Abstract
Rosai-Dorfman disease is a rare benign histiocytic disease that infrequently presents in the spine. We report a case of Rosai-Dorfman disease isolated to the epidural thoracic spine in a 26-year-old male. To our knowledge, this is the 15th reported case of isolated spinal disease and only the fourth case of isolated thoracic epidural disease. Given its rarity as well as non-specific symptoms and imaging findings, Rosai-Dorfman disease is often not considered and misdiagnosed on imaging studies. To help improve awareness of Rosai-Dorfman spinal disease, we review the literature and discuss the epidemiology, clinical presentation, imaging features, and treatment considerations for this condition.
Keywords: Rosai-Dorfman, spine, thoracic, epidural, sinus histiocytosis with massive lymphadenopathy, histiocytic disease, MRI
CASE REPORT
A 26-year-old man presented with two weeks of progressive bilateral lower extremity numbness, weakness, and gait difficulty. He denied bowel or bladder incontinence, back pain, fever, weight loss, or night sweats. There was no history of trauma. A review of personal and family medical history was unremarkable. He was not taking any medications. He endorsed occasional use of cigarettes and alcohol, but he denied use of intravenous drugs. On physical exam, the patient was afebrile. He had bilateral decreased sensation below the knees, decreased strength at the extensor hallucis longus, and patellar hyper-reflexia. His gait was unsteady. His exam demonstrated no other neurologic deficits or spinal tenderness. Complete blood count, basic metabolic panel, urinalysis, liver function tests, toxicology screen, vitamin B12, and thyroid stimulating hormone (TSH) were within reference range. Erythrocyte sedimentation rate (ESR) was mildly elevated at 19 mm/hr (ref: 0–15 mm/hr). A rapid human immunodeficiency virus (HIV) antibody test and rapid plasma reagin (RPR) were negative.
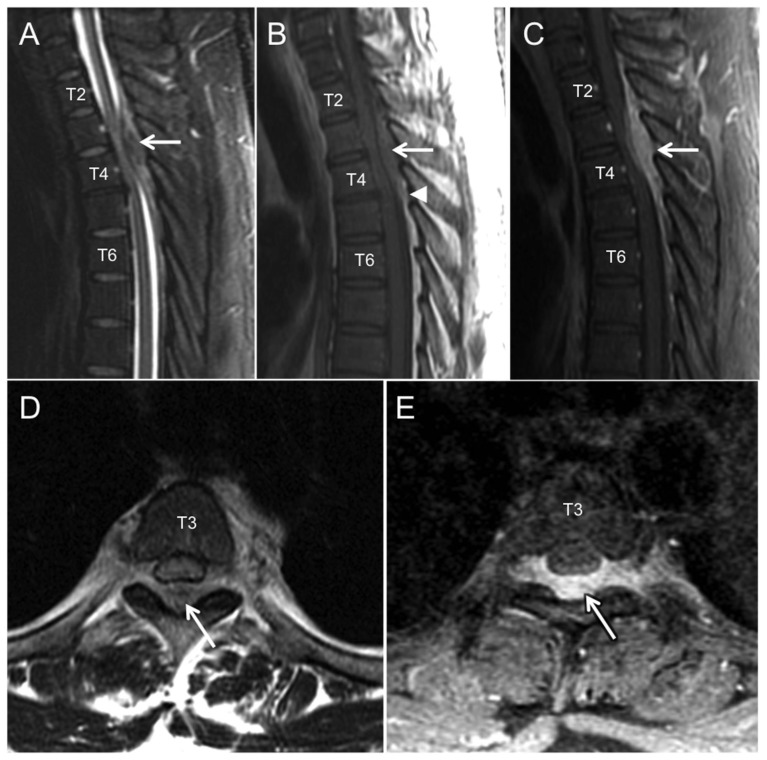
With his progressive neurologic symptoms indicating a possible myelopathy, magnetic resonance imaging (MRI) of his cervical, thoracic, and lumbar spine was performed. The MRI revealed a dorsal epidural mass extending from the T1 to T5 vertebral levels (Figures 1A–E). The lesion caused cord compression with a corresponding intramedullary spinal cord T2 hyper-intensity (Figures 1A and 1D). The lesion was iso-intense to the spinal cord on T1 weighted (T1WI) sequences (Figure 1B) and heterogeneously hypo-intense on T2 weighted (T2WI) sequences (Figures 1A and 1D) with homogenous enhancement following gadolinium administration (Figures 1C and 1E). No further abnormalities were observed in the vertebral column, spinal cord, or paraspinal soft tissues. With these imaging findings and the patient’s clinical picture, a malignant process (such as lymphoma or metastasis) was favored over an epidural hematoma or infectious etiology (abscess or phlegmon).
Figure 1.
26 year-old male with Rosai-Dorfman disease of the epidural thoracic spine.
Findings: On sagittal T2WI (A), sagittal T1WI (B), and sagittal T1WI post contrast (C) a mass spanning from the lower T1 to the T5 vertebral levels is observed in the dorsal epidural space (white arrows). The mass has heterogeneous T2 signal characteristics, iso-intense T1 signal relative to the spinal cord, and demonstrates avid, uniform enhancement. Mass effect includes severe narrowing of the spinal canal with spinal cord compression and subtle spinal cord intramedullary T2 hyper-intensity at the T3–T4 vertebral levels. Arrowhead in (B) shows the effacement of normal epidural fat by this mass at its inferior extent. Axial T2WI (D) and axial T1WI post contrast (E) MRI at the lower T3 level reveals the transverse extent of epidural mass (white arrows) with bilateral extension into the T3–T4 neural foramina and left greater than right extra-foraminal space. Selected vertebral levels are labeled within the vertebral body. No other abnormalities are observed in the vertebral column, spinal cord, or paraspinous soft tissues.
Technique: 1.5 Tesla General Electric (GE) Healthcare Genesis Signa HDxt scanner, software version 15 (GE Healthcare, Milwaukee, WI); sagittal T2 (TR 4016 ms, TE 105 ms, slice thickness 3 mm skip 1 mm), sagittal T1 (TR 500 ms, TE 13 ms, slice thickness 3 mm skip 1 mm), sagittal T1 post (TR 566 ms, TE 11 ms, slice thickness 3 mm skip 1 mm), axial T2 (TR 3666 ms, TE 106 ms, slice thickness 5 mm skip 1mm), axial T1 post (TR 616 ms, TE 19 ms, slice thickness 4 mm skip 0 mm).
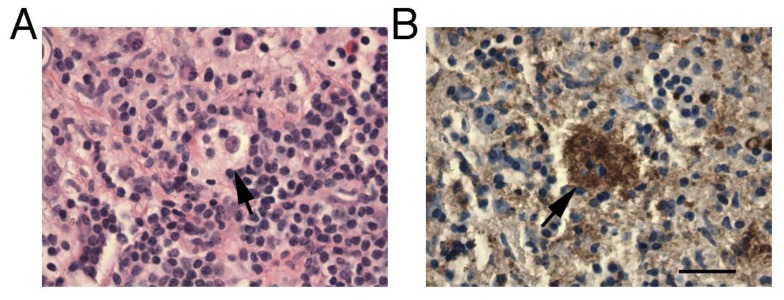
The patient was taken to the operating room to decompress the spinal canal and to obtain tissue for pathologic diagnosis. A laminectomy from T2–T5 was performed, which revealed a firm, well-encapsulated mass with high vascularity in the epidural space. This mass was resected. Pathology revealed fibroconnective tissue with a lymphoplasmacytic infiltrate and clusters of atypical histiocytes. These histiocytes demonstrated emperipolesis (Figure 2A). They also stained positive for S100 and negative for CD1a (Figure 2B). These findings were consistent with a diagnosis of Rosai-Dorfman disease. Flow cytometry and immunohistochemical analysis provided no evidence of lymphoma. A CT of the chest, abdomen, and pelvis as well as a brain MRI were performed to evaluate for other sites of disease in the body. No other lesions were identified.
Figure 2.
26 year-old male with Rosai-Dorfman disease of the epidural thoracic spine.
Findings: Histologic and immunohistochemical assessment demonstrating macrophages ingesting leukocytes (emperipolesis). A) Hematoxylin and Eosin staining of case show a mixed inflammatory infiltrate with a foamy macrophage containing other lymphoid cells (arrow). B) S-100 staining of sample showing positive cytoplasmic staining of foamy macrophage with emperipolesis. Bar= 100 um.
Following the resection, the patient had full resolution of his neurologic deficits. He only reported intermittent back pain, which began following the resection. Throughout a follow up period of 18 months, the patient remained otherwise asymptomatic.
DISCUSSION
Etiology & Demographics
Rosai-Dorfman disease, also known as sinus histiocytosis with massive lymphadenopathy, is a rare benign histiocytic disease. There are an estimated 100 new cases per year in the United States (Table I) [1]. Although Rosai-Dorfman disease has been reported in all age groups, it most frequently presents in children and young adults [1]. It is also slightly more common among males (1.4:1 male to female ratio) [2,3]. The etiology of Rosai-Dorfman disease is unknown. Although there is some evidence that viral infections (including human herpesvirus 6 [HHV-6] and Epstein-Barr virus [EBV]) or immune dysfunction may play a role, these etiologies have not been definitively established [2,4–10]. Molecular studies have demonstrated that Rosai-Dorfman disease is a polyclonal disorder, suggesting the disease is reactive rather than neoplastic [11].
Table 1.
Summary table for Rosai-Dorfman disease, both for extra-CNS disease and for isolated spinal disease.
| Extra-CNS Disease | Isolated Spinal Disease | |
|---|---|---|
| Etiology | Idiopathic | Idiopathic |
| Incidence | 100 cases per year in US | 15 reported cases (6 epidural, 7 intradural, 2 intramedullary) |
| Gender Ratio (M:F) | 1.4:1 | 1.5:2 |
| Age Predilection | Mean age 21 years | Mean age 40 years |
| Risk Factors | Unknown | Unknown |
| Clinical Symptoms |
|
|
| Laboratory Findings | Anemia, leukocytosis, elevated ESR, and/or polyclonal hypergammaglobulinemia reported in most patients | Anemia, leukocytosis, elevated ESR, polyclonal hypergammaglobulinemia occur variably |
| Imaging Findings | Variable CT and MRI findings depending on location |
|
| Treatment |
|
|
| Prognosis | Good | Good |
In Rosai-Dorfman disease, nonmalignant histiocytes proliferate and infiltrate nodal and extra-nodal tissue. It most often presents as massive and painless cervical lymphadenopathy with fever, malaise, weight loss, leukocytosis, polyclonal hypergammaglobulinemia, and an elevated ESR. Over 40% of patients develop extra-nodal disease, with sites including the skin, upper respiratory tract, salivary glands, orbit, eye, GI tract, breast, bone (including vertebral), and the heart [12–16].
Rosai-Dorman Disease in the Central Nervous System
Rosai-Dorfman disease within the central nervous system (CNS) is very rare, with less than 5% of reported cases documenting CNS involvement [17]. The majority of these cases have disease isolated to the CNS (>80%) and involvement of the intracranial space (>90%) [18]. Disease of the spine appears particularly uncommon [18]. In order to identify other case reports of spinal Rosai-Dorfman disease, we searched the English literature using PubMed for the period of 1969 to June 2015. Search terms included Rosai-Dorfman disease, sinus histiocytosis with massive lymphadenopathy, central nervous system, epidural, dural, and spine. Within the relevant manuscripts, we also reviewed all cited references to identify other case reports. Through this search, we identified less than 50 reported cases of Rosai-Dorfman disease with spinal involvement. There have been only 15 reported cases of disease isolated to the spine, including the present case (Tables II and III) [13,19–30]. Out of these 15 cases, six had epidural disease, seven had intradural disease, and two had intramedullary disease in the cord. The lesions most commonly occurred in the thoracic (ten cases) and/or cervical (seven cases) spine. To our knowledge, the current case represents just the fourth report of Rosai-Dorfman disease isolated to the epidural thoracic spine.
Table 2.
Case reports for Rosai-Dorfman disease isolated to the spine: summary of demographics and clinical symptoms.
| Author | Age | Gender | Spine Level | Location | Neurologic Symptoms | Systemic Symptoms |
|---|---|---|---|---|---|---|
| Andriko et al. 2001 | 51 | Male | Thoracic | Epidural | Acute onset paraplegia | N/A |
| Andriko et al. 2001 | 42 | Male | Thoracic | Epidural | 4–5 weeks of progressive numbness and weakness in lower extremities | N/A |
| Hargett et al. 2005 | 29 | Female | Thoracic | Epidural | 5 weeks of progressive gait difficulty, urinary and bowel difficulty, lower extremity numbness and weakness, back pain | No |
| Hollowell et al. 2000 | 78 | Male | Cervical, thoracic, lumbar | Epidural | 6 months of progressive back pain, sensory and strength deficits in left arm, leg weakness, urinary urgency | N/A |
| Maiti et al. 2011 | 19 | Female | Cervical | Epidural | 2 months of progressive neck pain, spastic quadriparesis | N/A |
| Bhandari et al. 2006 | 23 | Female | Cervical and thoracic | Intradural | 2.5 years of progressive lower extremity weakness, incontinence of urine and stool, sensory level at C6 | N/A |
| Chan et al. 1985 | 7 | Female | Cervical and thoracic | Intradural | 4 months of progressive weakness of all four limbs and neck stiffness | N/A |
| Wang et al. 2010 | 58 | Male | Thoracic | Intradural | 3 months of progressive leg weakness | N/A |
| Chen et al. 2012 | 16 | Female | Thoracic | Intradural | Acute onset of severe leg cramps, weakness, and numbness over days; urinary incontinence | N/A |
| Zhu et al. 2012 | 58 | Male | Thoracic | Intradural | Back pain and hypnalgia | N/A |
| Wu et al. 2014 | 43 | Male | Cervical | Intradural | 8 months of progressive back pain and numbness in hands | No |
| Fu et al. 2015 | 25 | Female | Cervical | Intradural | 6 months of progressive neck pain, weakness and numbness of upper extremities, unsteady gait | N/A |
| El-Molla et al. 2014 | 76 | Male | Cervical | Intramedullary | 10 weeks of progressive right arm weakness and right foot drop | N/A |
| Osenbach et al. 1996 | 35 | Male | Thoracic | Intramedullary | Several months of progressive numbness and weakness in lower extremities | No |
N/A = not reported in publication.
Table 3.
Case reports for Rosai-Dorfman disease isolated to the spine: summary of laboratory findings, imaging findings, treatment, and treatment outcomes.
| Author | Labs | MRI Findings | Treatment | Outcome |
|---|---|---|---|---|
| Andriko et al. 2001 | N/A | N/A | Resection | No recurrence at 3 months |
| Andriko et al. 2001 | N/A | N/A | Resection | No recurrence at 38 months |
| Hargett et al. 2005 | N/A | Homogenous enhancement ** | Resection and radiotherapy | Residual symptoms, no CNS recurrence at 4 years, later developed lymph node disease |
| Hollowell et al. 2000 | Mild anemia, elevated ESR, no leukocytosis, SPEP normal | T1WI iso-intense, T2WI diffusely hypo-intensity, heterogeneous enhancement | Resection | Residual symptoms, no recurrence at 1.5 years |
| Maiti et al. 2011 | Mild anemia, elevated ESR, leukocytosis, SPEP normal | T1WI iso-intense, T2WI heterogeneously hypo-intense, homogenous enhancement | Resection | No residual symptoms, no recurrence at 1 year |
| Bhandari et al. 2006 | No abnormalities | T1WI and T2WI hypo-intense, homogenous enhancement | Resection | N/A |
| Chan et al. 1985 | N/A | N/A | N/A | N/A |
| Wang et al. 2010 | N/A | T1WI and T2WI iso-intense, homogenous enhancement | Resection | No residual symptoms, no recurrence at 7 months |
| Chen et al. 2012 | Leukocytosis only | T1WI and T2WI intermediate intensity, enhancing* | Resection | No residual symptoms, no recurrence at 2 years |
| Zhu et al. 2012 | N/A | T1WI hyper-intensity, T2WI iso-intensity, homogenous enhancement | Resection | N/A |
| Wu et al. 2014 | N/A | Homogenous enhancement ** | Resection | No residual symptoms, no recurrence at 1.5 years |
| Fu et al. 2015 | No abnormalities | T1WI and T2WI hypo-intensity, homogenous enhancement | Resection | N/A |
| El-Molla et al. 2014 | N/A | Homogenous enhancement, cord expansion, edema ** | Resection | No residual symptoms, no recurrence at 1 years |
| Osenbach et al. 1996 | Elevated ESR only | Homogenous enhancement ** | Resection | Residual symptoms, residual abnormal signal without discrete lesion at 1 year |
N/A = Not reported in publication.
= Homogeneity of enhancement not reported in publication.
= T1WI and T2WI characteristics not reported in publication
Individuals with Rosai-Dorfman CNS disease tend to present at a later age (mean of 39 years) than those without CNS involvement (mean of 21 years) [13,18]. This was a consistent finding among those with disease isolated to the spine (median 35 years; range 7–78 years) or the epidural thoracic space (median 35.5 years, range 26–51 years) (Table I). Similar to systemic disease, spinal Rosai-Dorfman disease has occurred more frequently in males (nine cases) than females (six cases).
As seen with our patient, common presenting symptoms for patients with Rosai-Dorfman spinal disease include the progressive onset of limb paraparesis, limb sensory deficits, gait difficulty, urinary and bowel incontinence, and back pain (Table II). Generally, these symptoms develop over a course of weeks to months, although two cases reported the acute onset of symptoms over days [19,26]. While constitutional symptoms are frequently present in those with Rosai-Dorfman disease outside the CNS, constitutional symptoms are rare among those with isolated CNS disease [31]. In particular, none of the case reports of Rosai-Dorfman disease isolated to the spine documented the presence of constitutional symptoms, although only five of the reports specifically noted their absence. Laboratory abnormalities are variably observed among those with spinal Rosai-Dorfman disease. Of the seven cases reporting such data, ESR was elevated in four cases, leukocytosis occurred in two cases, and anemia was detected in two cases. No patient was reported to have polyclonal hypergammaglobulinemia.
Rosai-Dorfman disease within the CNS may also have implications for our understanding of CNS anatomy and physiology. Classic teaching maintains that the CNS is devoid of conventional lymphatic structures [32]. As discussed above, however, molecular studies indicate that Rosai-Dorfman disease is a reactive process, suggesting a lymphatic origin for the disease. Therefore, the presence of Rosai-Dorfman lesions within the CNS provides some evidence that CNS lymphatic structures indeed exist. Supportive of this conclusion is a recent study by Aspelund et al., which demonstrated the presence of dural-based lymphatic networks in transgenic mice [33].
Imaging Findings
Imaging findings for Rosai-Dorfman disease in the CNS are non-specific. As in the present case, isolated spinal disease appears as well-circumscribed dural or extra-dural masses on MRI. On T1WI, the majority of lesions are iso-intense to the spinal cord, although hyper-intense and hypo-intense lesions have been reported (Table III). On T2WI, the lesions are commonly hypo-intense or iso-intense. Homogeneous enhancement is observed following gadolinium administration.
These imaging findings for isolated spinal disease are consistent with published imaging findings for intracranial Rosai-Dorfman disease [34–37]. While computed tomography (CT) imaging was either not performed or not documented in the case reports for isolated spinal disease, Rosai-Dorfman lesions appear as hyper-dense and homogenously enhancing dural-based masses intra-cranially [13]. As was observed in the present case, a heterogeneous hypo-intensity on T2WI MRI has been reported for numerous cases of intracranial Rosai-Dorfman disease [13,35–39]. Several authors have proposed that this MRI feature may be helpful in distinguishing Rosai-Dorfman disease from meningiomas, which are more commonly iso- or hyper-intense on T2WI [40–42]. The foci of T2WI hypo-intensity may reflect the presence of free radicals produced by macrophages during phagocytosis, necrosis, fibrosis, or erythrophagocytosis [30]. Calcification is rare in Rosai-Dorfman disease and is not thought to contribute to the hypo-intense signal. Among cases of isolated spinal disease, only one other publication noted a heterogeneous T2WI hypo-intensity [22]. Yet, T2WI imaging findings or homogeneity of signal were unreported in a number of the case reports. Diffusion-weighted imaging (DWI) was not performed in our case. However, other authors have reported a heterogeneous signal on DWI with an increased signal on apparent diffusion coefficient (ADC) mapping for Rosai-Dorfman lesions within the CNS [43,44].
Differential Diagnosis
Because imaging findings for Rosai-Dorfman disease are non-specific, it is often misdiagnosed on imaging. As in the present case, the main differential diagnosis for Rosai-Dorfman disease localized to the epidural spine includes a hematoma, abscess or phlegmon, meningioma, metastasis, or lymphoma (Table IV). Although rarer, sarcoidosis and immunoglobulin G4 (IgG4)-related meningeal disease should also be considered. Early subacute or chronic hematomas can appear hypo-intense (sometimes with heterogeneity) on MRI T2WI; however, they are often associated with a history of trauma and typically do not homogenously enhance [45]. Epidural phlegmons diffusely enhance, while abscesses show peripheral enhancement [46]. These infectious processes are T2WI hyper-intense, and patients usually have constitutional symptoms. Characteristically, pyogenic infectious processes are hyper-intense on DWI with a corresponding low signal on ADC mapping [47]. Malignancies commonly homogenously enhance with contrast given their vascularity. Metastases demonstrate variable signal intensity of MRI depending on their cellularity, but they are often T1WI hypo-intense and T2WI hyper-intense [48]. These lesions tend to involve contiguous vertebral bodies. Lymphomas tend to be iso-intense on T1WI. On T2WI, they are usually iso- or hyper-intense; however, hyper-cellular lesions can display hypo-intensity [49]. Meningiomas show diffuse enhancement, T1WI iso-intensity, and T2WI iso- or hyper-intensity [50]. T2WI hypo-intensity occurs less frequently [13]. The vast majority of meningiomas are intradural rather than epidural [51]. Sarcoidosis can infrequently present with isolated spinal disease. In published case reports, MRI findings for extramedullary sarcoidosis include T1WI hypo- or iso-intensity, T2WI hyper-intensity, and homogenous enhancement [52,53]. Finally, there have been rare case reports of IgG4-related meningeal disease causing linear dural thickening or mass lesions within the spine [54–56]. On MRI, these lesions appear hypo- or hyper-intense on T2WI and they homogenously enhance.
Table 4.
Differential diagnosis for Rosai-Dorfman disease localized to the epidural spine and associated imaging findings on MRI.
| Disease | MRI |
|---|---|
| Rosai-Dorfman | T1WI iso- or hypo-intense; T2WI hypo- and/or iso-intense; homogenous enhancement |
| Meningioma | 95% intradural; T1WI iso-intense; T2WI iso-intense or hyper-intense > hypo-intense; homogenous enhancement |
| Metastasis | Vertebral involvement common; T1WI hypo-intense; T2WI hyper-intense > hypo-intense or iso- intense; homogenous enhancement |
| Sub-acute or Chronic Epidural Hematoma | Lentiform; early subacute = T1WI hyper-intense and T2WI hypo-intense; late subacute = T1WI and T2WI hyper-intense, chronic = T1WI and T2WI hypo-intense; peripheral or no enhancement |
| Epidural Abscess/phlegmon | T1WI iso- or hypo-intense; T2WI hyper-intense; peripheral enhancement (abscess); homogenous enhancement (phlegmon) |
| Lymphoma | T1WI iso-intense; T2WI iso- or hyper-intense > hypo-intense; homogenous enhancement |
| Sarcoidosis | T1WI hypo- or iso-intense; T2WI hyper-intense; homogenous enhancement |
| IgG4-Related Meningeal Disease | T2WI hypo- or hyper-intense; homogenous enhancement * |
= T1WI findings not reported
Pathologic Findings
Biopsy remains the gold standard for diagnosis. The histologic features of CNS Rosai-Dorfman disease include the presence of atypical histiocytes intermixed with plasma cells and lymphocytes. An increased background of collagen and/or reticulin fibers can be observed [19,20]. The histiocytes characteristically display emperipolesis. Emperipolesis is defined by the phagocytosis of intact lymphocytes by macrophages [22]. While this finding is consistently present in nodal disease, it is reported to occur in approximately 70% of cases with CNS disease [57]. Among the 13 cases of isolated spinal disease that reported pathology results, all had lesions demonstrating emperipolesis. Emperipolesis was previously seen rarely outside of Rosai-Dorfman disease. However, several studies have documented emperipolesis in rare cases B-cell lymphoma, autoimmune hemolytic anemia, myelosclerosis, carcinoma, neuroblastoma, multiple myeloma, and leukemia [20]. Histiocytes in Rosai-Dorfman disease additionally stain positive for the S100 protein and negative for the CD1a protein. Birbeck granules and calcification are not observed. These features help to exclude Langerhans cell histiocytosis, which typically shows positive staining for S100 and CD1a as well as the presence of Birbeck granules [25,41].
Treatment and Prognosis
There are no uniform treatment guidelines for Rosai-Dorfman disease given its rarity. Since it is a non-malignant disorder, treatment is generally advised only in those who are symptomatic or have vital organ involvement, such as the CNS. Spontaneous resolution has been documented in 20% of patients with Rosai-Dorfman disease; however, spontaneous resolution does not occur in patients with CNS lesions [18,20,23]. Surgical resection is usually the first line treatment for patients with CNS disease. Most patients generally remain disease free for prolonged periods of time following resection [23].
Among patients with isolated spinal disease, all were treated with surgical resection alone with the exception of one case. This individual also received radiotherapy given the extensiveness of her lesion [20]. Following resection, nearly all patients experienced either complete or incomplete resolution of their symptoms with no evidence of disease recurrence through the follow up period, which ranged from three months to four years. However, in one case, nodal disease eventually developed (Table III) [20].
While not reported for isolated spinal disease, disease recurrences have been uncommonly reported in cases of intracranial disease, particularly when lesions were not completely resected [58]. Radiosurgery, steroids, and chemotherapy agents (etoposide, methotrexate, 6-mercaptopurine, azathioprine) have been variably effective in recurrent cases or in cases requiring systemic therapy [13,18,34,38,58–70].
Given the benign nature of Rosai-Dorfman disease, the prognosis is generally good for both nodal and extra-nodal involvement [3]. However, rare fatalities have been reported, including in several patients with intracranial disease [19,58].
TEACHING POINT
Rosai-Dorman disease is a benign histiocytic disease that can very rarely present as an isolated epidural lesion (4 reported cases). Imaging findings are non-specific; however, Rosai-Dorfman disease should be considered in the differential for a spinal epidural mass when MRI demonstrates a hypo- or iso-intense (potentially heterogeneous) signal on T2 weighted sequences as well as homogenous enhancement following contrast administration.
ACKNOWLEDGEMENTS
Thank you to Dr. Stephen Nishimura for providing the pathology images for this report.
ABBREVIATIONS
- ADC
Apparent Diffusion Coefficient
- CNS
Central Nervous System
- CT
Computed Tomography
- DWI
Diffusion-weighted imaging
- EBV
Epstein-Barr Virus
- ESR
Erythrocyte Sedimentation Rate
- HHV-6
Human Herpesvirus 6
- HIV
Human Immunodeficiency Virus
- MRI
Magnetic Resonance Imaging
- RPR
Rapid Plasma Reagin
- T1WI
T1 Weighted Image
- T2WI
T2 Weighted Image
- TSH
Thyroid Stimulating Hormone
REFERENCES
- 1.Warrier R, Chauhan A, Jewan Y, Bansal S, Craver R. Rosai-dorfman disease with central nervous system involvement. Clin Adv Hematol Oncol. 2012;10(3):196–198. [PubMed] [Google Scholar]
- 2.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (rosai-dorfman disease): Review of the entity. Semin Diagn Pathol. 1990;7(1):19–73. [PubMed] [Google Scholar]
- 3.Zaveri J, La Q, Yarmish G, Neuman J. More than just langerhans cell histiocytosis: A radiologic review of histiocytic disorders. Radiographics. 2014;34(7):2008–2024. doi: 10.1148/rg.347130132. [DOI] [PubMed] [Google Scholar]
- 4.Zhao M, Li C, Zheng J, et al. Extranodal rosai-dorfman disease involving appendix and mesenteric nodes with a protracted course: Report of a rare case lacking relationship to IgG4-related disease and review of the literature. Int J Clin Exp Pathol. 2013;6(11):2569–2577. [PMC free article] [PubMed] [Google Scholar]
- 5.Noguchi S, Yatera K, Shimajiri S, et al. Intrathoracic rosai-dorfman disease with spontaneous remission: A clinical report and a review of the literature. Tohoku J Exp Med. 2012;227(3):231–325. doi: 10.1620/tjem.227.231. [DOI] [PubMed] [Google Scholar]
- 6.Mehraein Y, Wagner M, Remberger K, et al. Parvovirus B19 detected in rosai-dorfman disease in nodal and extranodal manifestations. J Clin Pathol. 2006;59(12):1320–1326. doi: 10.1136/jcp.2005.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luppi M, Barozzi P, Garber R, et al. Expression of human herpesvirus-6 antigens in benign and malignant lymphoproliferative diseases. Am J Pathol. 1998;153(3):815–823. doi: 10.1016/S0002-9440(10)65623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang WY, Yip TT, Chan JK. The rosai-dorfman disease histiocytes are not infected by epstein-barr virus. Histopathology. 1994;25(1):88–90. doi: 10.1111/j.1365-2559.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 9.Ortonne N, Fillet AM, Kosuge H, Bagot M, Frances C, Wechsler J. Cutaneous destombes-rosai-dorfman disease: Absence of detection of HHV-6 and HHV-8 in skin. J Cutan Pathol. 2002;29(2):113–118. doi: 10.1034/j.1600-0560.2002.290209.x. [DOI] [PubMed] [Google Scholar]
- 10.Becroft DM, Dix MR, Gillman JC, MacGregor BJ, Shaw RL. Benign sinus histiocytosis with massive lymphadenopathy: Transient immunological defects in a child with mediastinal involvement. J Clin Pathol. 1973;26(7):463–469. doi: 10.1136/jcp.26.7.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulli M, Bergamaschi G, Tonon L, et al. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (rosai-dorfman disease) Br J Haematol. 1995;91(2):415–418. doi: 10.1111/j.1365-2141.1995.tb05313.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta DK, Suri A, Mahapatra AK, et al. Intracranial rosai-dorfman disease in a child mimicking bilateral giant petroclival meningiomas: A case report and review of literature. Childs Nerv Syst. 2006;22(9):1194–1200. doi: 10.1007/s00381-006-0055-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Qiu LH, Dou YF, et al. Imaging characteristics of rosai-dorfman disease in the central nervous system. Eur J Radiol. 2012;81(6):1265–1272. doi: 10.1016/j.ejrad.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim DY, Park JH, Shin DA, et al. Rosai-dorfman disease in thoracic spine: a rare case of compression fracture. Korean J Spine. 2014;11(3):198–201. doi: 10.14245/kjs.2014.11.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yontz L, Franco A, Sharma S, Lewis K, McDonough C. A case of Rosai-Dorfman disease in a pediatric patient with cardiac involvement. J Radiol Case Rep. 2012;6(1):1–8. doi: 10.3941/jrcr.v6i1.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciacca S, Barkas K, Heptinstall L, McNamara C, Shetty R. Rosai-Dorfman disease with spinal cord compression: a diagnostic challenge. Eur Spine J. 2015;24:S529–535. doi: 10.1007/s00586-014-3671-6. [DOI] [PubMed] [Google Scholar]
- 17.Mahzoni P, Zavareh MH, Bagheri M, Hani N, Moqtader B. Intracranial ROSAI-DORFMAN disease. J Res Med Sci. 2012;17(3):304–307. [PMC free article] [PubMed] [Google Scholar]
- 18.Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-dorfman disease of the central nervous system: Report of 6 cases and review of the literature. Medicine (Baltimore) 2014;93(3):165–175. doi: 10.1097/MD.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-dorfman disease isolated to the central nervous system: A report of 11 cases. Mod Pathol. 2001;14(3):172–178. doi: 10.1038/modpathol.3880278. [DOI] [PubMed] [Google Scholar]
- 20.Hargett C, Bassett T. Atypical presentation of sinus histiocytosis with massive lymphadenopathy as an epidural spinal cord tumor: A case presentation and literature review. J Spinal Disord Tech. 2005;18(2):193–196. doi: 10.1097/01.bsd.0000137156.44689.c0. [DOI] [PubMed] [Google Scholar]
- 21.Hollowell JP, Wolfla CE, Shah NC, Mark LP, Whittaker MH. Rosai-dorman disease causing cervical myelopathy. Spine (Phila Pa 1976) 2000;25(11):1453–1456. doi: 10.1097/00007632-200006010-00020. [DOI] [PubMed] [Google Scholar]
- 22.Maiti TK, Gangadharan J, Mahadevan A, Arivazhagan A, Chandramouli BA, Shankar SK. Rosai-dorfman disease presenting as cervical extradural lesion: A case report with review of literature. Neurol India. 2011;59(3):438–442. doi: 10.4103/0028-3886.82769. [DOI] [PubMed] [Google Scholar]
- 23.Bhandari A, Patel PR, Patel MP. Extranodal rosai-dorfman disease with multiple spinal lesions: A rare presentation. Surg Neurol. 2006;65(3):308–311. doi: 10.1016/j.surneu.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Chan KW, Chow YY, Ghadially FN, Stansfeld AG, Woo CH. Rosai-dorfman disease presenting as spinal tumor. A case report with ultrastructural and immunohistochemical studies. J Bone Joint Surg Am. 1985;67(9):1427–1431. [PubMed] [Google Scholar]
- 25.Wang Y, Gao X, Tang W, Jiang C. Rosai-dorfman disease isolated to the central nervous system: A report of six cases. Neuropathology. 2010;30(2):154–158. doi: 10.1111/j.1440-1789.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen CW, Kachramanoglou C, Revesz T, Choi D. Rosai-dorfman disease presenting as a thoracic intradural extramedullary spinal tumor but without extraspinal manifestations. Acta Neurochir (Wien) 2012;154(2):367–368. doi: 10.1007/s00701-011-1176-1. [DOI] [PubMed] [Google Scholar]
- 27.El Molla M, Mahasneh T, Holmes SE, Al-Khawaja D. Rare presentation of rosai-dorfman disease mimicking a cervical intramedullary spinal cord tumor. World Neurosurg. 2014;81(2):442.e7–442.e9. doi: 10.1016/j.wneu.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Osenbach RK. Isolated extranodal sinus histiocytosis presenting as an intramedullary spinal cord tumor with paraplegia. case report. J Neurosurg. 1996;85(4):692–696. doi: 10.3171/jns.1996.85.4.0692. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Xu Y. Rosai-dorfman disease: A rare lesion with dura tail sign mimicking spinal meningioma. Spine J. 2014;14(12):3058–3059. doi: 10.1016/j.spinee.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Fu X, Jiang JH, Tian XY, Li Z. Isolated spinal rosai-dorfman disease misdiagnosed as lymphoplasmacyte-rich meningioma by intraoperative histological examination. Brain Tumor Pathol. 2015;32(1):72–75. doi: 10.1007/s10014-014-0181-5. [DOI] [PubMed] [Google Scholar]
- 31.Dalia S, Sagatys E, Sokol L, Kubal T. Rosai-dorfman disease: Tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21(4):322–327. doi: 10.1177/107327481402100408. [DOI] [PubMed] [Google Scholar]
- 32.Lliff JJ, Nedergaard M. Is there a cerebral vascular system? Stroke. 2013;44(6):S93–95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu F, Zhang JT, Xing XW, et al. Rosai-dorfman disease: A retrospective analysis of 13 cases. Am J Med Sci. 2013;345(3):200–210. doi: 10.1097/MAJ.0b013e3182553e2d. [DOI] [PubMed] [Google Scholar]
- 35.Raslan OA, Schellingerhout D, Fuller GN, Ketonen LM. Rosai-dorfman disease in neuroradiology: Imaging findings in a series of 10 patients. AJR Am J Roentgenol. 2011;196(2):W187–93. doi: 10.2214/AJR.10.4778. [DOI] [PubMed] [Google Scholar]
- 36.Geara AR, Ayoubi MA, Achram MC, Chamseddine NM. Rosai-dorfman disease mimicking neurofibromatosis: Case presentation and review of the literature. Clin Radiol. 2004;59(7):625–630. doi: 10.1016/j.crad.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Toh CH, Chen YL, Wong HF, Wei KC, Ng SH, Wan YL. Rosai-dorfman disease with dural sinus invasion. report of two cases. J Neurosurg. 2005;102(3):550–554. doi: 10.3171/jns.2005.102.3.0550. [DOI] [PubMed] [Google Scholar]
- 38.Udono H, Fukuyama K, Okamoto H, Tabuchi K. Rosai-dorfman disease presenting multiple intracranial lesions with unique findings on magnetic resonance imaging. case report. J Neurosurg. 1999;91(2):335–339. doi: 10.3171/jns.1999.91.2.0335. [DOI] [PubMed] [Google Scholar]
- 39.Simos M, Dimitrios P, Philip T. A new clinical entity mimicking meningioma diagnosed pathologically as rosai-dorfman disease. Skull Base Surg. 1998;8(2):87–92. doi: 10.1055/s-2008-1058581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Barge DV, 3rd, Salzman KL, Harnsberger HR, et al. Sinus histiocytosis with massive lymphadenopathy (rosai-dorfman disease): Imaging manifestations in the head and neck. AJR Am J Roentgenol. 2008;191(6):W299–306. doi: 10.2214/AJR.08.1114. [DOI] [PubMed] [Google Scholar]
- 41.Smith AB, Horkanyne-Szakaly I, Schroeder JW, Rushing EJ. From the radiologic pathology archives: Mass lesions of the dura: Beyond meningioma-radiologic-pathologic correlation. Radiographics. 2014;34(2):295–312. doi: 10.1148/rg.342130075. [DOI] [PubMed] [Google Scholar]
- 42.Sze G, Zimmerman RD. The magnetic resonance imaging of infections and inflammatory diseases. Radiol Clin North Am. 1988;26(4):839–859. [PubMed] [Google Scholar]
- 43.Fukushima T, Yachi K, Ogino A, et al. Isolated intracranial Rosai-Dorfman disease without dural attachment - case report. Neurol Med Chir. 2011;51(2):136–140. doi: 10.2176/nmc.51.136. [DOI] [PubMed] [Google Scholar]
- 44.Oner AY, Akpek S, Tali T. Rosai-Dorfman disease with epidural and spinal bone marrow involvement: magnetic resonance imaging and diffusion-weighted imaging features. Acta Radiol. 2007;48(3):331–334. doi: 10.1080/02841850701196930. [DOI] [PubMed] [Google Scholar]
- 45.Braun P, Kazmi K, Nogues-Melendez P, Mas-Estelles F, Aparici-Robles F. MRI findings in spinal subdural and epidural hematomas. Eur J Radiol. 2007;64(1):119–125. doi: 10.1016/j.ejrad.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep. 2014;16(11) doi: 10.1007/s11908-014-0436-7. 436-014-0436-7. [DOI] [PubMed] [Google Scholar]
- 47.Moritani T, Kim J, Capizzano AA, et al. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. Br J Radiol. 2014;87(1041):20140011. doi: 10.1259/bjr.20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah LM, Salzman KL. Imaging of spinal metastatic disease. Int J Surg Oncol. 2011;769753 doi: 10.1155/2011/769753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mascalchi M, Torselli P, Falaschi F, Dal Pozzo G. MRI of spinal epidural lymphoma. Neuroradiology. 1995;37(4):303–307. doi: 10.1007/BF00588341. [DOI] [PubMed] [Google Scholar]
- 50.Demir MK, Ozdemir H, Unlu E, Temizoz O, Genchellac H. Differential diagnosis of spinal epidural meningioma and hemangioma at MR imaging. Radiology. 2007;244(3):933. doi: 10.1148/radiol.2443061813. author reply 933–4. [DOI] [PubMed] [Google Scholar]
- 51.Frank BL, Harrop JS, Hanna A, Ratliff J. Cervical extradural meningioma: Case report and literature review. J Spinal Cord Med. 2008;31(3):302–305. doi: 10.1080/10790268.2008.11760727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lidar M, Dori A, Levy Y, et al. Sarcoidosis presenting as a “corset-like” myelopathy: a description of six cases and literature review. Clin Rev Allergy Immunol. 2010;38(2):270–275. doi: 10.1007/s12016-009-8156-8. [DOI] [PubMed] [Google Scholar]
- 53.Lury KM, Smith JK, Matheus MG, Castillo M. Neurosarcoidosis - review of imaging findings. Semin Roentgenol. 2004;39(4):495–504. doi: 10.1016/j.ro.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Chan SK, Cheuk W, Chan KT, Chan JK. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol. 2009;33(8):1249–1252. doi: 10.1097/PAS.0b013e3181abdfc2. [DOI] [PubMed] [Google Scholar]
- 55.Choi SH, Lee SH, Khang SK, Jeon SR. IgG4-related sclerosing pachymeningitis causing spinal cord compression. Neurology. 2010;75(15):1388–1390. doi: 10.1212/WNL.0b013e3181f73614. [DOI] [PubMed] [Google Scholar]
- 56.Kim SH, Kang Y, Oh SH, Paik S, Kim JS. Paraplegia in a Patient With IgG4-Related Sclerosing Disease: A Case Report. Ann Rehabil Med. 2014;38(6):856–860. doi: 10.5535/arm.2014.38.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabbay LB, Leite Cda C, Andriola RS, Pinho Pda C, Lucato LT. Histiocytosis: A review focusing on neuroimaging findings. Arq Neuropsiquiatr. 2014;72(7):548–558. doi: 10.1590/0004-282x20140063. [DOI] [PubMed] [Google Scholar]
- 58.Petzold A, Thom M, Powell M, Plant GT. Relapsing intracranial rosai-dorfman disease. J Neurol Neurosurg Psychiatry. 2001;71(4):538–541. doi: 10.1136/jnnp.71.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadjipanayis CG, Bejjani G, Wiley C, Hasegawa T, Maddock M, Kondziolka D. Intracranial rosai-dorfman disease treated with microsurgical resection and stereotactic radiosurgery. case report. J Neurosurg. 2003;98(1):165–168. doi: 10.3171/jns.2003.98.1.0165. [DOI] [PubMed] [Google Scholar]
- 60.Sato A, Sakurada K, Sonoda Y, et al. Rosai-dorfman disease presenting with multiple intracranial and intraspinal masses: A case report. No Shinkei Geka. 2003;31(11):1199–1204. [PubMed] [Google Scholar]
- 61.Kidd DP, Revesz T, Miller NR. Rosai-dorfman disease presenting with widespread intracranial and spinal cord involvement. Neurology. 2006;67(9):1551–1555. doi: 10.1212/01.wnl.0000242893.55416.8e. [DOI] [PubMed] [Google Scholar]
- 62.Franco-Paredes C, Martin K. Extranodal rosai-dorfman disease involving the meninges. South Med J. 2002;95(9):1101–1102. [PubMed] [Google Scholar]
- 63.Shaver EG, Rebsamen SL, Yachnis AT, Sutton LN. Isolated extranodal intracranial sinus histiocytosis in a 5-year-old boy. case report. J Neurosurg. 1993;79(5):769–773. doi: 10.3171/jns.1993.79.5.0769. [DOI] [PubMed] [Google Scholar]
- 64.Bernard F, Sarran N, Serre I, et al. Sinus histiocytosis (destombes-rosai-dorfman disease) revealed by extranodal spinal involvement. Arch Pediatr. 1999;6(2):173–177. doi: 10.1016/s0929-693x(99)80205-x. [DOI] [PubMed] [Google Scholar]
- 65.Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (rosai-dorfman disease): Report of a case and literature review. Am J Hematol. 2002;69(1):67–71. doi: 10.1002/ajh.10008. [DOI] [PubMed] [Google Scholar]
- 66.Purav P, Ganapathy K, Mallikarjuna VS, et al. Rosai-dorfman disease of the central nervous system. J Clin Neurosci. 2005;12(6):656–659. doi: 10.1016/j.jocn.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Foucar E, Rosai J, Dorfman RF, Brynes RK. The neurologic manifestations of sinus histiocytosis with massive lymphadenopathy. Neurology. 1982;32(4):365–372. doi: 10.1212/wnl.32.4.365. [DOI] [PubMed] [Google Scholar]
- 68.Forest F, N’guyen AT, Fesselet J, et al. Meningeal rosai-dorfman disease mimicking meningioma. Ann Hematol. 2014;93(6):937–940. doi: 10.1007/s00277-013-1994-8. [DOI] [PubMed] [Google Scholar]
- 69.Cooper SL, Jenrette JM. Rosai-dorfman disease: Management of CNS and systemic involvement. Clin Adv Hematol Oncol. 2012;10(3):199–202. [PubMed] [Google Scholar]
- 70.McPherson CM, Brown J, Kim AW, DeMonte F. Regression of intracranial rosai-dorfman disease following corticosteroid therapy. Case report. J Neurosurg. 2006;104(5):840–844. doi: 10.3171/jns.2006.104.5.840. [DOI] [PubMed] [Google Scholar]