triplicate wells in each experiment.
EC50, half maximal effective concentration. Concentration required to inhibit T. gondii-induced cytopathic effect by 50%.
CC50, half maximal cytotoxic concentration. Concentration required to reduce Vero cell growth by 50%.
Selectivity index=CC50/EC50
DISCUSSION
Our study showed that sterculic acid and its analogues all exhibited anti-T. gondii activities in vitro in 3 different assays – egress, proliferation, and MTS. Among the compounds examined, sterculic acid exhibited the lowest EC50 value against T. gondii by the MTS assay while it was also most toxic to Vero cells, hindering us from testing its effect in the proliferation assay with a higher concentration. The effect of sterculic acid on Vero cells or HFFs has not been reported previously. Our results showed that sterculic acid is cytotoxic at a high concentration, which was similar to a report that the viability of differentiated adipocyte cultures was decreased at higher levels of sterculic acid (100 μM or 200 μM) [10]. In contrast, the methyl esters did not exhibited negative effects on Vero cells and even increased the cell viability in low concentrations. Increased cell proliferation under methyl sterculate treatment was also observed in early reports, and methyl ester of sterculic acid has been identified as one of the mitogenic principles [11,12]. INT21, the only methoxy analogue, was found to have little cytotoxicity and the most active in the inhibition of T. gondii growth among the methyl esters, which consisted with a previous report that the compound was the most active against P. falciparum among 7 methyl esters tested [6].
Sterculic acid directly inhibits stearoyl-coenzyme A desaturase (SCD) activity without affecting the processes required for adipocyte differentiation, scd gene expression or SCD protein translation in 3T3-L1 adipocytes [13]. Sterculic acid methyl ester also inhibits Δ9-desaturase of the bacteria Rhodococcus opacus, leading to an almost complete suppression of growth at 0.25 mM and even cell lysis at 0.75 mM [14]. Methyl esters of sterculic acid have also been shown to inhibit SCD of P. falciparum and exhibited antimalarial activities in vitro [6]. By sequence alignment with P. falciparum SCD in the ToxoDB database, a putative TgSCD (TGGT1_238950) was predicted to exist in T. gondii, retaining classic Δ9-desaturase amino acid sequences, such as 3 Histidine-Boxes and 8 conserved His residues. Neither the transcription nor the translation of T. gondii SCD was affected in the presence of the test compounds in our research, determined by qPCR and western blot (data not shown). We implied that sterculic acid and its analogues might only inhibit the SCD activity in T. gondii.
SCD is the endoplasmic reticulum-resident enzyme that catalyzes the introduction of the first double bond in the cis-Δ9 position of several saturated fatty acyl-CoAs, principally palmitic acid (C16:0) and stearic acid (C18:0), to render palmitoleic acid (C16:1) and oleic acid (C18:1). The presence of SCD has been described in a wide variety of organisms [15]. SCD1, an isoform of SCD in mammalian organisms, apart from its role in monounsaturated fatty acids synthesis, has been recently revealed a novel function in the modulation of metabolic and signaling processes related to cell proliferation, survival, and malignant transformation to cancer [16]. In apicomplexan parasites, SCD has not been deeply studied but only identified in P. falciparum. It converted C18:0 to C18:1 oleic acid and was important for the blood-stage survival [6]. However, C18:1 in T. gondii was presumably synthesized from the elongation of C16:1 palmitoleic acid by ELO-A (1 of 3 elongases in the fatty acid elongation system) [17]. Besides de novo synthesis of the fatty acids, T. gondii can also salvage lipids from the host cells and the environment [18,19]. It is possible that T. gondii desaturates salvaged C18:0 to C18:1 by SCD. Moreover, SCD can also catalyze the synthesis of C16:1 using C16:0 as the substrate [20], suggesting that the synthesis of C16:1 palmitoleic acid may be mediated by SCD in T. gondii. Therefore, TgSCD might be an essential enzyme in the mono-unsaturated fatty acid synthesis in this parasite. We inferred that the anti-T. gondii activity of sterculic acid and its analogues is most likely mediated by inhibiting TgSCD.
In conclusion, sterculic acid and its analogues inhibited T. gondii growth in vitro, most likely by targeting the synthesis of unsaturated fatty acid, which is essential for the development and proliferation of T. gondii. In vivo anti-T. gondii effects of sterculic acid analogues and other Δ9-desaturase inhibitors warrant further research. The function of TgSCD also requires further investigation.
Acknowledgments
This work was financed through the National Natural Science Foundation of China (No. 31372424).
Footnotes
We have no conflict of interest related to this work.
REFERENCES
- 1.Lüder CG, Bohne W, Soldati D. Toxoplasmosis: a persisting challenge. Trends Parasitol. 2001;17:460–463. doi: 10.1016/s1471-4922(01)02093-1. [DOI] [PubMed] [Google Scholar]
- 2.Martins-Duarte ES, Urbina JA, de Souza W, Vommaro RC. Antiproliferative activities of two novel quinuclidine inhibitors against Toxoplasma gondii tachyzoites in vitro. J Antimicrob Chemother. 2006;58:59–65. doi: 10.1093/jac/dkl180. [DOI] [PubMed] [Google Scholar]
- 3.Ferra B, Holec-Gasior L, Kur J. Serodiagnosis of Toxoplasma gondii infection in farm animals (horses, swine, and sheep) by enzyme-linked immunosorbent assay using chimeric antigens. Parasitol Int. 2015;64:288–294. doi: 10.1016/j.parint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Kavitha N, Noordin R, Chan KL, Sasidharan S. In vitro anti-Toxoplasma gondii activity of root extract/fractions of Eurycoma longifolia Jack. BMC Complement Altern Med. 2012;12:91. doi: 10.1186/1472-6882-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffcoat R, Pollard MR. Studies on the inhibition of the desaturases by cyclopropenoid fatty acids. Lipids. 1977;12:480–485. doi: 10.1007/BF02535446. [DOI] [PubMed] [Google Scholar]
- 6.Gratraud P, Huws E, Falkard B, Adjalley S, Fidock DA, Berry L, Jacobs WR, Jr, Baird MS, Vial H, Kremer L. Oleic acid biosynthesis in Plasmodium falciparum: characterization of the stearoyl-CoA desaturase and investigation as a potential therapeutic target. PLoS One. 2009;4: doi: 10.1371/journal.pone.0006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird MS, Dale CM, Lytollis W, Simpson MJ. A new approach to cyclopropene fatty acids. Tetrahedron Lett. 1992;33:1521–1522. [Google Scholar]
- 8.Baird MS, Grehan B. A new approach to cyclopropene fatty acids involving 1,2-deiodination. J Chem Soc Perkin Trans. 1993;1:1547–1548. [Google Scholar]
- 9.Weiss LM, Kim K. Toxoplasma gondii - the Model Apicomplexan: Perspectives and Methods. 2nd ed. Salt Lake City, USA: Academic Press; 2014. pp. 594–595. [Google Scholar]
- 10.Kadegowda AK, Burns TA, Pratt SL, Duckett SK. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids. 2013;48:967–976. doi: 10.1007/s11745-013-3823-1. [DOI] [PubMed] [Google Scholar]
- 11.Scarpelli DG. Mitogenic activity of sterculic acid, a cyclopropenoid fatty acid. Science. 1974;185:958–960. doi: 10.1126/science.185.4155.958. [DOI] [PubMed] [Google Scholar]
- 12.Leveille AS, Fischer-Dzoga K. The mitogenic effects of methyl sterculate on aortic smooth muscle cells. Artery. 1982;11:207–224. [PubMed] [Google Scholar]
- 13.Gomez FE, Bauman DE, Ntambi JM, Fox BG. Effects of sterculic acid on stearoyl-CoA desaturase in differentiating 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;300:316–326. doi: 10.1016/s0006-291x(02)02842-5. [DOI] [PubMed] [Google Scholar]
- 14.Waltermann M, Steinbuchel A. In vitro effects of sterculic acid on lipid biosynthesis in Rhodococcus opacus strain PD630 and isolation of mutants defective in fatty acid desaturation. FEMS Microbiol Lett. 2000;190:45–50. doi: 10.1111/j.1574-6968.2000.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 15.Scaglia N, Igal RA. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem. 2005;280:25339–25349. doi: 10.1074/jbc.M501159200. [DOI] [PubMed] [Google Scholar]
- 16.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–1515. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren GG, Hiltunen JK, Kastaniotis AJ, McConville MJ, Striepen B. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2012;287:4957–4971. doi: 10.1074/jbc.M111.310144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charron AJ, Sibley LD. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- 19.Mazumdar J, Wilson EH, Masek K, Hunter CA, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poloni S, Blom HJ, Schwartz IV. Stearoyl-CoA Desaturase-1: is it the link between sulfur amino acids and lipid metabolism? Biology. 2015;4:383–396. doi: 10.3390/biology4020383. [DOI] [PMC free article] [PubMed] [Google Scholar]