Abstract
Background
Decreased CFTR-mediated chloride (Cl) secretion across mucosal surfaces contributes to the development of airway disease by depleting airway surface liquid, increasing mucus viscosity and adhesion, and consequently hindering mucociliary clearance. We serendipitously discovered during testing of drugs solubilized in low concentrations ethanol (0.25%, 43mM) that the control vehicle produced robust activation of CFTR-mediated Cl− transport. The objective of the current study is to investigate low concentrations ethanol for effects on Cl− secretion and ciliary beat frequency (CBF).
Methods
Wild type (WT) and transgenic CFTR−/− primary murine nasoseptal epithelial (MNSE) and WT and F508del/F508del human sinonasal epithelial (HSNE) cultures were subjected to transepithelial ion transport measurements using pharmacologic manipulation in Ussing chambers. CBF activation was also monitored. Murine nasal potential difference (NPD) was measured in vivo.
Results
Ussing chamber tracings revealed ethanol activated CFTR-mediated Cl transport in a dose-dependent fashion in WT MNSE (n=4, p<0.05) and HSNE (n=4, p<0.05). Ethanol also significantly increased CBF (fold-change) in WT MNSE cultures in a dose dependent fashion [PBS, 1.33+/−0.04; 0.25% Ethanol, 1.37+/−0.09; 0.5% Ethanol, 1.53+/−0.06 (p<0.05), 1% Ethanol, 1.62+/−0.1 (p<0.05)]. Lack of stimulation in CFTR−/− and F508del/F508del cultures indicated activity was dependent on the presence of intact functional CFTR. Ethanol perfusion (0.5%) resulted in a significant −3.5mV mean NPD polarization when compared to control solution (p<0.05).
Conclusion
The observation that brief exposure of ethanol stimulated Cl− secretion via CFTR-mediated pathways indicates possible use as topical aerosol delivered alone or in combination with other CFTR activators for diseases of dysfunctional MCC in CRS.
Keywords: CFTR, Chronic rhinosinusitis, Ethanol, Drug delivery, Transepithelial Ion Transport, Sinusitis, Chloride Secretion, Murine Nasal Culture, Sinus Epithelium, Mucociliary Clearance, Ciliary beat
INTRODUCTION
Dysfunctional mucociliary clearance (MCC) and inflammation are hallmarks of many airway diseases, including cystic fibrosis and chronic rhinosinusitis (CRS).1,2 Enhancing transepithelial Cl− secretion in the upper airways by topical administration of cystic fibrosis transmembrane conductance regulator (CFTR) potentiators or other Cl− secretagogues represents a novel strategy that has not been investigated previously in either CF or non-CF CRS, but could provide considerable advantages over conventional therapies for sinus disease.3,4
A number of molecules that modulate CFTR function have been identified through high throughput drug screening. However, many exhibit limited solubility in water and are therefore dissolved in solvents such as dimethyl sulfoxide (DMSO) or ethanol for experimental testing. In terms of nasal delivery, several studies have reported the use of ethanol based microemulsions as a topical drug delivery system.5,6 Short-chain alcohols such as ethanol have been used as the co-surfactant in microemulsions for intranasal topical drug delivery because the onset of drug activity is the fastest and the duration is the longest due to solubility being substantially enhanced by ethanol.6 During testing of candidate CFTR modulators, we serendipitously discovered low concentrations of ethanol in the control vehicles produce robust activation of wild type CFTR-mediated Cl− transport in well characterized primary cell culture models of murine nasal septal (MNSE) and human sinonasal respiratory epithelium (HSNE). Enhancing transepithelial Cl− secretion with low concentrations of a common and safe vehicle (already present in numerous aerosolized pharmaceuticals) represents a reasonable therapeutic approach to treatment of mucociliary dysfunction.
The objectives of the current study are to investigate low concentrations of ethanol for effects on transepithelial Cl− secretion and ciliary beat frequency (CBF) and to characterize the mechanism that underlies this type of CFTR modulation.
METHODS
Primary Cell Culture
Institutional Review Board and Institutional Animal Care and Use Committee approval were obtained prior to initiating these studies. MNSE and HSNE cells were harvested, grown on Costar 6.5-mm diameter permeable filter supports (Corning Life Sciences, Lowell, MA), and submerged in culture medium as previously described.7–10 Media was removed from the monolayers on day 4 after the epithelium reached confluence, and cells fed via the basal chamber. Transgenic CFTR−/− MNSE cultures were also investigated. Differentiation and ciliogenesis occurred in all cultures within 10 to 14 days. Cultures were used for experiments when fully differentiated with widespread ciliogenesis with transepithelial resistances (Rt) >300 Ω·cm2.
Ussing Chamber Analysis
Transwell inserts (Costar) were mounted in Ussing chambers to investigate pharmacologic manipulation of vectorial ion transport as previously described.1,2,4,11–17 Monolayers were evaluated under short circuit current conditions following fluid resistance compensation using automatic voltage clamping (VCC 600; Instruments, San Diego, CA). Serosal bath solutions contained (in mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. Baths for transwell filters were warmed to 37°C and gassed continuously with a 95%O2-5%CO2 mixture that provides a pH of 7.4 under conditions studied here. Chemicals were obtained from Sigma (St. Louis, MO). Ussing chamber experiments were performed with a nominal mucosal Cl2 in which 6 mM replaced NaCl in the above solution. The following pharmacologic agents were used: amiloride (100 mM) - blocks epithelial Na+ channels, as a means to isolate changes in short-circuit current (ΔISC) secondary to effects on Cl− channel activity18; ethanol (0.25% (43mM), 0.5% (85.5mM) or 1% (171mM)) – activates CFTR; forskolin (20 mM) - activates CFTR by elevating intracellular cAMP, which results in protein kinase A (PKA)-dependent phosphorylation of the CFTR regulatory domain (R-D); INH-172 (10 mM)- a CFTR inhibitor that permits determination of CFTR-dependent contributions to ISC.12 1% of ethanol is equal to 171mM of ethanol. Each solution was produced as 1,000 x stock and studied at 1x the Ussing chamber or by CBF analysis. The ISC was continuously assessed at one current measurement per second. By convention, a positive deflection in ISC under conditions tested here was defined as the net movement of anions from the serosal to mucosal direction.
Lactate Dehydrogenase (LDH) assay
To determine the cytotoxicity of cells after exposure to ethanol, culture medium was tested for the presence of released lactate dehydrogenase (LDH). A 96-well LDH Cytotoxicity Assay Kit (Abcam, Cambridge, MA) was used, with the following modifications: on the day of the assay, 50 μL of culture medium was removed and added to 50 μL of Substrate Mix. Absorbance at 450 nm was obtained with Epx Precision Microplate Reader (Molecular Devices, Sunnyvale, CA). We measured the LDH levels 30mins, 4, 8 and 24 hours after ethanol exposure at each dose (0.25%, 0.5% and 1%).
Nasal Potential Difference (NPD) Assay
Nasal cavities of anesthetized mice (C57/BL6) were perfused sequentially with 1) Ringer’s solution containing 140mM NaCl, 5mM KCl, 1mM MgCl2, 2mM CaCl2, 10mM HEPES, and amiloride 50μM (pH 7.3); 2) amiloride + a low-Cl−-containing solution (NMDG, 6 mM Cl−, pH 7.3); and 3) amiloride + low-Cl−-containing solution + ethanol, forskolin 20 uM, or DMSO control.10 Activity was measured from the stable baseline to the highest point of hyperpolarization.
CFTR R-Domain Phosphorylation and cAMP Measurements
Cellular cAMP was measured using an ELISA-based detection kit (Cayman Chemicals, Ann Arbor, MI) as previously described.4 To confirm absence of a cAMP/PKA dependent mechanism, polyclonal NIH-3T3 cells expressing HA-tagged R-domain were treated with ethanol for 20 minutes, and compared to forskolin (100 nM) x 5 minutes as a positive control and DMSO as negative control. Following lysis, equal amounts of protein (50 mg) from total cell lysate were electrophoresed through a 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and immunoblotted with antibody to the HA tag (Covance, Cumberland, VA). Phosphorylation of the R-domain was visualized as a 2–4 kD shift in migration, as previously described.19
Ciliary Beat Frequency Analysis
Images were visualized using a 20X objective on an inverted scope (Fisher Scientific, Pittsburgh, Pa.). Data was captured using a Model A602f-2 Basler area scan high-speed monochromatic digital video camera (Basler AG, Ahrensburg, Germany) at a sampling rate of 100 frames per second and a resolution of 640 x 480 pixels. Images were analyzed using the Sisson-Ammons Video Analysis (SAVA) system version 2.1.6 and virtual instrumentation software for CBF monitoring. All recordings were made at 200× magnification. Experiments were all performed at ambient temperature (23°C). A baseline recording of CBF was conducted for each cell monolayer prior to apical administration of test solution and compared to the corresponding control using vehicle alone. To demonstrate whether the CBF increase is dependent on nitric oxide (NO) production, CBF was measured with and without L-NAME (L-NG-Nitroarginine methyl ester, Cayman, Ann Arbor, MI), nitric oxide synthase (NOS) inhibitor. Whole field analysis was performed with each point measured representing one cilia and CBF recordings in both baseline and ethanol at each dose were made for at least 40 minutes. Each analysis was normalized to fold-change over baseline CBF Hz (treatment/baseline) and average fold-changes were compared for significance.
Statistical Analysis
Statistical analyses were conducted using Excel 2010 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized unpaired Student t tests for electrophysiology data and analysis of variance followed by Tukey-Kramer multiple comparison test if necessary for LDH, CBF, and cAMP assay. Data is expressed +/− standard error of the mean.
RESULTS
Low Dose Ethanol does not induce cytotoxicity
Time-dependent and dose-dependent toxicity measured in MNSE by LDH based protocols demonstrated no significant difference from physiological buffer saline (PBS) controls at all time and concentration endpoints (Figure 1). Low dose ethanol does not induce cytotoxicity to nasal epithelial cells.
Figure 1. Low dose ethanol does not induce cytotoxicity.
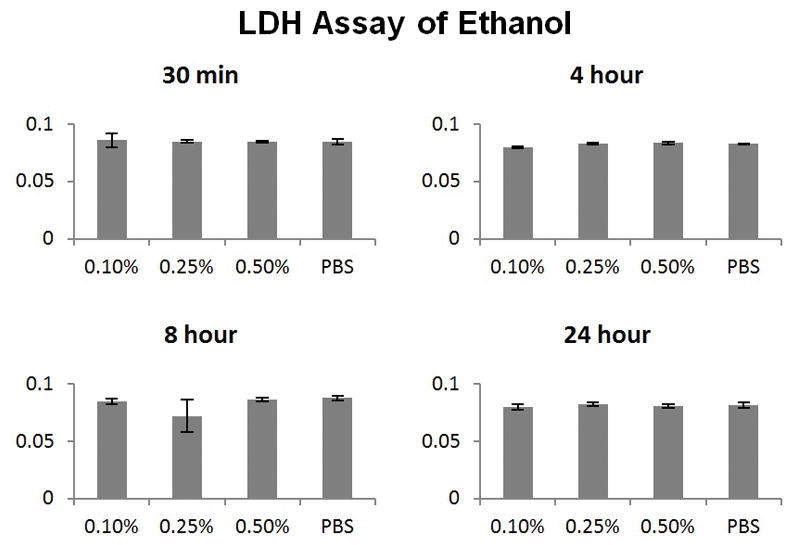
Time-dependent and dose-dependent toxicity measured in murine nasal epithelium by LDH based protocols demonstrated no significant difference from PBS controls at all time and concentration endpoints.
Ethanol Stimulates CFTR-mediated Cl− Transport in WT MNSE in vitro
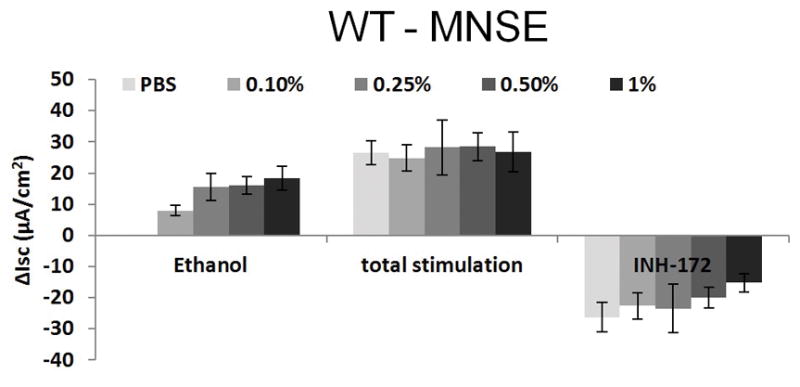
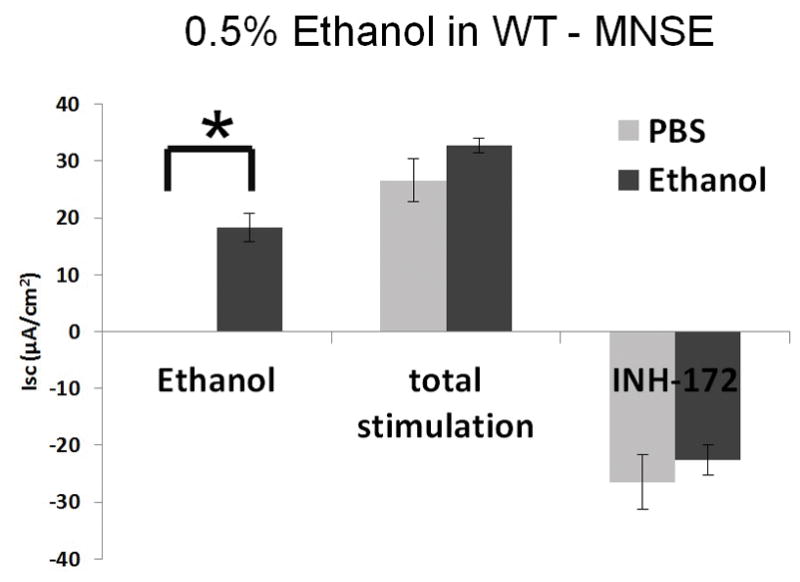
Ussing chamber tracings reveal ethanol activation of Cl− transport in a dose-dependent fashion (Figure 2A). Figure 2B represents the summary of ethanol stimulation versus total stimulation [ethanol + forskolin (100 nM)] of transepithelial Cl− conductance (n = 4, per condition). PBS did not stimulate Cl− secretion, whereas ethanol (0.5%) significantly increased Cl− transport when compared to PBS control (PBS = 0 ± 0 μA/cm2 (n = 3), ethanol (0.5%) = 18.2 ± 2.5 μA/cm2 (n = 3), p = 0.0018) (Figure 2C). Total stimulated ΔISC and inhibition of CFTR-mediated ISC following application of INH-172 with [ethanol + forskolin] were higher than those with [PBS + forskolin] (total stimulated ΔISC of ethanol + forskolin (n = 3) = 32.7 ± 2.2 μA/cm2; total stimulated ΔISC of PBS + forskolin (n = 3) = 26.6 ± 3.8 μA/cm2, p = 0.24). However, statistical significance was lacking. Throughout the Ussing chamber tracing, transepithelial resistances (Rt) was maintained above 250 Ω·cm2 in all tracings.
Figure 2. Ethanol Stimulates CFTR-mediated Cl− Transport in WT MNSE in vitro.
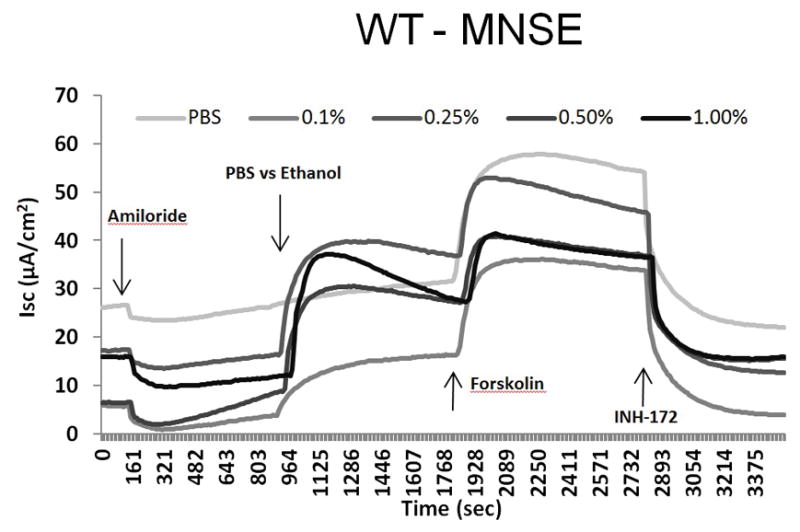
A: Representative Ussing chamber tracings reveal ethanol activation of Cl− transport in a dose-dependent fashion. By convention, a positive deflection in the tracing (ΔISC) represents movement of an anion (i.e. Cl−) in the serosal to mucosal direction.
B: Summary representation of ethanol stimulation vs. total stimulation [ethanol+ forskolin (100 nM)] of transepithelial Cl− conductance (n = 4 per condition). Ethanol concentrations > 0.5% results in decreased total stimulation.
C: Ethanol (0.5%) significantly increased Cl− transport when compared to PBS control (n = 3; p = 0.0018). Total stimulated ΔISC and inhibition of CFTR-mediated ISC following application of INH-172 with [ethanol + forskolin] were higher than those with [PBS + forskolin] (total stimulated ΔISC of ethanol + forskolin (n = 3) = 32.7 ± 2.2 μA/cm2; total stimulated ΔISC of PBS + forskolin (n = 3) = 26.6 ± 3.8 μA/cm2, p = 0.24). However, statistical significant was lacking. * Statistical significance (p < 0.05)
Ethanol Stimulates CFTR-mediated Cl− Transport in HSNE in vitro
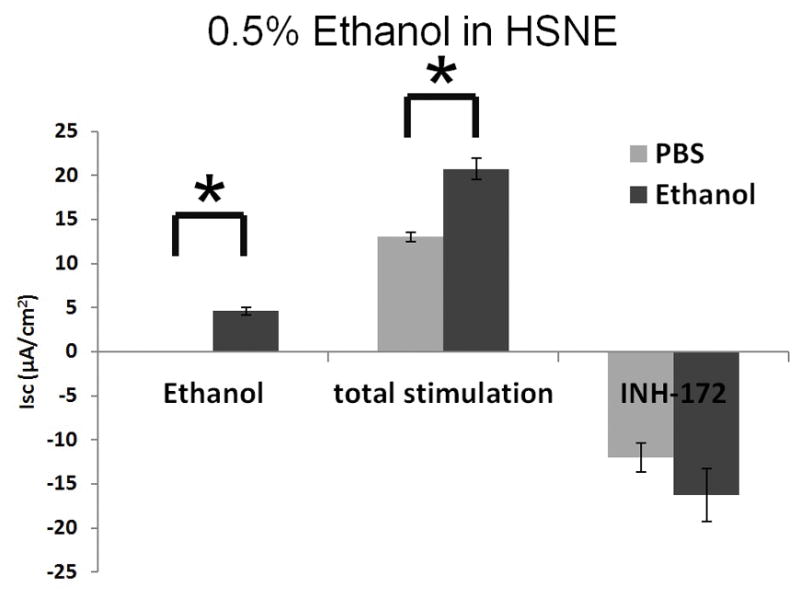
Ethanol also activates Cl− transport in HSNE in a dose-dependent fashion (Figure 3A). Total stimulation [ethanol+ forskolin] of transepithelial Cl− conductance (n = 5 per condition) indicates ethanol concentration at 1.0% results in decreased total stimulation compared to concentration at 0.5% (85.5mM), which was not statistically significant (p > 0.05). As seen in MNSE, ethanol (0.5%) markedly increased Cl− transport when compared to PBS control (PBS (n = 4); 0 ± 0 μA/cm2 vs. ethanol (n = 9); 4.6 ± 0.5 μA/cm2: p = 0.0001) (Figure 3B). Total stimulated ΔISC following application of ethanol (0.05%) + forskolin was statistically different from those with PBS + forskolin [20.7 ± 1.2 μA/cm2 (n=9) vs. 13.0 ± 0.5 μA/cm2 (n=4) respectively; p = 0.002). Throughout the Ussing chamber tracing, transepithelial resistances (Rt) was maintained above 250 Ω·cm2 in all tracings.
Figure 3. Ethanol Stimulates CFTR-mediated Cl− Transport in HSNE in vitro.
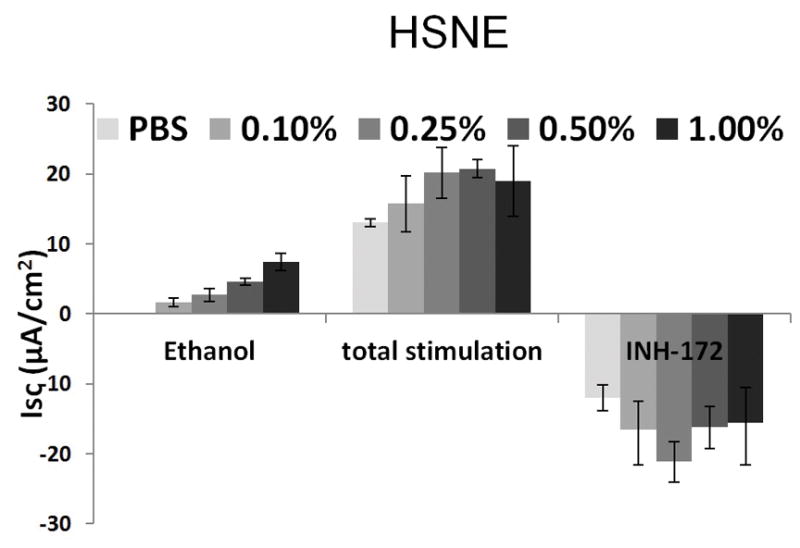
A: Ethanol stimulation vs. total stimulation [ethanol+ forskolin (0.5%)] of transepithelial Cl− conductance (n = 9 per condition) indicates ethanol concentrations > 0.5% result in decreased total stimulation.
B: Ethanol (0.5%) significantly increased Cl− transport when compared to PBS control (n = 9; p<0.05). Total stimulated ΔISC following application of [ethanol (0.05%) + forskolin] were statistically different from those with [PBS + forskolin] (total stimulated ΔISC of ethanol + forskolin (n = 9) = 20.7 ± 1.2 μA/cm2; total stimulated ΔISC of PBS + forskolin (n = 4) = 13.0 ± 0.5 μA/cm2, p = 0.002). * Statistical significance (p < 0.05)
Activation of Transepithelial Cl− Conductance is Dependent on the Presence of Functional CFTR
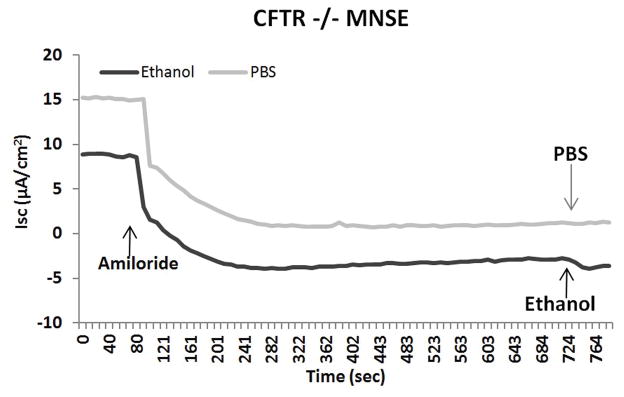
Another means to investigate overall contribution of CFTR channel activation to ethanol is by using CFTR knock out cells (CFTR−/−). We observed that CFTR−/− MNSE (Figure 4A) and F508del/F508del HSNE (Figure 4B) primary cell cultures demonstrate no stimulation with the administration of ethanol in Ussing chamber tracings. Ethanol activates CFTR-mediated Cl− transport with apical administration only (Figure 4C). These findings suggest that the major component of ΔISC stimulated by the ethanol is attributable to CFTR-mediated Cl− secretion.
Figure 4. Ethanol 0.5% Activation of Transepithelial Cl− Conductance is Dependent on Presence of CFTR and Apical Administration.
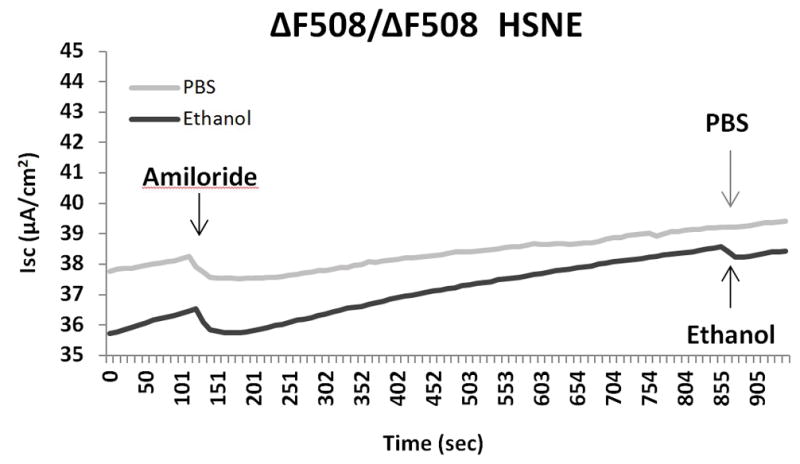
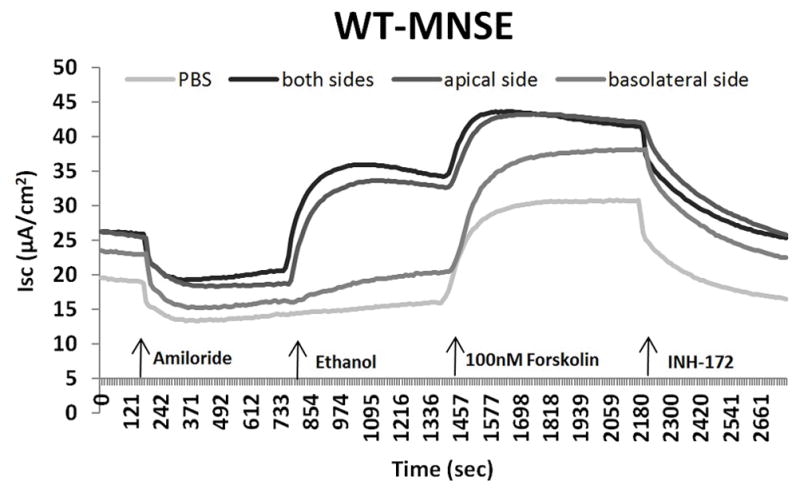
CFTR−/− MNSE (A) and F508del/F508del HSNE (B) primary cultures demonstrate no stimulation with the administration of ethanol in Ussing chamber tracings.
C: Ethanol activates CFTR-mediated Cl− transport with apical administration only.
Murine Nasal Potential Difference Measurements Demonstrate Activation of Cl− Transport in vivo
Mice underwent a standardized NPD protocol with the addition of 0.5% Ethanol (or vehicle control) in the final perfusion solution. The tracing (Figure 5A) is representative from C57 mice stimulated with ethanol in the final perfusate. Ethanol perfusion resulted in a −3.5mV mean NPD polarization that was significantly different than mice receiving vehicle alone (p<0.05) (Figure 5B).
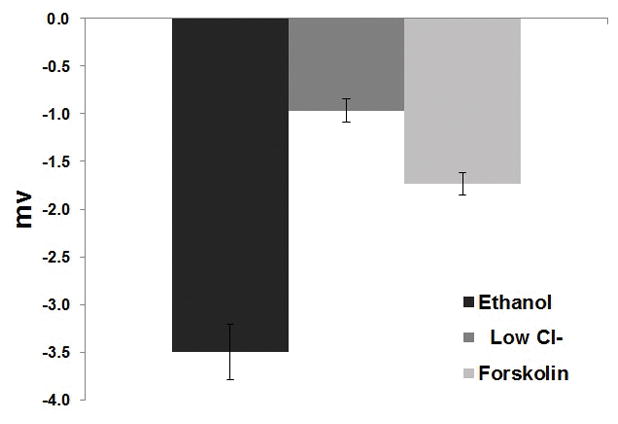
Figure 5. Murine Nasal Potential Difference Measurements Demonstrate Activation of Cl− Transport In Vivo.
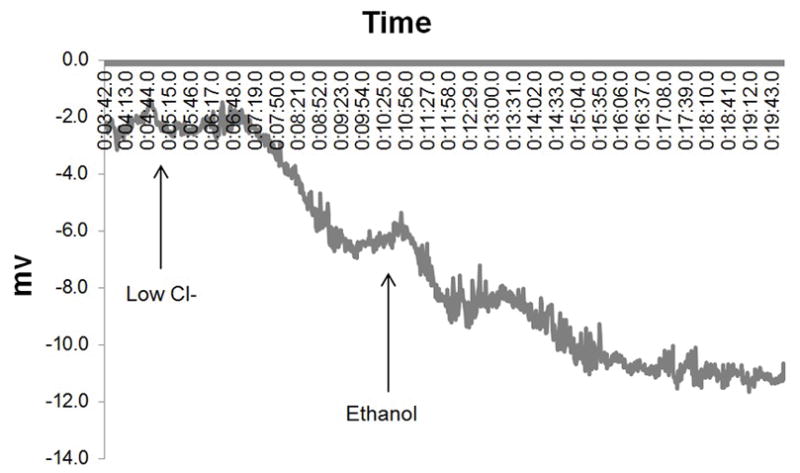
A: Mice underwent a standardized NPD protocol with the addition of 0.5% Ethanol (or vehicle control) in the final perfusion solution. The tracing above is representative from C57 mice stimulated with ethanol in the final perfusate.
B: Ethanol perfusion resulted in a −3.5mV mean NPD polarization that was significantly different than in mice receiving vehicle alone (p<0.05).
Ethanol does not increase cellular cAMP or mediate phosphorylation of the CFTR R-domain
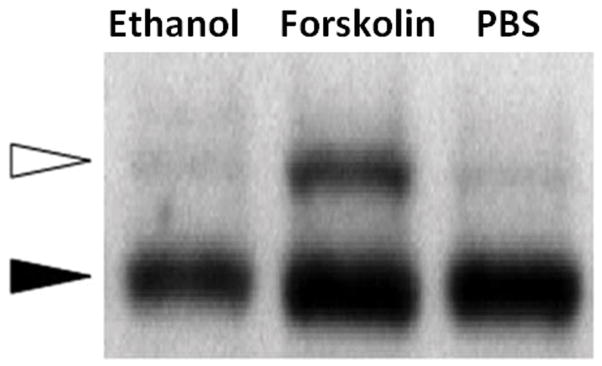
CFTR-mediated anion secretion is regulated by cAMP/PKAdependent second messenger signaling, which results in phosphorylation of the regulatory (R)-domain. To determine whether ethanol works via these second messenger pathways, intracellular cAMP, and a gel shift assay used to detect R-domain phosphorylation were utilized. Ethanol applied to MNSE cultures at 0.25, 0.5, and 1.0% (20 minutes) did not increase cAMP (in pmol/ml) in contrast to that seen with the positive control 100 nM forskolin (Figure 6A). There was no elevation of cellular cAMP in ethanol treated cells compared to PBS control, but the forskolin positive control was markedly increased (forskolin (n = 3) = 389.3 ± 5.1 pmol/ml) compared to PBS control and PBS (n = 3) = 0.56 ± 0.02 pmol/ml; 0.1% ethanol (n = 3) = 0.53 ± 0.2 pmol/ml; 0.25% ethanol (n = 3) = 0.47 ± 0.02 pmol/ml; 0.5% ethanol (n = 3) = 0.64 ± 0.03 pmol/ml, p <0.05; Post-hoc Tukey Q = 4.66). Additionally, ethanol did not create a 2–4 kD shift and thus does not confer phosphorylation of the CFTR R-domain (Figure 6B).
Figure 6. Ethanol does not increase cellular cAMP or mediate phosphorylation of the CFTR R-domain.
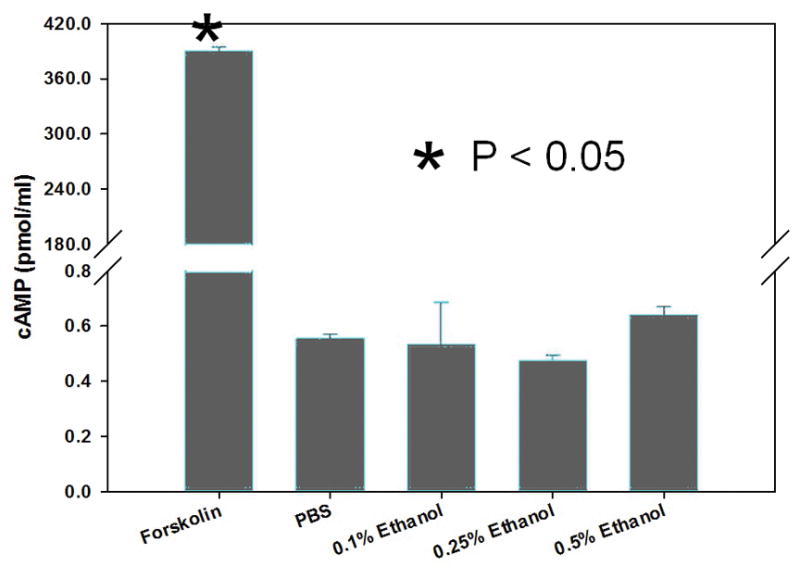
A: Cellular cAMP was minimal in the presence of ethanol and not significantly changed when compared to PBS controls. Forskolin serves as a positive control. * Statistical significance (p < 0.05)
B: Polyclonal NIH-3T3 cells expressing HA-tagged R-domain (R-D) were treated with ethanol for 20 minutes, and compared to the adenylate cyclase agonist forskolin (100 nM) or PBS control in an assay of R-D phosphorylation. Phosphorylation results in a 2–4 kD shift in migration (white arrow).
Ethanol Activates CBF in vitro
CBF is an important component of MCC and to determine whether ethanol activates CBF, the drug was applied to the apical membranes and compared to corresponding vehicle controls. Ethanol significantly increased CBF in wild type MNSE cultures in a dose dependent fashion compared to control [PBS, 1.33 ± 0.04 CBF fold changes; 0.25% Ethanol, 1.37 ± 0.09 CBF fold changes; 0.5% Ethanol, 1.53 ± 0.06 CBF fold changes (p = 0.03), 1% Ethanol, 1.62 ± 0.1 CBF fold changes (p = 0.04)] (Figure 7). To demonstrate the contribution of NO in stimulation of CBF, CBF was also measured with and without L-NAME with stimulation of 1% Ethanol. There was no statistical difference in CBF with stimulation of 1% Ethanol with and without L-NAME (1mM) [1% Ethanol without L-NAME = 1.47 ± 0.08 CBF fold changes, 1% Ethanol with L-NAME = 1.52 ± 0.14 CBF fold changes, p = 0.81].
Figure 7. Ethanol Activates CBF.
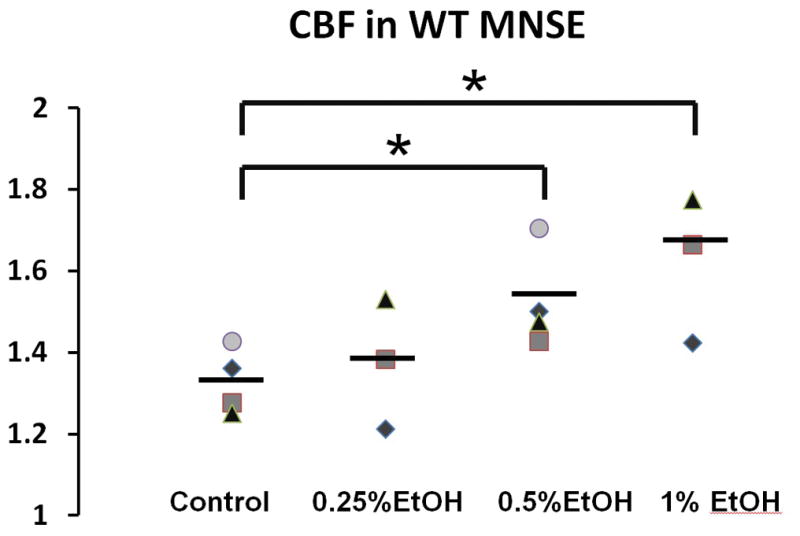
Ethanol significantly increased CBF in wild type MNSE cultures in a dose dependent fashion [PBS, 1.33 ± 0.04 CBF fold changes; 0.25% Ethanol, 1.37 ± 0.09 CBF fold changes; 0.5% Ethanol, 1.53 ± 0.06 CBF fold changes (p = 0.03), 1% Ethanol, 1.62 ± 0.1 CBF fold changes (p = 0.04)]. * Statistical significance (p < 0.05)
DISCUSSION
Airway epithelial ion transport regulates the homeostasis of airway surface liquid, essential for airway mucosal immunity and sinonasal host defense. Here, air-liquid interface cultures of murine and human epithelial cells were briefly exposed to physiologically relevant conditions of ethanol (0.25% (43mM), 0.5% (85.5mM) and 1.0% (171mM)) and Cl− transport was measured using an Ussing chamber. To our knowledge, this is the first paper to demonstrate that brief exposures of ethanol stimulate transepithelial Cl− secretion through CFTR-mediated pathways. This finding is supported by: 1) a significantly decrease in ΔIsc with chemical inhibition (INH-172) in WT airway epithelial monolayers from both murine and human cells, 2) an absence of Cl− secretion in CFTR−/− MNSE and HSNE lacking CFTR and 3) a significant polarization after infusing ethanol during murine nasal potential difference measurements in vivo. CFTR-mediated anion secretion is regulated by cAMP/PKAdependent second messenger signaling, which results in phosphorylation of the regulatory (R)-domain. In order to determine whether ethanol works via these second messenger pathways, cAMP and a gel shift assay used to detect R-domain phosphorylation were utilized. However, ethanol did not increase cAMP in contrast to that seen with the positive control forskolin. Additionally, ethanol did not confer phosphorylation of the CFTR R-domain.
The effect of ethanol on ciliary beating was first described by Maurer et al. in 1988.20 In their study, high concentrations of ethanol have been shown to slow or arrest ciliary beating but an unexpected stimulation of ciliary beating was noticed at low levels of ethanol from 0.01% to 1%. Since then, moderate brief alcohol exposure has been known to activate ciliary motility through a nitric oxide- and protein kinase-dependent pathway, which in theory should enhance MCC. 21–23 In an isolated demembranated cilia model from bovine trachea, ethanol rapidly stimulated axoneme beating 40% above baseline at very low concentrations (1–10 mM).24 This activation required the synthesis of nitric oxide (NO), the activation of soluble adenylyl cyclase (sAC) and the activation of both cAMP- and cGMP-dependent kinases (PKA and PKG). However, our experiment (at higher concentration, 1% of Ethanol = 171mM) found that ethanol does not increase cellular cAMP but stimulates CFTR-mediated Cl− transport independent of R-D phosphorylation, indicating the mechanistic effects of this compound may act through more direct opening of CFTR pore forming elements.25 Given the absence of stimulation of calcium-activated Cl− channels in monolayers lacking functional CFTR, stimulation of nitric oxide pathways and release of intracellular calcium seems unlikely. However, to demonstrate the possible role of NO in stimulation of CBF, CBF was measured with and without NOS inhibitor (L-NAME). There was no statistical difference in CBF with and without L-NAME with stimulation of 1% ethanol. The effects of ethanol on CFTR appear to be a direct stimulation on channel itself, independent of NO production. Importantly, the effects of brief exposures described in this study are in direct contrast to heavy and prolonged alcohol intake which is known to impair mucociliary clearance through down-regulation of the same cyclic nucleotide-dependent kinase-signaling pathway described previously.26 In addition, Raju et al. demonstrated that basolateral ethanol exposure (0, 25, 50 and 100 mM) for 24 hours reduced epithelial short-circuit currents (ISC) in a dose-dependent manner.27
In figure 3, total stimulated ΔISC following application of ethanol + forskolin was statistically higher than those with PBS + forskolin (p = 0.002). We may hypothesize that specific interactions between CFTR and ethanol might also play a role in the CFTR trafficking process in nasal epithelial cells. Further investigation is warranted to delineate this interesting interaction.
Ethanol-based formulations have been widely used in airway nebulizer preparations because the solvent provides a simple means of generating isotonic liposome preparations with reliable physical stability and output rate.28,29 The observation that ethanol stimulates transepithelial Cl− secretion via CFTR and increases CBF indicates possible use as topical aerosol delivered alone or in combination with other CFTR activators for diseases of dysfunctional MCC. The same intervention could be useful to activate residual CFTR (e.g. in the setting of F508del-CFTR pharmacologic correction) and ameliorate underlying mucus obstruction in CF-associated sinusitis. In addition, it should be noted that CFTR activation by potentiators dissolved in ethanol may overestimate potency, and our findings emphasize the need for stringent vehicle controls during experimental testing of CFTR modulators in vitro and in vivo.
CONCLUSIONS
The observation that brief exposure of ethanol stimulated Cl− secretion via CFTR and significantly increased CBF indicates possible use as topical aerosol delivered alone or in combination with other CFTR activators for diseases of dysfunctional MCC. Augmenting fluid and electrolyte secretion represents a means of improving mucus clearance in individuals affected by acute or chronic rhinosinusitis, specifically in the setting of WT CFTR.
Footnotes
Manuscript presented at the American Rhinologic Society Meeting for COSM, Boston, MA, April 24th, 2015.
The authors have no conflicting financial interests to disclose.
References
- 1.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. The Laryngoscope. 2012;122:1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. The Laryngoscope. 2011;121:1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EH, Tang XX, Shah VS, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. International forum of allergy & rhinology. 2014 doi: 10.1002/alr.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PloS one. 2014;9:e104090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Nandi I, Kim KH. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. International journal of pharmaceutics. 2002;237:77–85. doi: 10.1016/s0378-5173(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 6.Lu HT, Chen RN, Sheu MT, Chang CC, Chou PY, Ho HO. Rapid-onset sildenafil nasal spray carried by microemulsion systems: in vitro evaluation and in vivo pharmacokinetic studies in rabbits. Xenobiotica; the fate of foreign compounds in biological systems. 2011;41:567–577. doi: 10.3109/00498254.2011.563877. [DOI] [PubMed] [Google Scholar]
- 7.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. American journal of rhinology. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 8.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. American journal of rhinology. 2007;21:533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 9.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:235–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 10.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. BioTechniques. 2007;43:195–196. 198. doi: 10.2144/000112531. 200 passim. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23:149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 13.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. The Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 15.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. The Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. The Laryngoscope. 2011;121:1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:307–312. doi: 10.2500/ajra.2011.25.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazrak A, Jurkuvenaite A, Ness EC, et al. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. American journal of respiratory cell and molecular biology. 2014;50:953–962. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PloS one. 2013;8:e81589. doi: 10.1371/journal.pone.0081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer DR, Liebman J. Effects of ethanol on in vitro ciliary motility. Journal of applied physiology. 1988;65:1617–1620. doi: 10.1152/jappl.1988.65.4.1617. [DOI] [PubMed] [Google Scholar]
- 21.Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. The American journal of physiology. 1995;268:L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- 22.Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcoholism, clinical and experimental research. 1999;23:1528–1533. [PubMed] [Google Scholar]
- 23.Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. The American journal of pathology. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcoholism, clinical and experimental research. 2009;33:610–616. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cellular and molecular life sciences: CMLS. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PubMed] [Google Scholar]
- 26.Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcoholism, clinical and experimental research. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raju SV, Wang G. Suppression of adenosine-activated chloride transport by ethanol in airway epithelia. PloS one. 2012;7:e32112. doi: 10.1371/journal.pone.0032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhissi A, Gill H, Ahmed W, Taylor K. Vibrating-mesh nebulization of liposomes generated using an ethanol-based proliposome technology. Journal of liposome research. 2011;21:173–180. doi: 10.3109/08982104.2010.505574. [DOI] [PubMed] [Google Scholar]
- 29.Elhissi AM, Karnam KK, Danesh-Azari MR, Gill HS, Taylor KM. Formulations generated from ethanol-based proliposomes for delivery via medical nebulizers. The Journal of pharmacy and pharmacology. 2006;58:887–894. doi: 10.1211/jpp.58.7.0002. [DOI] [PubMed] [Google Scholar]