Abstract
Nasal vaccines are very effective but the olfactory organ provides direct access of antigens to the brain. Infectious hematopoietic necrosis virus (IHNV) is known to cause high mortalities in salmonids. The purpose of this study is to evaluate the safety of a live attenuated IHNV nasal (I.N) vaccine in rainbow trout (Oncorhynchus mykiss). In the olfactory organ, the vaccine was detected 1 and 4 days after primary I.N vaccination but not in the intramuscular (i.m) or control groups. In the brain, IHNV was detected by RT-qPCR 4 and 21 days after i.m primary vaccination. One i.m and one I.N vaccinated trout were positive at days 4 and 28 days post-boost, respectively. Presence of IHNV in the brain of i.m vaccinated fish correlated with moderate increases in IL-1β and TNF-α expression in this tissue. These results demonstrate that IHNV vaccine lasts for 4 days in the local nasal environment and that nasal vaccination appears to be safe to the CNS of rainbow trout.
Keywords: Safety, Nasal vaccines, Rainbow trout, Central nervous system, Infectious hematopoietic necrosis virus
1. Introduction
Vaccination induces protective immunity through activation of the host's immune system and ideally has no adverse effects. Killed vaccines are usually very safe, but do not mimic the immune response elicited by a live pathogen such as an attenuated vaccine. While live attenuated vaccines are highly immunogenic and lead to high levels of protection, they may cause safety issues particularly in immunocompromised hosts [1].
Nasal vaccines are licensed for use in humans and other animals (cats, dogs, etc.). However, the usage of nasal vaccines in humans has been demonstrated to negatively affect the CNS. The notable Bell's palsy cases reported in Switzerland in 2000–2001 showed inflammation of the CNS due to ganglioside-binding Escherichia coli heat labile toxin adjuvant present in the influenza virus vaccine formulation [2,3]. While concern has arisen in instances of nasal vaccination such as the Bell's Palsy case, nasal vaccination continues to remain widely used.
Fish vaccination plays an important role in controlling infectious diseases that threaten the fish farming industry [4,5]. While several delivery methods of vaccination are available (including immersion, oral delivery and injection vaccination) [6], injection vaccination is the most widely used vaccination method for disease control in aquaculture [7,8]. Recently, a fourth delivery method, the nasal vaccination, has been shown to be potentially useful in aquaculture [5,9].
Infectious hematopoietic necrosis virus (IHNV) is a virus of the genus Novirhabdovirus [10] and the causative agent of infectious hematopoietic necrosis (IHN), one of the most serious threats to salmonid fishes. IHN outbreaks can cause more than 80% mortality rates in certain cases [11]. Interestingly, IHN can have both hematopoietic and neurotropic manifestations [12]. We have previously shown that the nasal route is extremely effective at protecting rainbow trout against IHNV when using a live attenuated IHNV vaccine [5,9]. However, due to the direct connection of the olfactory system to the CNS via the olfactory bulb, as well as the live nature of the vaccine and the neurotropic potential of this virus, we asked whether nasal vaccination leads to antigen access to the CNS of rainbow trout. We report here that nasal vaccination of 5 g rainbow trout with live attenuated IHNV vaccine is overall safe to the CNS based on molecular and histological studies.
2. Materials and methods
2.1. Animals and vaccination trials
Specific-pathogen-free (SPF) rainbow trout (mean weight, 5 g) were obtained from Clear Springs Foods, Inc. Fish maintenance and rearing conditions as well as live attenuated IHNV viral vaccination trials were conducted as previously reported [5]. Briefly, specific-pathogen-free (spf) rainbow trout (4 g mean weight) were obtained from Clear Springs Foods Inc. (Buhl, Idaho). Fish were maintained in 378 L tanks that received single-pass spf spring water at a constant temperature of 14.5 °C and a dissolved oxygen content of 9.2 ppm. Fish were fed twice daily a commercial rainbow trout diet (Clear Springs Foods, Inc.).
The three experimental groups included mock vaccinated (saline I.N and i.m), I.N (attenuated vaccine) and i.m (attenuated vaccine) vaccinated groups. Fish received either a primary vaccination alone or an additional booster vaccination 28 days after the primary vaccination using the same vaccine delivery method. Boosting was performed on day 28 since at this point rainbow trout are known to have established an efficient adaptive immune response. Moreover, although a recommendation for humans, the Centers for Disease Control and Prevention (CDC) recommends spacing live viral vaccine administration at least 28 days apart. Fish were vaccinated by pipetting 25 µl of live attenuated IHNV into the right nare (I.N) or through injection of 25 µl of the same vaccine into the dorsal musculature (i.m) anterior to the dorsal fin as previously described [5]. Both olfactory rosettes and the entire brain of each fish (n = 5–6) were dissected out using sterile forceps and scalpel. Fish were sampled at days 1, 4, 7, 14, 21 and 28 days post-primary immunization (dpi), and after boosting fish were sampled (n = 6) at days 4,14, and 28 days post-boost (dpb) in order to reflect the kinetics of ectothermic vertebrates innate (7 dpi) and adaptive (28 dpi) immune responses.
2.2. Detection of IHNV and pro-inflammatory cytokines
RNA was extracted as explained elsewhere [5], cDNA was synthesized and RT-qPCRs were performed as previously described [13]. Positive IHNV detection was then confirmed for the IHNV G protein amplicon, 113 bp [14], via RT-PCR. Products were run in a 2% agarose gel in order to confirm that the detection was accurate. Specific primer sequences (5′–3′) were used to determine the presence of IHNV (IHNV-G1035F: CATGTCCATCCCCCAGAACT; IHNV-G1147R: GGACAACTGTTCCACCTTGTGTT; Accession Number: L40883) [14]. All RT-qPCR positive samples for IHNV were confirmed positive by RT-PCR.
To measure the expression of pro-inflammatory cytokines, trout elongation factor EF-1α (primers 5′–3′: EF-1aF: CAACGATATCCGTCGTGGCA; EF-1aR: ACAGCGAAACGACCAAGAGG; Accession number: AF498320) was used as a control. IL-1β was chosen as a marker for brain inflammation since it is known to be the major pro-inflammatory cytokine triggered by rhabdoviruses in brain tissue of other animal species [15]. Additionally, the expression of two other pro-inflammatory cytokine genes, TNF-α and IFNγ, was also quantified. Specific primer sequences (5′–3′) used were: IL1bF: ACATTGCCAACCTCATCATCG; IL1bR: TTGAGCAGGTCCTTGTCCTTG; TNFaF: GGGGACAAACTGTGGACTGA; TNFaR: GAAGTTCTTGCCCTGCTCTG; IFNgF: GCTGTTCAACGGAAAACCTGTTT; IFNgR: TCACTGTCCTCAAACGTG [16]. The relative expression level of the cytokine genes was determined using the Pfaffl method [17] and qPCR results were analyzed by T-test to identify statistically significant differences between groups (p < 0.05).
2.3. Histology
The brains (n = 6) of trout from each experimental group were sampled after primary vaccination as described above. Brains were fixed in 10% neutral buffered formalin overnight and then transferred to 70% ethanol. Tissues were embedded in paraffin and 5 µm thick sections (ten sections per sample) were stained using routine hematoxylin-eosin stain. Slides were observed under a Zeiss Axioscope microscope coupled to a digital camera using the Axiovision V.2 software.
3. Results
3.1. Detection of IHNV RNA in the olfactory organ following vaccination
We measured the presence or absence of the live attenuated IHNV vaccine in the olfactory organ of trout in all groups. All (100%) I.N vaccinated fish tested positive for the vaccine RNA 1 dpi. Additionally, 4/5 (80%) fish tested positive for the IHNV RNA 4 dpi (Table 1). The vaccine was no longer detectable in the olfactory system 7 dpi and beyond. In the i.m vaccinated group, no fish tested positive for IHNV RNA at any of the sampling points, similar to the control group. In the booster vaccination portion of the trial no fish tested positive for IHNV RNA in either treatment group.
Table 1.
Presence of IHNV RNA in rainbow trout olfactory tissue following primary or booster i.m or I.N vaccination.
| Days | 1 | 4 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|---|
| i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
|
| Primary | 0/5 5/5 |
0/5 4/5 |
0/5 0/5 |
0/5 0/5 |
0/5 0/5 |
0/5 0/5 |
| Booster | Not tested | 0/6 0/6 |
Not tested | 0/6 0/6 |
Not tested | 0/5 0/5 |
3.2. Detection of IHNV in the CNS following vaccination
In order to examine the ability of a live attenuated IHNV vaccine to cross the blood brain barrier, brain tissue from mock, I.N, and i.m. vaccinated trout was examined for the presence of the IHNV G protein transcripts by RT-qPCR. IHNV RNA was detected in the brain tissue of 3/5 (60%) i.m vaccinated fish 4 dpi and in 1/5 (20%) of the i.m vaccinated fish at 21 dpi (Table 2). All I.N vaccinated fish were negative for IHNV G protein transcripts. Following booster vaccination, IHNV RNA was detected in 1/6 (16.6%) i.m vaccinated (4 dpb) and 1/6 (16.6%) I.N. vaccinated trout (28 dpb). In both the primary and booster vaccination trial all mock-vaccinated trout tested negative.
Table 2.
Presence of IHNV RNA in rainbow trout brain following primary and booster i.m or I.N vaccination.
| Days | 1 | 4 | 7 | 14 | 21 | 28 |
|---|---|---|---|---|---|---|
| i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
i.m I.N |
|
| Primary | 0/5 0/5 |
3/5 0/5 |
0/5 0/5 |
0/5 0/5 |
1/5 0/5 |
0/5 0/5 |
| Booster | Not tested | 1/6 0/6 |
Not tested | 0/6 0/6 |
Not tested | 0/4 1/6 |
3.3. Expression of pro-inflammatory cytokines in the brain of rainbow trout following i.m or I.N IHNV vaccination
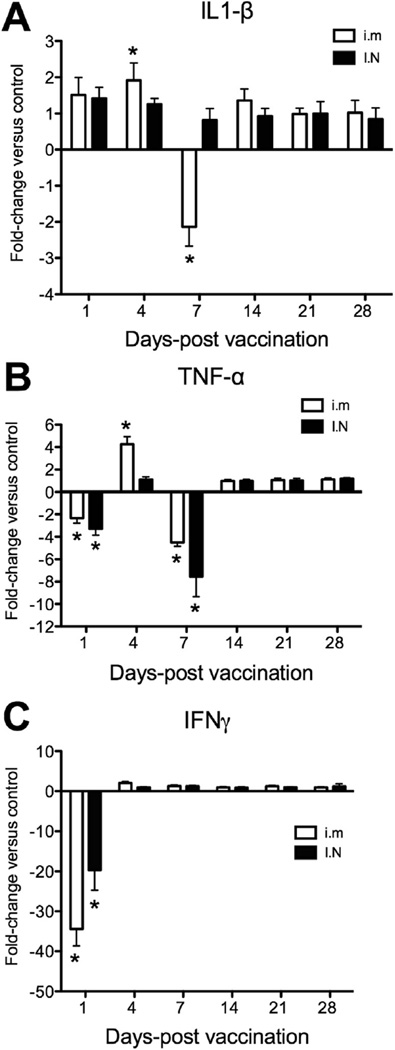
We examined the modulation of three pro-inflammatory cytokines in the CNS of rainbow trout following primary vaccination with live attenuated iHNV. Approximately a 2-fold up-regulation of IL-1β was detected in 4/5 (80%) i.m. vaccinated trout at 4 dpi, whereas a ~2-fold down-regulation occurred in 4/5 (80%) i.m vaccinated fish at 7 dpi (Fig. 1A). Trout that received an I.N. vaccination experienced no significant changes in IL-1β expression levels compared to the control group (Fig.1A). With regards to TNF-α, we observed a significant down-regulation in expression in both i.m and I.N groups on day 1 (≈2-fold and 3-fold, respectively) (Fig. 1B). On day 4, TNF-α expression was significantly up-regulated (4.25-fold) in the i.m but not I.N vaccinated group. On day 7, a second down-regulation in expression was observed in both vaccinated groups (approximately 4.5-fold) with no further changes in expression observed subsequently (Fig. 1B). IFNγ expression was significantly down-regulated on day 1 in both the i.m vaccinated group (32-fold) and I.N vaccinated group (14-fold) (Fig. 1C). Similar to what was observed for the other two genes, the i.m but not the I.N vaccinated group showed a significant change in IFNγ expression in the brain 4 dpi, with a 2-fold increase in expression compared to control fish. No changes in expression were recorded thereafter (Fig. 1C).
Fig. 1.
Fold-change expression of A) IL1-β B) TNF-α and C) IFNγ in the brain of I.N or i.m IHNV vaccinated rainbow trout compared to mock-vaccinated trout at different time points following primary vaccination. Bars represent mean fold change ± standard error (N = 5). Asterisks denote statistically significant differences (p < 0.05).
Histological examination of the brain tissue of all groups failed to identify any signs of tissue damage or inflammatory cell infiltration into the CNS (not shown) although no markers for immune genes or leucocytes were used in the present study.
4. Discussion
Vaccines must pose no threats to the host and safety testing is essential prior to vaccine licensing. Vaccines delivered intranasally can be effective both in aquatic and terrestrial animals but the safety of nasal vaccination in fish had not been evaluated to date. Since cases of cerebral Bell's palsy linked to influenza virus nasal vaccination were reported in humans, we were interested in determining possible safety issues of nasal vaccination with IHNV in rainbow trout.
Our results demonstrate that following nasal vaccination, the IHNV vaccine remains at detectable levels in the olfactory organ for at least 4 days but then is cleared, likely by a number of mechanisms, including uptake by infiltrating immune cells. This kinetics mirrors our previous results where we showed that the peak of the NALT innate immune response at the transcriptome level takes place 4 days post nasal vaccination with the same vaccine [5]. In the booster experiment, we could not detect IHNV RNA at 4 dpb, an indication that faster antigen clearance takes place in trout upon secondary encounter with the antigen (memory response). Interestingly, we found one I.N vaccinated fish (16.6%) with detectable IHNV RNA at 28 dpb. Larger sampling sizes may have revealed different proportions but in any case, this result suggests a few proportion of the animals may mount insufficient memory immune responses to the initial vaccination. However, given that 83.3% of the fish given a booster vaccination were able to clear the antigen successfully indicating that, in a majority of the cases, local memory immunity is effective at clearing the antigen upon secondary encounter.
Live attenuated viral vaccines, particularly neurotropic viruses, may pose a problem for the host's nervous system if the vaccine travels via the olfactory bulb to the brain. Our results clearly demonstrate that I.N vaccination is a safe vaccination method for the CNS of rainbow trout at the age group tested in this study. These findings indicate that following nasal vaccination the olfactory organ and/or the olfactory bulb do not allow the live vaccine to reach the CNS. This is in agreement with the observation that despite the olfactory mucosa serving as a conduit for a number of viruses to enter the brain, infections in the CNS rarely occur [18]. However, previous work in our laboratory found vaccine-associated mortalities when nasal vaccination with IHNV was performed in very young trout (~2.3 g), indicating the possibility that such mechanisms are not fully functional in younger fish [19].
Surprisingly, we found that primary i.m. vaccination can lead to presence of viral RNA in the trout CNS and to modulation in the expression of three different pro-inflammatory genes (IL-1β, TNF–α and IFNγ). Since IHNV is neurotropic, these results indicate that systemic delivery of the vaccine is sufficient for the live attenuated virus to reach the CNS. On the other hand, I.N vaccination did not result in the presence of viral RNA in the CNS. Despite the absence of the antigen, we still recorded a significant down-regulation in the gene expression of TNF-α and IFNγ in the brain of I.N vaccinated fish. I.N vaccination did not, however, lead to increased proinflammatory cytokine expression in the brain. Thus, CNS cytokine response following nasal vaccination must be triggered via other mechanisms. The CNS of teleosts contains large numbers of leucocytes, mostly lymphocytes and macrophages [20], therefore it is likely that CNS resident leucocytes are sources of IL-1β, TNF-α and IFNγ, although neuronal sources cannot be ruled out. Additionally, no obvious signs of inflammation were observed after histological examination, thus it is likely that the increases in IL-1β and TNF-α expression recorded do not result in serious damage to the CNS of rainbow trout. Alternatively, we cannot rule out that tissue damage may occur at a later time point that when gene expression is detected. In any case, mild inflammation in the CNS as a result of vaccination may be beneficial to the host if it occurs under controlled conditions and may protect the host against viral infection as shown in a mouse model [21].
5. Conclusion
In conclusion, we demonstrate that nasal vaccination with a live attenuated IHNV vaccine is a safe vaccination method for rainbow trout (5 g) whereas mild inflammatory responses can occur in the CNS following i.m injection vaccination. Further studies will address whether induction of mild inflammatory responses in the CNS can be advantageous to the host and help clear neurotropic IHN upon secondary encounter with the pathogen.
Acknowledgments
This work was funded by USDA AFRI Grant# 2DN70-2RDN7 to IS. ETL was funded by the Initiatives to Maximize Student Development (IMSD) program.
Abbreviations
- i.m
intramuscular
- I.N
intranasal
- CNS
central nervous system
- IHNV
infectious hematopoietic necrosis virus
- spf
specific-pathogen-free
- dpi
days post-immunization
- dpb
days post booster
Footnotes
The authors declare no conflict of interest.
Contributor Information
Erin T. Larragoite, Email: Erin.Larragoite@utah.edu.
Luca Tacchi, Email: ltacchi@unm.edu.
Scott E. LaPatra, Email: scott.lapatra@clearsprings.com.
Irene Salinas, Email: isalinas@unm.edu.
References
- 1.Medical Advisory Committee of the Immune Deficiency Foundation. Shearer WT, Fleisher TA, Buckley RH, Ballas Z, Ballow M, et al. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J. Allergy Clin. Immunol. 2014;133:961–966. doi: 10.1016/j.jaci.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's Palsy in Switzerland. N. Engl. J. Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DJM, Zhiming H, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. Transient facial nerve paralysis (Bell's Palsy) following intransal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PloS One. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duff DCB. The oral immunization of trout against Bacterium salmonicida. J. Immunol. 1942;44:87–94. [Google Scholar]
- 5.Tacchi L, Musharrafieh R, Larragoite ET, Crossey K, Erhardt EB, Martin SA, LaPatra SE, Salinas I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014;5:5205. doi: 10.1038/ncomms6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinitantharat S, Kjersti G, Greger E. Fish vaccines. In: Schultz RD, editor. Advances in Veterinary Medicine. New York: Elsevier; 1999. pp. 539–550. [DOI] [PubMed] [Google Scholar]
- 7.Gudding R, Lillehaug A, Evensen O. Recent developments in fish vaccinology. Vet. Immunol. Immunopathol. 1999;72:203–212. doi: 10.1016/s0165-2427(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 8.Plant KP, LaPatra SE. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011;35:1256–1262. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 9.LaPatra SE, Kao S, Erhardt EB, Salinas I. Evaluation of dual nasal delivery of infectious hematopoietic necrosis virus and enteric red mouth vaccines in rainbow trout (Oncorhynchus mykiss) Vaccine. 2015;33:771–776. doi: 10.1016/j.vaccine.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki M, Kim HJ, Kasai H, Nishizawa T, Yoshimizu M. Virulence change of infectious hematopoietic necrosis virus against rainbow trout Oncorhynchus mykiss with viral molecular evolution. Jap. Soc. Fish. Pathol. 2009;44:159–165. [Google Scholar]
- 11.Wolf K. Infectious hematopoietic necrosis. In: Wolf K, editor. Fish Viruses and Viral Fish Diseases. Ithaca: Cornell University Press; 1988. pp. 83–114. [Google Scholar]
- 12.LaPatra SE, Lauda KA, Jones GR, Walker SC, Shewmaker BS, Morton AW. Characterization of IHNV isolates associated with neurotropism. Vet. Res. 1995;26:433–437. [PubMed] [Google Scholar]
- 13.Tacchi L, Larragoite E, Salinas I. Discovery of J chain in African lungfish (Protopterus dolloi, Sarcopterygii) using high throughput transcriptome sequencing: implications in mucosal immunity. PloS One. 2013;8:e70650. doi: 10.1371/journal.pone.0070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhar AK, Bowers RM, Licon KS, LaPatra SE. Detection and quantification of infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss) by SYBR green real-time reverse transcriptase-polymerase chain reaction. J. Virol. Methods. 2008;147:157–166. doi: 10.1016/j.jviromet.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Marquette C, Van Dam AM, Ceccaldi PE, Weber P, Haour F, Tsiang H. Induction of immunoreactive interleukin-1 beta and tumor necrosis factor-alpha in the brains of rabies virus infected rats. J. Neuroimmunol. 1996;68:45–51. doi: 10.1016/0165-5728(96)00056-2. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Grabowski PS, Cunningham C, Secombes CJ. Molecular cloning of interleukin 1β from rainbow trout Oncorhynchus mykiss reveals no evidence of an ICE cut site. Cytokine. 1999;11:552–560. doi: 10.1006/cyto.1998.0470. [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori I, Nishiyama Y, Yokochi T, Kimura Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- 19.Salinas I, Erhardt EB, LaPatra SE. Nasal vaccination of young rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis and enteric red mouth disease. Dev. Comp. Immunol. 2015;53:105–111. doi: 10.1016/j.dci.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Dowding AJ, Scholes J. Lymphocytes and macrophages outnumber oligodendroglia in normal fish spinal cord. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10183–10187. doi: 10.1073/pnas.90.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 2007;196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]