Abstract
The impetus behind our study was to establish a quantitative comparison between the IRIS collimator and the InCise multileaf collimator (MLC) (Accuray Inc. Synnyvale, CA) for prostate stereotactic body radiation therapy (SBRT). Treatment plans for ten prostate cancer patients were performed on MultiPlan™ 5.1.2 treatment planning system utilizing MLC and IRIS for 36.25 Gy in five fractions. To reduce the magnitude of variations between cases, the planning tumor volume (PTV) was defined and outlined for treating prostate gland only, assuming no seminal vesicle or ex-capsule involvement. Evaluation indices of each plan include PTV coverage, conformity index (CI), Paddick's new CI, homogeneity index, and gradient index. Organ at risk (OAR) dose sparing was analyzed by the bladder wall Dmax and V37Gy, rectum Dmax and V36Gy. The radiobiological response was evaluated by tumor control probability and normal tissue complication probability based on equivalent uniform dose. The dose delivery efficiency was evaluated on the basis of planned monitor units (MUs) and the reported treatment time per fraction. Statistical significance was tested using the Wilcoxon signed rank test. The studies indicated that CyberKnife M6™ IRIS and InCise™ MLC produce equivalent SBRT prostate treatment plans in terms of dosimetry, radiobiology, and OAR sparing, except that the MLC plans offer improvement of the dose fall-off gradient by 29% over IRIS. The main advantage of replacing the IRIS collimator with MLC is the improved efficiency, determined from the reduction of MUs by 42%, and a 36% faster delivery time.
Keywords: CyberKnife, IRIS, multileaf collimator, prostate stereotactic body radiation therapy
Introduction
Prostate cancer is the second most common cancer among American men after skin cancer. According to the American Cancer Society, there will be more than 220,800 new prostate cancer cases and approximately 27,540 men will die from prostate cancer in 2015.[1] The standard treatments are radical prostatectomy, interstitial or external radiation therapy, and/or hormone (androgen deprivation) therapy. The choice of the treatment mainly depends on the stage of cancer, patient's age, and urologist's and patient's preferences. Regardless of the specific treatment modality, widely accepted external beam radiotherapy regime involves fractionated daily doses of 1.8–2.0 Gy for 7–8 weeks.[2,3] Such conventional fractionated dose scheme with prolonged treatment course is somewhat challenging for patients. The disruption of physical and social lifestyles has urged clinicians to consider other effective alternatives, including hypofractionated radiotherapy.[4,5]
In addition, the well-studied radiobiology of prostate cancer with a low α/β ratio due to slowly proliferating cells supports hypofractionated dose scheme.[6,7,8] This has established the CyberKnife (CK) robotic system as a standard treatment modality for prostate stereotactic body radiation therapy (SBRT) due to its wide range of noncoplanar beams for a confined dose coverage to planning tumor volume (PTV) and normal tissue dose sparing, as well as its ability for intrafractional tumor tracking.[9,10,11] CK with dose scheme of 35 Gy or 36.25 Gy in five fractions has been proven to be one of the best options for prostate SBRT due to its excellent clinical outcome with a high local tumor control and low-grade toxicities.[12,13,14,15]
In order to avoid collimator exchange time, CK treatment plans were frequently prepared using a single or two fixed collimators. Previous studies have suggested that small collimator scan achieves high dose conformity and steep dose gradient, while large collimators tend to minimize the total monitor units (MUs) and treatment beams.[16,17] With a combination of two collimators, the total MUs in the treatment plan can also be reduced by an average of 31% compared to the single collimator.[18] Such improvements in prostate plan dosimetry and treatment efficiency are immediately realized by using the more advanced IRIS variable collimator which was introduced in 2008. The IRIS™ variable collimator consists of 30° offset two banks of hexagon-shaped collimators resulting in a dodecagon-shaped beam.[19] The IRIS™ collimator automatically replicates 12 discrete collimators ranging from 5 to 60 mm with next to none pausing time for collimator exchange. Therefore, the IRIS™ collimator provides considerable time advantage compared to multiple fixed collimator treatment plans. It has been reported that prostate plans generated by IRIS™ collimator had demonstrated a reduction of total MUs by 25% and a reduction of treatment beams by 28% compared to fixed collimators.[20] Similarly, it also showed lower mean critical structure doses, fewer treatment beams, and MUs for head and neck, lung, pancreas, and axilla treatment plans.[21] In addition, it had been shown that the IRIS™ collimator can provide good dose linearity and MU stability for all the collimators in the medium and large MU range for stereotactic fields.[22]
More recently, a simulation study showed that CK with a virtually equipped mini-multileaf collimator (mMLC) generated more time efficient, high-quality lung cancer treatment plans than those created with the IRIS™ variable collimator.[23] The mMLC allows three-dimensional conformal radiotherapy using beam's eye view projection of the target volume reducing the required number of beams and MUs.
During the first quarter of 2015, the CK M6™ series[24] equipped with InCise™ multileaf collimator (MLC) revealed its initial success in the clinic. MLC provides the benefit of irregularly shaped beam projections which traditional conical beams were unable to offer. The current generation of InCise™ MLC consists of 41 tungsten leaf pairs with 2.5 mm leaf width, capable of forming a maximum field size of 10 cm by 12 cm.[22] Introduction of the MLC option has brought the obvious speculation for higher delivery efficiency without compromising its inherent superior normal structure dose sparing and intrafractional tumor tracking. The addition of MLC modality may also expand the patient eligibility for CK treatment for larger and more irregular-shaped tumors.[25]
The purpose of this study is to quantitatively compare the radiation dose characteristics and the estimated treatment efficiencies of CK M6™ using InCise™ MLC versus the IRIS™ collimators for prostate SBRT.
Materials and Methods
Case selections
Ten low-risk prostate cancer cases were randomly selected from our institutional database for previously treated patients with the standard fractionations. All the patients were diagnosed by 12-quadrant transrectal ultrasound-guided prostate biopsy and the pathological report as prostate adenocarcinoma at the clinical stage between T1bN0M0 and T2cN0M0. The planning computed tomography (CT) sets with contours of clinical target volume (CTV), bladder, and rectum were imported to the current planning system via DICOM file exchange, in which CTV sizes ranged from 32.2 to 89.9 cm3 (median, 51.5 cm3).
CyberKnife treatment planning
On MultiPlan 5.1.2 (Accuray Inc., Sunnyvale, CA, USA) system, two treatment plans with similar dose constraints were generated for each case one using the CK M6™ InCise™ MLC and another using IRIS™ variable collimator. The longitudinal resolution for the planning CT is 2 mm or better. The PTV was created by expanding the CTV by 3 mm posteriorly and 5 mm to all other dimensions.[12] The size of PTV ranged from 61.4 to 139.2 cm3 (median 86.9 cm3). A bladder wall with 2 mm thickness and rectum were generated as critical structures. Dose prescription and the organ at risk (OAR) objectives are given in Table 1.
Table 1.
Dose prescription and organ at risk objectives

The prescription dose to the PTV was 36.25 Gy delivered in five fractions, and the PTV coverage was at least 95%.[24] The prescription isodose line was >84%, which limited the maximum prostatic dose to 119%. The rectal volume receiving 36 Gy was limited to <1 cm3 and the bladder volume receiving 37 Gy was limited to <10 cm3.[26] The plans generated with MLC allow full body path using nonisocentric beams with 1 mm leaf margin, while for the plans generated with the IRIS™ collimator all available field sizes were selected and the optimization software decided which it would utilize. Finally, full body path for robotic movements was allowed. In addition, beam reduction was performed upon the optimization up to 5% of the total number of MUs as well as reduction of MLC segments, without compromising PTV coverage. The resultant final plans ensured the prescribed dose coverage for 95% PTV or better.
Comparison of plans
The two types of plans were compared on the basis of dosimetry, radiobiology, and delivery efficiency. The dosimetry of the plans was categorized into target coverage, conformity index (CI), new conformity index (nCI), homogeneity index (HI), gradient index (GI), maximum dose to bladder wall and rectum, bladder wall V37Gy, and rectum V36Gy. The radiobiological characteristics of the plans were compared in terms of tumor equivalent uniform dose (EUD) and tumor control probability (TCP), as well as normal structure EUD and normal tissue complication probability (NTCP). The treatment delivery efficiency was compared on the basis of total number of delivered MUs and treatment time per fraction.

CI is defined as the ratio of the prescription isodose volume PV to the tumor volume covered by the prescription isodose TVPV. CI is reported in the CKs MultiPlan and it is the reciprocal of healthy tissue conformity index proposed by Lomax and Scheib.[27]

This index evaluates the quality of the target coverage and the healthy tissue dose. PV is the tissue volume that receives the prescription isodose; TV is the tumor volume (PTV) and TVPV is the tumor volume (PTV) covered by the prescription isodose. An ideal plan would have TVPV = TV = PV, yielding a nCI and CI = 1.0. nCI is reported in the CKs MultiPlan and it is the reciprocal of CI proposed by Paddick.[28]

This ratio evaluates the uniformity of dose within the target volume. Dmax is the maximum target dose (100% isodose in CK planning) and Dpres is the prescription dose. The ideal value is 1.0 while HI>1 indicates poor homogeneity. This value is also reported in the CKs MultiPlan, proposed by the Radiation Therapy Oncology Group for evaluation of stereotactic radiotherapy plans.[29] We need to stress that for calculation of both CI and HI, for consistency purposes, we used the same normalization isodose line for each patient for both IRIS and MLC in order to achieve 95% coverage.
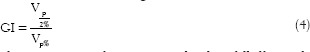
This ratio gives information on the dose falloff outside the prescription isodose volume. Vp/2% is the tissue volume enclosed by half of the prescription isodose and Vp% is the tissue volume enclosed by the prescription isodose. GI objectively measures the dose falloff outside the target volume and can be used to demonstrate the optimal prescription isodose.[30]

In this equation, vi is the ith partial volume receiving dose Di, and α is a parameter which is negative for tumor and positive for normal tissue. EUD of the normal tissue represents the uniform dose that has the same probability of damage as the actual inhomogeneous dose. The tumor EUD represents the uniform dose which has the same probability of tumor control as the actual inhomogeneous dose. The generalized EUD model was introduced by Niemierko.[31,32] The vi and Di values are obtained from the Dose Volume Histograms and the values of α from Rana and Cheng.[33]

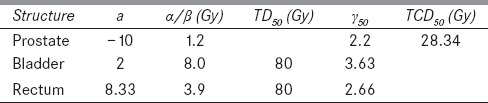
TCP is defined as the probability that no clonogenic cells survive after the treatment. EUD is calculated from equation (5), TCD50 is the tumor dose to control 50% of the tumor when irradiated homogeneously, and γ50 is the slope of the dose-response curve which is specific to the normal tissue structure or tumor. EUD-based NTCP and TCP models were proposed by Niemierko in 1999.[32] TCD50 is obtained from Rana and Cheng,[33] and γ50 values from Park et al.[34]

NTCP is based on a linear-quadratic model, it is a function of the delivered dose and irradiated normal tissue volume. TD50 is the tolerance dose of bladder and rectum for a 50% complication rate at a specific time interval (5 years). TD50 values are obtained from the model of Emami et al.[35]
The radiobiological parameters used for EUD, TCP, and NTCP calculations are given in Table 2. The Matlab-2014a software (MathWorks Inc., Natick, MA, USA) was utilized for the numerical calculations.[36]
Table 2.
Radiobiological parameters
Statistical analysis
A pairwise Wilcoxon signed rank test (XLSTAT, Addinsoft) was performed to evaluate the statistical significance of the differences between IRIS™ and MLC prostate treatment plans.
Results
CK's IRIS™ collimator and InCise™ MLC plans of 10 localized prostate cancer patients were generated according to the clinical acceptance criteria. Figure 1 illustrates the transverse dose distribution of the IRIS and MLC plans a patient's treatment plan. The orange isodose line represents the prescription dose of 3625 cGy when normalized to the 87% dose line. The maximum dose line of 4167 cGy corresponds 100% nonnormalized isodose.
Figure 1.
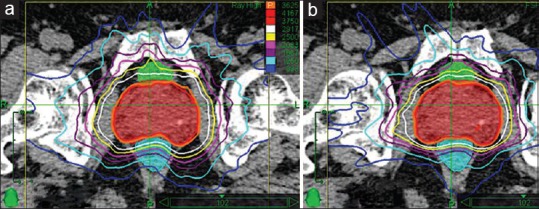
Transverse view of the dose distributions of (a) IRIS and (b) multileaf collimator plans for a selected case
From Figure 1 it is apparent that both plans have similar target coverage (DPTV95 =3625 cGy). It is worth mentioning that the MLC plan has tighter isodose lines compared to the IRIS plan, which results in a rapid dose falloff beyond the target.
Dosimetric comparison
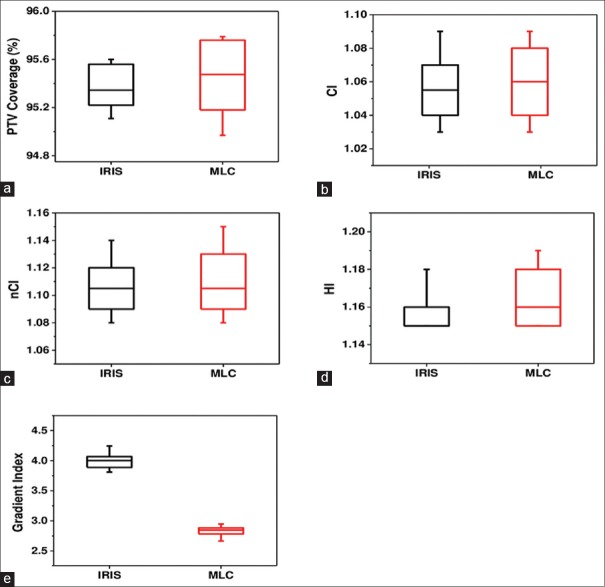
Figure 2 illustrates the PTV coverage, CI, nCI, HI, and GI for both, the IRIS collimator and MLC treatment plans. The average values of these parameters are listed in Table 3.
Figure 2.
Dosimetric indices of the CyberKnife's IRIS™ collimator and InCise™ multileaf collimator plans for ten patients: (a) Planning tumor volume coverage, (b) conformity index, (c) new conformity index, (d) homogeneity index, and (e) gradient index
Table 3.
Average values of the target coverage, conformity index, new conformity index, homogeneity index, and gradient index for IRIS and multileaf collimator treatment plans
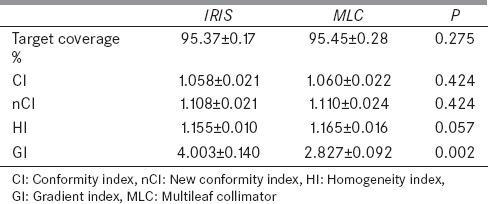
Our results indicate that the average values of the target coverage, CI, nCI, and HI were found similar for both MLC and IRIS plans (P>0.05). The main difference of the dose distribution between the two sets of plans is the dose falloff beyond the target volume which is verified by the GI of the MLC plans (29% less than IRIS plans).
Organ at risk sparing
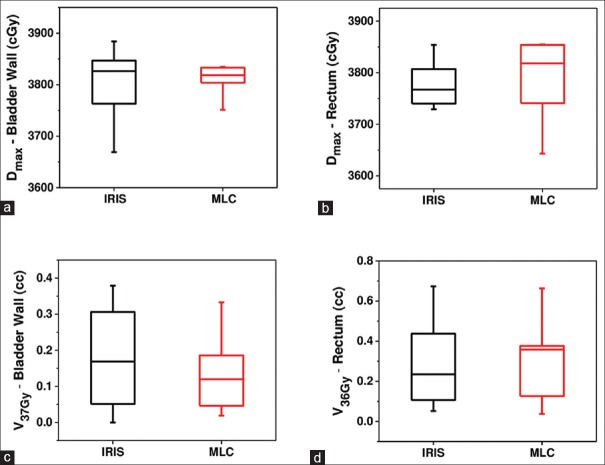
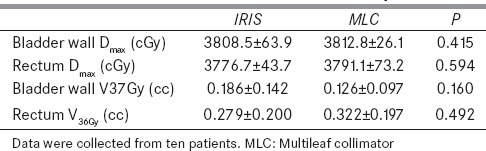
The maximum doses (Dmax) of the bladder wall and rectum, as well as the bladder wall V37Gy, and rectum V36Gy are plotted in Figure 3.
Figure 3.
Comparison of organ at risks parameters of IRIS and multileaf collimator plans for ten patients: (a) Dmax of bladder wall, (b) Dmax of rectum, (c) V37Gy of bladder wall, and (d) V36Gy of rectum
Table 4 reports the average values of these parameters for the MLC and IRIS plans. Both normal tissue doses and dose volumes were found to be not statistically significantly different, based on the Wilcoxon signed rank test. The bladder wall and rectum maximum doses of IRIS plans are marginally lower compared to the MLC plans. MLC plans are superior in terms of bladder wall V37Gy (32%), while IRIS plans are improved in terms of rectum V36Gy (13%).
Table 4.
Comparison of the average values of maximum doses of bladder wall and rectum, V37Gy of bladder wall, and V36Gy of rectum for the multileaf collimator and IRIS treatment plans
Radiobiological comparison
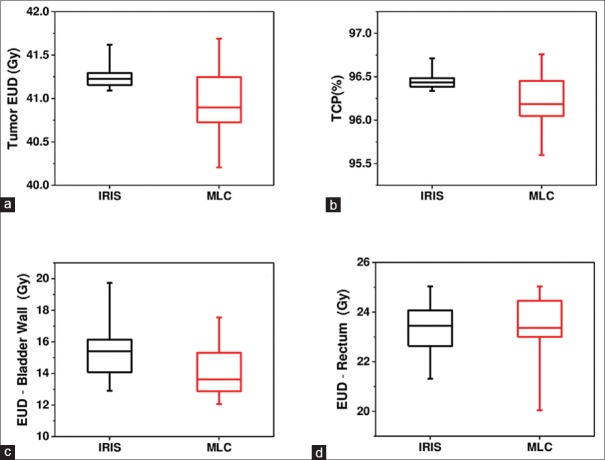
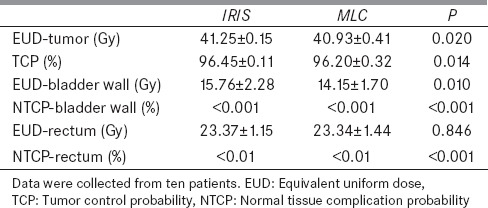
Comparison of the radiobiological parameters of tumor EUD, TCP, and the EUD of the bladder wall and rectum between MLC and IRIS plans are displayed in Figure 4.
Figure 4.
Comparison of radiobiological parameters obtained from IRIS collimator and multileaf collimator for tumor bladder wall and rectum: (a) Tumor equivalent uniform dose, (b) tumor control probability, (c) equivalent uniform dose of bladder wall, and (d) equivalent uniform dose of rectum
The average values of all radiobiological parameters are listed in Table 5. The average values of the tumor EUDs, the TCPs, and the EUD of rectum are similar in both plans, while the EUD of the bladder wall is lower for the MLC plans than in the IRIS. The NTCP of the bladder wall and rectum is <0.001% and 0.01%, respectively, for both plans.
Table 5.
Comparison of tumor equivalent uniform dose, tumor control probability, and equivalent uniform dose and normal tissue complication probability of bladder wall and rectum between multileaf collimator and IRIS plans
Delivery efficiency
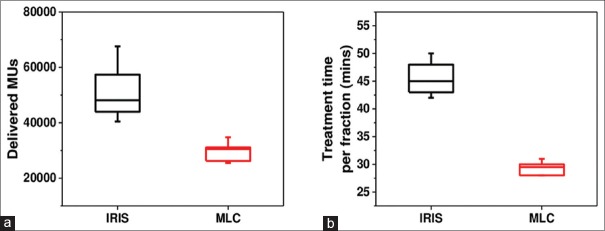
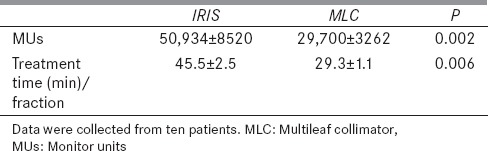
The delivered MUs and treatment time per fraction of the MLC and IRIS plans for the ten patients are plotted in Figure 5.
Figure 5.
Comparison of delivery efficiency parameters between the IRIS collimator and multileaf collimator plans: (a) Delivered monitor units and (b) treatment time per fraction
The average values of MUs and treatment times are reported in Table 6. It is apparent that fast and efficient dose delivery is the main feature of the MLC plans. The average delivered MUs are lower by 42% in plans using MLC than the IRIS collimator, while the average treatment time per fraction is lower by 36%. It worth mentioning that the treatment time per fraction includes 5 min set up time and the imaging time during the treatment.
Table 6.
Comparison of delivered monitor units and treatment time per fraction between multileaf collimator and IRIS plans
Discussion
This is the first study of a comprehensive comparison between CK's IRIS collimator and InCise MLC for prostate SBRT. One of the reasons for the limited availability in literature for dosimetric comparisons between InCise MLC and other treatment delivery modalities might be the difficulty in quantifying the dosimetrical parameters among the highly inconsistent tumor shapes between cases. It is evident that geometric characters and proximity to risk organs may vary drastically in metastatic lesions at lungs, spine, and intracranial sites. When the CTVs are small or spherically shaped, the advantages of MLC will dissipate since its treatment can be easily accomplished with a single collimator. In contrary, prostate cancer cases often present relatively consistent PTV and a similar relationship with the adjacent risk organs, such as the rectum and the bladder. As selected in this study, the prostate cancer without seminal vesicle and extra-capsule invasions is ideal for a reliable dosimetric comparison.
The studies indicated that the HI of IRIS plans (1.155) is slightly better than that of MLC plans (1.165) for similar target coverage and conformity indices. This could be attributed to the higher number of beams of IRIS plans, which allows greater flexibility to the dose distribution. It is reported by Ceylan et al.[37] that CK prostate plans generated using fixed collimators (median 20 mm) had a mean HI of 1.33 and CI of 1.23. These values are higher compared to our findings because their median prescription isodose line was 75%. Another study has reported a mean HI of 1.45 and CI of 1.18 based on eight prostate plans generated using two fixed collimators (10–35 mm).[38] Similarly, these HI and CI values are higher compared to our findings due to the 69% mean prescription isodose line.
As mentioned in the results section, the main difference of the dose distribution between the two plans is found in the dose falloff beyond the target volume. The average value of GI of the MLC plans is 42% lower when copared to that of IRIS plans (2.827 vs. 4.003) and hence is superior. Lin et al.[39] have reported that two groups of ten CK prostate plans generated had a mean GI of 5.39 and 4.91. In addition, the OAR (bladder wall and rectum) sparing of IRIS and MLC plans is comparable. Similar Dmax (3808.5 cGy vs. 3812.8 cGy) and rectum Dmax (3776.7 cGy vs. 3791.1 cGy) were found for IRIS and MLC plans, respectively. The corresponding values reported by Lin et al.[39] are 4137 cGy and 3894 cGy (normalized to our prescription, 3625 cGy) for a group of ten CK prostate plans generated with fixed collimators (≤3). These Dmax values are higher compared to our findings for a similar set of OAR constraints (bladder V100 <5 cm3, and rectum V36Gy <1 cm3). Furthermore, Fuller et al.[40] had found higher bladder Dmax (4083 cGy) and lower rectal wall Dmax (3558 cGy) for a group of ten CK prostate plans generated with fixed collimators. Table 4 shows that the average V37Gy of the bladder wall is superior in MLC plans (0.186cc vs. 0.126cc), while V36Gy of rectum is superior in IRIS plans (0.279cc vs. 0.322cc). Feng et al.[41] have reported a similar trend of V37Gy of bladder wall (3.09 cc vs. 1.82cc) and V36Gy of rectum (0.38cc vs. 0.52cc) for prostate SBRT using G4™ circular collimators and M6™ MLC. These reported OAR volumes are higher compared to our findings.
The radiobiological parameters, tumor EUD and TCP, are similar for both IRIS and MLC plans (41.25 Gy vs. 40.93 Gy, and 96.45% vs. 96.20%), [Table 5]. Moreover, NTCP of bladder wall and rectum is <0.001% and 0.01%, respectively. To our knowledge, no published results of EUD based TCP and NTCP calculations exist for prostate SBRT. Rana and Cheng[33] had found TCP of 98.3%, bladder NTCP of 0.01%, and rectum NTCP of 2.21% for conventionally fractionated RapidArc prostate treatments, while Fu et al.[42] have reported TCP of 86.35%, bladder NTCP of 0.00%, and rectum NTCP of 7.59% for IMRT prostate treatments.
The most important finding of our current study is the treatment efficiency, which was evaluated based on delivered MUs and treatment time per fraction. The delivered MUs and treatment time per fraction were significantly lower for MLC than IRIS plans (50,934 vs. 29,700 and 45.5 min vs. 29.3 min, P < 0.01), [Table 6]. Preliminary results of Feng et al.[41] have reported a reduction in delivered MUs (24,228 from 32,347) and treatment time per fraction (29.5 min from 45 min) when replacing CK G4™ circular collimators with CK M6™ MLC for prostate SBRT. The mean treatment time per fraction is comparable to our findings while the mean delivered MUs of IRIS collimator is higher compared to circular collimators. Fahimian et al.[25] have reported a 38% ±10% reduction of delivered MUs and treatment time, by replacing the IRIS with MLC of CK M6™ for prostate, partial breast, and periorbit carcinomas.
The main advantage of replacing the IRIS collimator with MLC in CK M6™ appears to be the improved efficiency, as demonstrated from the reduction of MUs by 42% resulting to a 36% faster delivery time. Reduced number of MUs per treatment would result in reduced peripheral dose, leading in decreased risk of secondary cancer, which could be an influencing factor for the long-term survival of the patients. Further, shorter treatment time would benefit patient comfort and accurate treatment delivery by reducing the patient motion.
Conclusion
To sum up, the InCise™ MLC of CK M6™ was able to produce dosimetrically comparable plans with the IRIS™ collimator for prostate SBRT. Comparing with IRIS™ collimator, prostate plans using InCise™ MLC demonstrated significant reductions in the total MUs (42%), estimated treatment time (36%), and improvement in planning dose gradient (29%). The authors would like to further confirm the estimated treatment efficiency with real-time dose delivery during the clinical practice, which may be affected by robot motion and MLC or IRIS changing speed. The studies for other treatment sites are also warranted during future investigations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Key Statistics about Prostate Cancer, American Cancer Society. 2014. [Last accessed on 2016 Apr 24]. Available from: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics .
- 2.Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 3.Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet. 1999;353:267–72. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–8. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton AS, Stanford JL, Gilliland FD, Albertsen PC, Stephenson RA, Hoffman RM, et al. Health outcomes after external-beam radiation therapy for clinically localized prostate cancer: Results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:2517–26. doi: 10.1200/JCO.2001.19.9.2517. [DOI] [PubMed] [Google Scholar]
- 6.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 7.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 8.Sudahar H, Kurup PG, Murali V, Mahadev P, Velmurugan J. Equivalent normalized total dose estimates in cyberknife radiotherapy dose delivery in prostate cancer hypofractionation regimens. J Med Phys. 2012;37:90–6. doi: 10.4103/0971-6203.94743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexibility of Prostate Treatment Planning and Delivery for Cyber Knife Radiosurgery. Accuray Inc. 2008. [Last accessed on 2016 Apr 24]. Available from: http://www.cyberknife.com/uploadedFiles/For_Your_Doctor/500345%20B%20HDR%20Whitepaper.pdf .
- 10.King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: Rationale and technical feasibility. Technol Cancer Res Treat. 2003;2:25–30. doi: 10.1177/153303460300200104. [DOI] [PubMed] [Google Scholar]
- 11.Ganapathy K, Kurup PG, Murali V, Muthukumaran M, Subramanian SB, Velmurugan J. A study on rectal dose measurement in phantom and in vivo using Gafchromic EBT3 film in IMRT and CyberKnife treatments of carcinoma of prostate. J Med Phys. 2013;38:132–8. doi: 10.4103/0971-6203.116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC., Jr Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043–8. doi: 10.1016/j.ijrobp.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 13.King CR, Brooks JD, Gill H, Presti JC., Jr Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–82. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. doi: 10.1186/1748-717X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IRIS Variable Aperture Collimatr for the CYberKnife Robotic Radiosurgery System. Accuray Inc. 2008. [Last accessed on 2016 Apr 24]. Available from: http://printable.p1nnacle.com/accuray2/500345B.pdf .
- 17.Sudahar H, Kurup PG, Murali V, Velmurugan J. Comparative analysis between 5 mm and 7.5 mm collimators in CyberKnife radiosurgery for trigeminal neuralgia. J Med Phys. 2013;38:120–4. doi: 10.4103/0971-6203.116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pöll JJ, Hoogeman MS, Prévost JB, Nuyttens JJ, Levendag PC, Heijmen BJ. Reducing monitor units for robotic radiosurgery by optimized use of multiple collimators. Med Phys. 2008;35:2294–9. doi: 10.1118/1.2919090. [DOI] [PubMed] [Google Scholar]
- 19.Echner GG, Kilby W, Lee M, Earnst E, Sayeh S, Schlaefer A, et al. The design, physical properties and clinical utility of an iris collimator for robotic radiosurgery. Phys Med Biol. 2009;54:5359–80. doi: 10.1088/0031-9155/54/18/001. [DOI] [PubMed] [Google Scholar]
- 20.Fuller D, Lee C, Mardirossian G. Comparison of virtual HDR prostate CyberKnife Treatment Plans: Fixed Collimator with MultiPlan v1.6-2.1 Versus Iris™ Variable Collimator with Sequential Optimizationin MultiPlan v3.0. CyberKnife Users’ Meeting; Hollywood, FL. 2009. [Google Scholar]
- 21.Lee C, Mardirossian G, Jin H. Comparison of Clinical CyberKnife Treatment Plans Created using Fixed Collimators in MultiPlan v2.1 and the Iris™ Variable Aperture Collimator in MultiPlan v3.0. CyberKnife Users’ Meeting; Hollywood, FL. 2009. [Google Scholar]
- 22.Sudahar H, Kurup PG, Murali V, Velmurugan J. Dose linearity and monitor unit stability of a G4 type cyberknife robotic stereotactic radiosurgery system. J Med Phys. 2012;37:4–7. doi: 10.4103/0971-6203.92714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Water S, Hoogeman MS, Breedveld S, Nuyttens JJ, Schaart DR, Heijmen BJ. Variable circular collimator in robotic radiosurgery: A time-efficient alternative to a mini-multileaf collimator? Int J Radiat Oncol Biol Phys. 2011;81:863–70. doi: 10.1016/j.ijrobp.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 24.CyberKnife M6TM Series brochure, Acuuray Inc. [Last accessed on 2016 Apr 24]. available from: http://www.accuray.com/sites/default/files/ck_m6_series_brochure_en_501004b.pdf .
- 25.Fahimian B, Soltys S, Xing L, Gibbs I, Chang S, Wang L. Evaluation of MLC-based robotic radiotherapy. Med Phys. 2013;40:344. [Google Scholar]
- 26.Friedland JL, Freeman DE, Masterson-McGary ME, Spellberg DM. Stereotactic body radiotherapy: An emerging treatment approach for localized prostate cancer. Technol Cancer Res Treat. 2009;8:387–92. doi: 10.1177/153303460900800509. [DOI] [PubMed] [Google Scholar]
- 27.Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 2003;55:1409–19. doi: 10.1016/s0360-3016(02)04599-6. [DOI] [PubMed] [Google Scholar]
- 28.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219–22. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 29.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation Therapy Oncology Group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–9. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 30.Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105(Suppl):194–201. doi: 10.3171/sup.2006.105.7.194. [DOI] [PubMed] [Google Scholar]
- 31.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–10. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 32.Niemierko A. A generalized concept of equivalent uniform dose (EUD) Med Phys. 1999;26:1100. [Google Scholar]
- 33.Rana S, Cheng CY. Radiobiological impact of planning techniques for prostate cancer in terms of tumor control probability and normal tissue complication probability. Ann Med Health Sci Res. 2014;4:167–72. doi: 10.4103/2141-9248.129023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JY, Lee JW, Chung JB, Choi KS, Kim YL, Park BM, et al. Radiobiological model-based bio-anatomical quality assurance in intensity-modulated radiation therapy for prostate cancer. J Radiat Res. 2012;53:978–88. doi: 10.1093/jrr/rrs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 36.Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Technical note. Phys Med. 2007;23:115–25. doi: 10.1016/j.ejmp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Ceylan C, Kucuk N, Bas Ayata H, Guden M, Engin K. Dosimetric and physical comparison of IMRT and CyberKnife plans in the treatment of localized prostate cancer. Rep Pract Oncol Radiother. 2010;15:181–9. doi: 10.1016/j.rpor.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hossain S, Xia P, Huang K, Descovich M, Chuang C, Gottschalk AR, et al. Dose gradient near target-normal structure interface for nonisocentric CyberKnife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:58–63. doi: 10.1016/j.ijrobp.2009.07.1752. [DOI] [PubMed] [Google Scholar]
- 39.Lin YW, Lin KH, Ho HW, Lin HM, Lin LC, Lee SP, et al. Treatment plan comparison between stereotactic body radiation therapy techniques for prostate cancer: Non-isocentric CyberKnife versus isocentric RapidArc. Phys Med. 2014;30:654–61. doi: 10.1016/j.ejmp.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Fuller DB, Naitoh J, Lee C, Hardy S, Jin H. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: Dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70:1588–97. doi: 10.1016/j.ijrobp.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 41.Feng J, Yang J, Lamond J, Lavere N, Laciano R, Ding W, et al. A Dosimetric Comparison of Robotic Prostatic Radiosugery Using Multi-Leaf Collimation Vs Circular Collimators. Med Phys. 2014;41:223. [Google Scholar]
- 42.Fu W, Yang Y, Li X, Heron DE, Huq MS, Yue NJ. Dosimetric effects of patient rotational setup errors on prostate IMRT treatments. Phys Med Biol. 2006;51:5321–31. doi: 10.1088/0031-9155/51/20/016. [DOI] [PubMed] [Google Scholar]