Abstract
Purpose
To assess the effect of vertebroplasty (VP) on the risk of further radiologically apparent vertebral fracture within two years of the procedure.
Methods
We conducted a randomised placebo-controlled trial of VP in people with acute osteoporotic vertebral fracture. Eligible participants were randomly assigned to VP (n=38) or placebo (n=40). Cement volume and leakage were recorded for the VP group. Plain thoracolumbar radiographs were taken at baseline, 12 and 24 months. Two independent radiologists assessed these for new and progressed fractures at the same, adjacent and non-adjacent levels.
Results
At 12 and 24 months, radiographs were available for 45 (58%) and 47 (60%) participants respectively. There were no between-group differences for new or progressed fractures: 32 and 40 in the VP group after 12 and 24 months compared with 21 and 33 in the placebo group (hazard ratio (HR) 1.80, 95% confidence interval (CI) 0.82 to 3.94). Similar results were seen when considering only adjacent (HR (95% CI): 2.30 (0.57 to 9.29)), and non-adjacent (HR (95% CI): 1.45 (0.55 to 3.81) levels. In all comparisons there was a consistent trend towards higher risk of any type of fracture in the group undergoing VP. Within the VP group, fracture risk was unrelated to total (HR (95% CI): 0.91 (0.71 to 1.17)) or relative (HR (95% CI): 1.31 (0.15 to 11.48)) cement volume, or cement leakage (HR (95% CI): 1.20 (0.63 to 2.31)).
Conclusion
For patients undergoing VP our study did not demonstrate significant increases in subsequent fracture risk beyond that experienced by those with vertebral fractures who did not undergo the procedure. However, because of the non-significant numerical increases observed, studies with adequate power are needed to draw definite conclusions about fracture risk.
Keywords: Vertebroplasty, placebo-controlled, randomised trial
Introduction
Vertebroplasty (VP) is a minimally invasive procedure used to palliate pain from painful osteoporotic vertebral compression fractures. It was first introduced in the late 1980s but recent evidence has questioned its efficacy [1, 2], while data regarding its long-term safety is limited and inconclusive. Serious complications, including subsequent vertebral fractures, pulmonary and cerebral embolism, infection, cardiac perforation and cement leakage resulting in neurological deficit, have been reported [3–5].
Retrospective reviews of patients undergoing VP for osteoporotic compression fractures have reported that between 6% and 34% had new vertebral fractures following the procedure [6, 7], but without the fracture risk for an appropriate comparison group, this information has limited value. Those with a prevalent fracture are at higher risk of a new vertebral fracture therefore it is important to determine whether VP increases the fracture risk beyond that expected for patients not having VP.
Treated vertebral fractures can undergo height loss and persistent oedema [8] while vertebrae adjacent to the treated level appear to be more susceptible to incident fracture following vertebroplasty [7, 9–12], particularly in the short term, but the time frame for the occurrence of these subsequent fractures is unclear. A prevalent osteoporotic vertebral fracture also increases the risk of adjacent fractures [13] as there are spinal zones where risk is considered to be increased across several levels simultaneously [14].
A higher fracture risk for patients having VP compared with patients choosing alternative management has been reported by some [15, 16] but not all studies [10, 17, 18]. Fracture risk may also be related to the volume of cement injected, cement leakage, or patient characteristics such as age or bone mineral density (BMD). A meta-analysis of 16 case-control studies found that the risk of new vertebral fractures was higher for patients with low BMD, low body mass index and cement leakage but was not associated with age or cement volume [3].
Two recent blinded, randomised controlled trials (RCTs) showed no benefit of VP compared with a placebo procedure for improving pain or function in the short term [1, 2]. Adequately-powered sub-group analyses based on these two RCTs also showed no differences in pain improvement between the groups up to one month following the trial procedure for patients with acute pain (less than 6 weeks) or severe pain (>8 on a 0 to10 VAS pain scale) [19]. The US-based trial allowed cross-over after one month complicating the assessment of the risk of subsequent vertebral fracture [2]. In the Australian-based trial there was no difference between the groups in new clinical vertebral fractures reported to six months [1]. Two-year follow-up also demonstrated no pain or function benefit of VP over the placebo procedure[20].
We report here the 24-month independent radiology review of outcomes. The incidence of radiologically evident new and progressed vertebral fractures at the procedure level and adjacent and non-adjacent vertebrae is compared for the two groups. We also investigate the risk of new or progressed incident fractures in relation to cement volume, cement leakage and certain baseline patient clinical characteristics.
Methods
Between April 2004 and October 2010, we conducted a participant and outcome assessor blinded placebo controlled trial of VP. Full protocol details [21], and efficacy and safety results have been reported elsewhere [1, 19]. Briefly, eligible participants had one or two acute osteoporotic vertebral compression fractures (OVCFs) of at least grade 1 [22] confirmed by history, radiograph and the presence of bone oedema on MRI. If MRI was contraindicated or could not be performed, a bone scan was required to determine the presence of increased uptake compatible with recent fracture. This applied to 12 (15%) of the 78 participants. Eligible participants were randomised to vertebroplasty or a placebo procedure stratified by study centre, sex and duration of symptoms (< 6 weeks or ≥ 6 weeks). Participants and all study personnel other than the treating radiologist were blinded to treatment allocation. The human research ethics committees at each participating centre approved the study and all participants provided written informed consent. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN012605000079640).
For the VP intervention, the participant was sedated with midazolam and fentanyl and local anaesthetic infiltrated into the skin overlying the target pedicle and the periosteum of the posterior lamina prior to making a small incision in the skin. Under image guidance, prepared polymethylmethacrylate (PMMA) cement was slowly injected into the vertebral body via the pedicle(s), using a 13-gauge needle, until substantial resistance was met or the cement reached the posterior quarter of the vertebral body. Fourteen (31%) of the 45 treated levels used a bipedicular approach. The total volume of cement injected and any cement leakage was determined during cement injection and assessed on final AP and lateral images post procedure. The placebo procedure was identical up to the skin incision. A 13-gauge needle was introduced through the excision to rest on the lamina.
The lamina was gently tapped with a blunt stylet, but not penetrated, to mimic the VP procedure and a small quantity of PMMA was prepared so that its smell permeated the room.
Baseline data collected included lumbar and femoral neck bone mineral density (current or in the past year) and use of bisphosphonates and oral glucocorticoids. Plain radiograph and either MRI or bone scintigraphy and CT scans were performed at baseline to assess eligibility for the trial. Antero-posterior and lateral images were taken after the injection of cement in the active treatment group and all participants had plain radiographs taken at 12 and 24 months post-procedure. At the end of the study, the baseline plain radiographs and MRI or bone and CT scans, and the follow-up radiographs were digitised. CDs containing the digitised images were couriered by registered mail to two independent radiologists (BMH & MDR) at the Mayo Clinic for review. Any plain radiographs taken to confirm symptomatic fractures occurring during the follow-up period were also digitised and sent for review. Any confirmed fractures were included in the analysis with the confirmed fracture date.
The radiologists reviewed all available imaging and recorded vertebral body compression fractures of the visualized spine. CT, MRI, bone scan, and radiographs were reviewed together to identify the fractures. The fractures were categorized as mild, moderate, and severe with morphology of wedge, biconcave, or crush according to Genant’s semi-quantitative technique [22]. The 12- and 24-month radiographs were assessed for new vertebral fractures and any progression of pre-existing vertebral fractures. New fractures were defined as development of abnormal vertebral body morphology with loss of normal height. Progression of pre-existing fractures was defined as increased loss of vertebral body height, or change in fracture morphology according to this semi-quantitative technique. For participants who had undergone VP, the amount of cement injected was assessed from the radiograph at the time of the procedure as the ratio of the length of cement to the total length of the vertebra in each of the craniocaudal, anterior-posterior and medial-lateral directions. The relative volume of cement injected was then calculated as the product of the three ratios. The values used in the analyses were the mean scores for the two radiologists.
Because of the radio-opacity of PMMA it was not possible to blind the radiologists reviewing the follow-up films. The radiologists reviewed the films independently and discrepancies were resolved by consensus at the completion of the review process.
The risk of new and progressed fractures at the treated, adjacent and non-adjacent vertebral levels was compared for both treatment groups using Kaplan-Meier survival analysis. The event date was taken as the date of the x-ray and the censor date was the date of the 24-month x-ray or the date of the final questionnaire completed if no x-ray was available. Because an individual could experience multiple fracture events, participants remained in the risk set if a fracture event occurred. If a new fracture was assessed as progressed at one time point it was not included again at the later time.
Possible predictors of overall fracture risk were assessed using Cox proportional hazards models including sex, site of treated level (thoracic or lumbar) and baseline values of age, lumbar and femoral neck T score ≤−2.5 or >2.5, previous spinal fracture, glucocorticoid use and duration, use of osteoporosis medication at baseline and current use collected at one and two years.
The influence of total cement volume, relative cement volume and cement leakage on the risk of fracture was also investigated for the VP group using Cox modelling.
We calculated that a total sample of 164 participants (82/group) would be needed to show a three-fold increase in the risk of further vertebral fractures at 24 months assuming a 10% event rate in the placebo group [21]. However trial enrolment was terminated prior to reaching the planned sample size for long-term outcomes due to recruitment difficulties. The decision was made with the knowledge that the study’s power was sufficient to address the primary outcome. The decision to curtail recruitment was made without knowledge of any outcome results.
Results
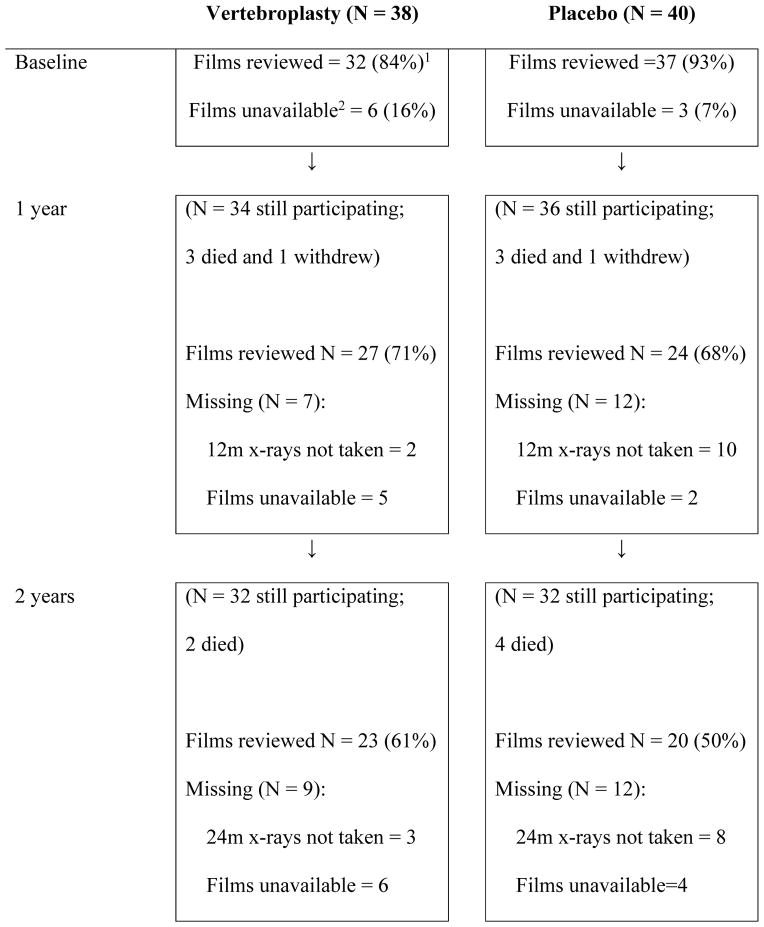
A total of 92 vertebral levels were treated in the trial; 45 levels for the 38 participants having the VP procedure and 47 levels for the 40 participants undergoing placebo treatment. Treated levels were 15 thoracic (T6-T8: 6 placebo; 9 VP), 60 thoracolumbar (T7-L2: 29 placebo; 31 VP) and 17 lumbar (L3–L4: 12 placebo; 5 VP. Not all pre-treatment MRI and/or bone scans were available for review. Some participants did not attend for follow-up radiographs or did not return their films and copies were not available from the imaging facility. Table 1 shows the availability of films sent for independent radiological review at baseline and each follow-up time point.
Table 1.
Flow diagram of radiographs available for review at each time point
All percentages shown are percent of all patients randomised to the group.
Unavailability of films indicates films were unable to be retrieved from participant or imaging facility.
Of the 68 (74%) pre-treatment levels reviewed (29 (64%) VP, 39 (83%) placebo), bone marrow oedema and/or increased uptake on bone scintigraphy was detected in 61 (90%) levels (27 (90%) were in the VP levels and 34 (87%) were in the placebo procedure levels). The mean (SD) cement volume per level for the treated group was 2.8 (1.2) ml and cement leakage occurred at 18 (40%) treated levels. The mean (SD) relative cement volume was 34% (16%) for the 36 levels assessed at the radiology review. At one year, 60.6% of the VP group and 70.6% of the placebo group (65.7% overall) (p=0.34) reported taking treatment for osteoporosis that included bisphosphonates (93%) and raloxifene (7%); at two years this had changed to 65.5% for the VP group and 67.9% for the placebo group (66.7% overall: 87% bisphosphonates, 11% raloxifene, 2% strontium ranelate) (p=0.85).
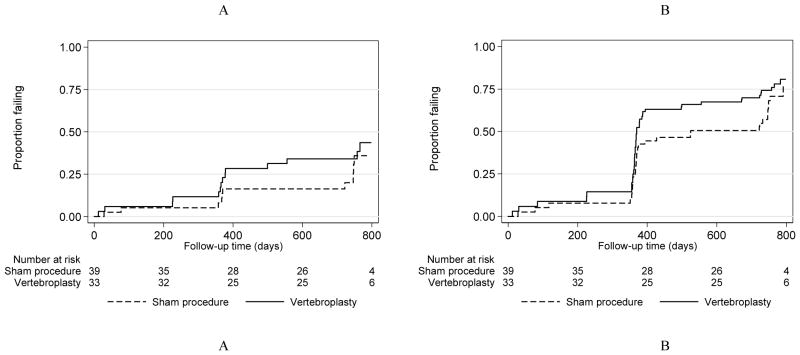
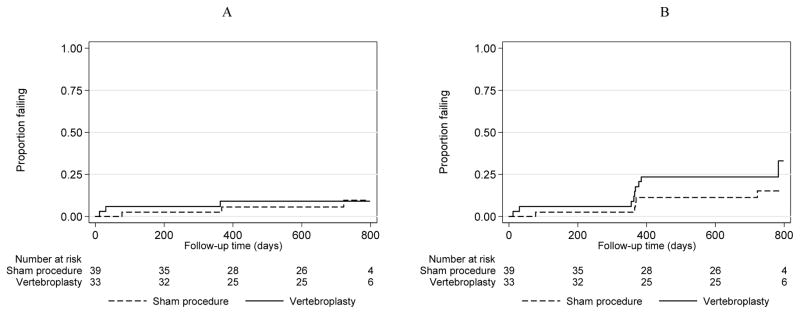
Subsequent fractures for each group are shown in Table 2 by position, type and time after the procedure. Within the two-year follow-up period, there were 17 new fractures and 23 progressed fractures in the VP group and 10 new and 23 progressed fractures in the placebo group. There were no significant differences between the groups for the risk of new or new and progressed fractures combined at any level, adjacent or non-adjacent (Table 3). Similarly, there were no significant differences between the groups for new or new and progressed fractures combined at the procedure level. In all comparisons, however, there was a consistent trend towards higher risk of any type of fracture in the group undergoing VP. Kaplan-Meier curves for new (Figure 1A) and progressed fractures (Figure 1B) at any level by treatment group are shown in Figure 1. Figure 2 shows Kaplan-Meier curves for new (2A) and new and progressed (2B) adjacent level fractures by group.
Table 2.
New and progressed vertebral fractures in each treatment group during follow up according to level and treatment1
| Vertebroplasty group | |||||||
|---|---|---|---|---|---|---|---|
| Fracture type | 1 week | 1 month | 3 months | 6 months | 1 year (N = 34) | 2 years (N = 36) | Total |
| New treated level | 12 | 1 | |||||
| New adjacent level | 2 | 1 | 3 | 6 | |||
| New non-adjacent level | 7 | 1 | 2 | 10 | |||
| Progressed treated level | 1 | 6 | 5 | 1 | 13 | ||
| Progressed adjacent level | 3 | 0 | 1 | 4 | |||
| Progressed non-adjacent level | 3 | 1 | 2 | 6 | |||
| Total | 2 | 1 | 20 | 8 | 9 | 40 | |
| Placebo group | |||||||
|---|---|---|---|---|---|---|---|
| Fracture type | 1 week | 1 month | 3 months | 6 months | 1 year (N = 32) | 2 years (N = 32) | Total |
| New treated level | |||||||
| New adjacent | 1 | 1 | 1 | 3 | |||
| New non-adjacent | 1 | 3 | 3 | 7 | |||
| Progressed treated level | 1 | 4 | 3 | 4 | 12 | ||
| Progressed adjacent level | 2 | 2 | |||||
| Progressed non-adjacent level | 4 | 1 | 4 | 9 | |||
| Total | 1 | 1 | 1 | 14 | 4 | 12 | 33 |
Radiographs obtained prior to the one year follow up were available only for select participants who presented with new onset pain and radiographs were performed to look for further fractures.
This fracture at a treated level was considered to be a new fracture rather than progression of an existing fracture
Table 3.
Absolute number of fractures and hazard ratios (95% confidence intervals) for increased risk in the VP group for new and progressed fractures or new fractures only at any level and according to level and treatment
| New and progressed fractures | VP group fractures | Placebo group fractures | Hazard Ratio | (95% Confidence Interval) |
|---|---|---|---|---|
| Any level | 40 | 33 | 1.29 | (0.80, 2.08) |
| Adjacent level | 10 | 5 | 2.18 | (0.74, 6.42) |
| Non-adjacent level | 16 | 16 | 1.18 | (0.58, 2.43) |
| Treated level | 14 | 12 | 1.05 | (0.47, 2.34) |
| Untreated level | 26 | 21 | 1.44 | (0.80, 2.61) |
| New fractures only | ||||
| Any level | 17 | 10 | 1.80 | (0.82, 3.94) |
| Adjacent level | 6 | 3 | 2.30 | (0.57, 9.29) |
| Non-adjacent level | 10 | 7 | 1.45 | (0.55, 3.81) |
| Treated level1 | 1 | 0 | - | - |
| Untreated level | 16 | 10 | 1.69 | (0.77, 3.74) |
No new fractures at treated level in the placebo group.
Figure 1.
New (A) and New or progressed (B) fractures at any site
Figure 2.
New (A) and New or progressed (B) fractures at adjacent levels
Cox regression also indicated no association between the risk of new or progressed fractures at adjacent, non-adjacent or procedure level and age, sex, site of treated level, use of bisphosphonates at baseline or current bisphosphonate use, glucocorticoid use or duration of use, previous vertebral fracture, BMD, total or relative cement volume or cement leakage (Table 4).
Table 4.
Risk of new and progressed fractures (hazard ratios and 95% confidence intervals) for various predictor variables at any level and according to level and treatment
| Predictor variable | Hazard Ratio | (95% Confidence Intervals) |
|---|---|---|
| Any level | ||
| Age (years) | 1.02 | (1.00, 1.05) |
| Female | 1.03 | (0.54, 1.98) |
| Bisphosphonate use at baseline | 1.12 | (0.55, 2.27) |
| Current treatment for osteoporosis* | 0.96 | (0.56, 1.64) |
| Glucocorticoid use at baseline | 1.26 | (0.78, 2.05) |
| Duration of glucocorticoids | 1.01 | (0.98, 1.03) |
| Previous spinal fracture | 1.39 | (0.85, 2.26) |
| Lumbar spine T score | 0.91 | (0.76, 1.09) |
| Lumbar spine T score ≤ −2.5 | 1.24 | (0.67, 2.29) |
| Femoral neck T score | 0.92 | (0.72, 1.19) |
| Femoral neck T score ≤ −2.5 | 1.14 | (0.69, 1.90) |
| Total cement volume† | 0.91 | (0.71, 1.17) |
| Relative cement volume† | 1.31 | (0.15, 11.5) |
| Any cement leakage† | 1.20 | (0.63, 2.31) |
| Treated lumbar fracture‡ | 0.69 | (0.43, 1.10) |
| Adjacent level | ||
| Age (years) | 1.06 | (0.99, 1.13) |
| Female | 0.95 | (0.20, 4.40) |
| Bisphosphonate use at baseline | - | - |
| Current treatment for osteoporosis* | 0.86 | (0.29, 2.59) |
| Glucocorticoid use at baseline | 0.70 | (0.22, 2.21) |
| Duration of glucocorticoids | 0.97 | (0.84, 1.11) |
| Previous spinal fracture | 1.08 | (0.39, 2.99) |
| Lumbar spine T score | 0.81 | (0.51, 1.29) |
| Lumbar spine T score ≤ −2.5 | 0.92 | (0.24, 3.48) |
| Femoral neck T score | 0.71 | (0.39, 1.30) |
| Femoral neck T score ≤ −2.5 | 1.89 | (0.56, 6.36) |
| Total cement volume† | 1.06 | (0.66, 1.70) |
| Relative cement volume† | 4.45 | (0.07, 296) |
| Any cement leakage† | 0.85 | (0.21, 3.42) |
| Treated lumbar fracture‡ | 1.06 | (0.37, 3.03) |
| Non-adjacent level | ||
| Age (years) | 1.02 | (0.98, 1.06) |
| Female | 1.09 | (0.41, 2.87) |
| Bisphosphonate use at baseline | 1.56 | (0.47, 5.17) |
| Current treatment for osteoporosis* | 1.10 | (0.47, 2.58) |
| Glucocorticoid use at baseline | 1.65 | (0.80, 3.39) |
| Duration of glucocorticoids | 1.02 | (0.99, 1.04) |
| Previous spinal fracture | 2.82 | (1.20, 6.61) |
| Lumbar spine T score | 0.85 | (0.66, 1.10) |
| Lumbar spine T score ≤ −2.5 | 1.66 | (0.63, 4.36) |
| Femoral neck T score | 0.95 | (0.66, 1.36) |
| Femoral neck T score ≤ −2.5 | 0.85 | (0.41, 1.78) |
| Total cement volume† | 0.77 | (0.51, 1.16) |
| Relative cement volume† | 3.52 | (0.09, 131) |
| Any cement leakage† | 0.69 | (0.24, 1.99) |
| Treated lumbar fracture‡ | 0.52 | (0.24, 1.11) |
| Treated level | ||
| Age (years) | 1.01 | (0.97, 1.05) |
| Female | 1.00 | (0.34, 2.96) |
| Bisphosphonate use at baseline | 0.52 | (0.21, 1.33) |
| Current treatment for osteoporosis* | 0.88 | (0.37, 2.14) |
| Glucocorticoid use at baseline | 1.26 | (0.56, 2.84) |
| Duration of glucocorticoids | 0.97 | (0.89, 1.06) |
| Previous spinal fracture | 0.77 | (0.35, 1.73) |
| Lumbar spine T score | 1.05 | (0.78, 1.43) |
| Lumbar spine T score ≤ −2.5 | 1.00 | (0.36, 2.78) |
| Femoral neck T score | 1.02 | (0.66, 1.57) |
| Femoral neck T score ≤ −2.5 | 1.33 | (0.54, 3.27) |
| Total cement volume† | 0.99 | (0.64, 1.53) |
| Relative cement volume† | 0.22 | (0.01, 8.80) |
| Any cement leakage† | 3.14 | (0.94, 10.4) |
| Treated lumbar fracture‡ | 0.87 | (0.39, 1.94) |
| Untreated level | ||
| Age (years) | 1.03 | (1.00, 1.06) |
| Female | 1.04 | (0.46, 2.37) |
| Bisphosphonate use at baseline | 2.31 | (0.71, 7.51) |
| Current treatment for osteoporosis* | 1.01 | (0.52, 1.97) |
| Glucocorticoid use at baseline | 1.27 | (0.70, 2.31) |
| Duration of glucocorticoids | 1.01 | (0.99, 1.04) |
| Previous spinal fracture | 1.96 | (1.04, 3.70) |
| Lumbar spine T score | 0.84 | (0.67, 1.05) |
| Lumbar spine T score ≤ −2.5 | 1.39 | (0.64, 3.01) |
| Femoral neck T score | 0.88 | (0.64, 1.20) |
| Femoral neck T score ≤ −2.5 | 1.06 | (0.57, 1.97) |
| Total cement volume† | 0.87 | (0.64, 1.19) |
| Relative cement volume† | 3.89 | (0.25, 60.2) |
| Any cement leakage† | 0.74 | (0.32, 1.73) |
| Treated lumbar fracture‡ | 0.66 | (0.36, 1.21) |
Includes bisphosphonates, raloxifene and strontium ranelate
Vertebroplasty patients only included in analysis.
compared with treated thoracic fracture
Discussion
In this randomised-controlled trial with 24-month follow-up, there were no statistically significant differences in the risk of new or progressed radiographically apparent fractures in participants who received VP compared with those who received a placebo procedure. However in all comparisons there was a consistent trend towards higher risk of any type of fracture in the group undergoing VP. Neither the volume of cement injected, cement leakage or vertebroplasty level was associated with the risk of subsequent fractures. In addition, none of the patient characteristics we examined were linked with subsequent fracture risk.
Results from randomised controlled trials that have compared risk of subsequent vertebral fracture in participants receiving VP compared with those receiving conservative treatment have been inconsistent. One trial reported a significantly increased risk of radiologically apparent vertebral fractures associated with VP in comparison to usual care over 12 months follow up (29 new radiologic vertebral fractures observed in 17 of 64 patients treated with VP compared with 8 in 8 of 61 patients treated with usual care; Odds Ratio (OR) 2.78 (95% CI 1.02 to 7.62)) [9]. They also reported a significantly higher risk of symptomatic vertebral fractures (OR 25.67 (95% CI 3.04 to 216.8)). Another trial reported a significantly lower risk of symptomatic vertebral fractures among patients receiving VP compared with usual care over two years of follow up (2.2% versus 13.3%, p<0.01) but did not report risk of radiologically apparent fractures [18]. Two trials did not observe an increased risk of radiologically apparent fractures with VP. Rousing et al. [23, 24] found no between-group difference in new radiologically apparent fractures at either 3 months (3 new fractures in the VP group versus 1 new fracture in the usual care group; Relative Risk (RR) 2.9 (95% CI 0.3 to 25.7)) or 12 months (4 and 3 new fractures respectively; RR 1.3 (95% CI not reported)). Klazen et al. [25] reported 18 new radiologically apparent fractures in 15 of 91 participants treated with VP compared with 30 new fractures in 21 of 85 participants treated conservatively (P=0.44) after a mean of 11.4 months follow up (median 12.0, range 1 to 24 months; 12 people refused radiographs during follow up.
The main strength of our study is the randomised and blinded allocation of participants to treatment group. This minimises the selection bias associated with participant or clinician choice of the procedure and gives a more appropriate assessment of the true effect of VP. Although the presence of bone marrow oedema was an eligibility criterion for inclusion in the study, independent radiology review confirmed the presence of oedema in only 90%. Given that bone marrow oedema can be quite subtle and subject to false positive diagnosis, there will be unavoidable inter-observer variability. Wang et al. [26] reported a kappa statistic for inter-rater reliability for Modic changes on vertebral MRIs of 0.79 and several studies have reported inter-rater agreement to be no higher than around 75% [27, 28].
Our sample size to assess safety outcomes was limited due to the early termination of recruitment and we had limited power to detect small to moderately increased risk of further vertebral fracture. With a total sample size of 78 we had only 50% power to demonstrate the hypothesised three-fold increase in fracture risk associated with VP. The hazard ratios seen in our study did not differ significantly from unity, ranging from 1.05 to 2.30 with wide 95% confidence intervals. While all comparisons consistently indicated an increased risk we have insufficient power to draw definite conclusions.
Another limitation is that we had standardised long-term radiological follow-up of only 60% of all participants and 69% of those still participating. We also included radiologically-confirmed fractures identified at non-standard follow-up points for patients with back pain but as we have no information from those without radiological follow-up, we have no way of determining whether follow-up was related to fracture outcomes.
We sought to determine whether VP increased the risk of any subsequent vertebral fracture. Our radiological review confirmed 73 new or progressed fractures but only around one third of these (N=27) were clinically apparent [20]. Under diagnosis (false negative) rates of up to 46% have been reported for radiologically-confirmed vertebral fractures [29]. Delmas et al also reported discrepancies between local and subsequent central radiology review indicating a 34% false negative. Discrepancies between the two independent reviewers in our study were adjudicated to obtain consensus. Symptomatic, or clinically apparent, fractures are likely to cause the patient greater distress, but morbidity and mortality are increased following both symptomatic and asymptomatic fractures [30–32]. By either measure there were no statistically significant differences between participants having the VP or the placebo procedure.
Objective outcomes such as radiologically confirmed fractures should be less prone to bias in outcome assessment but the radio-opacity of the cement makes blinded assessment impossible. Therefore, the two radiologists (BMH & MDR), who were not otherwise involved in the trial, would have been unblinded to the presence of cement in treated vertebrae at follow-up x-rays. If either radiologist had a pre-conceived belief, either positive or negative, about fracture risk following VP this could have resulted in bias of either direction.
To conclusively establish whether or not there is an increased risk of further vertebral fracture associated with VP would require further randomised placebo-controlled trials. These studies would need to be large and have extended follow-up to demonstrate even a moderate risk increase. However in the absence of proven benefits over placebo, it is questionable whether further placebo-controlled trials should be pursued. Meta-analyses of our study and other randomised placebo-controlled trials could also provide some insights.
Population-based studies may be an alternative option but these need to be analysed carefully to account for differences in patient selection leading to biases in who gets treatment [33]. McCullough et al. examined a large database of US Medicare claims for patients with newly diagnosed vertebral fractures and illustrated how selection biases within claims data can alter results in this type of research; those undergoing vertebroplasty differed from those not having vertebral augmentation. After adjusting for pre-treatment pain and function, age, comorbidities, socio-economic status and access to healthcare, factors that may influence patient or physician choice of treatment, they found increased healthcare utilisation in the augmented group but no differences between the augmented and control groups in mortality or major medical complications at one year.
Conclusions
While VP in placebo-controlled studies has failed to provide superior pain relief or functional benefit compared with placebo, our study did not observe an increase in subsequent fracture risk beyond that experienced by those with vertebral fractures who do not undergo the procedure although lack of sufficient power precludes drawing definitive conclusions about fracture risk.
Acknowledgments
The authors would like to acknowledge the valuable contribution of Dr Leigh Gray from the Department of Radiology, Mayo Clinic College of Medicine, Rochester, MN, USA. The Mayo Clinic received funding from NIH (R01 AR49373) for the work submitted. Rachelle Buchbinder is supported by an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship.
Footnotes
Conflict of Interest
Margaret Staples, Matthew Howe, Michael Ringler, Peter Mitchell, Christian Wreidt, John Wark, Peter Ebeling, Richard Osborne, David Kallmes, and Rachelle Buchbinder declare that they have no conflict of interest. The Mayo Clinic has received research funding from Benvenue Medical, Inc for an unrelated spine augmentation project.
Contributor Information
MP Staples, Email: margaret.staples@monash.edu, Cabrini Institute & Department of Clinical Epidemiology, Cabrini Hospital, 183 Wattletree Rd. Malvern, Victoria, 3144, Australia.
BM Howe, Email: Howe.Matthew@mayo.edu, Department of Radiology, Mayo Clinic College of Medicine, Rochester, MN, USA.
MD Ringler, Email: Ringler.Michael@mayo.edu, Department of Radiology, Mayo Clinic College of Medicine, Rochester, MN, USA.
P Mitchell, Email: peter.mitchell@mh.org.au, Department of Radiology, The University of Melbourne, Royal Melbourne Hospital, Melbourne, Victoria, Australia.
CHR Wriedt, Email: cjwriedt@bigpond.net.au, MIA Radiology, 540 Springvale Rd, Glen Waverly, Victoria, 3150, Australia.
JD Wark, Email: jdwark@unimelb.edu.au, Department of Medicine (Royal Melbourne Hospital), Royal Parade, Parkville, Victoria, 3050, Australia.
PR Ebeling, Email: peter.ebeling@monash.edu, Department of Medicine, School of Clinical Sciences, Monash University, Monash Medical Centre, Clayton 3168, Victoria, Australia.
RH Osborne, Email: richard.osborne@deakin.edu.au, School of Health and Social Development, Deakin University, Melbourne, Victoria, Australia.
DF Kallmes, Email: Kallmes.David@mayo.edu, Department of Radiology, Mayo Clinic College of Medicine, Rochester, MN, USA.
R Buchbinder, Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Victoria, Australia, Monash Department of Clinical Epidemiology, Cabrini Hospital, Malvern, Vic.
References
- 1.Buchbinder R, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–68. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 2.Kallmes DF, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, et al. Risk Factors for New Osteoporotic Vertebral Compression Fractures After Vertebroplasty: A Systematic Review and Meta-Analysis. J Spinal Disord Tech. 2012 doi: 10.1097/BSD.0b013e31827412a5. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nakshabandi NA. Percutaneous vertebroplasty complications. Ann Saudi Med. 2011;31(3):294–7. doi: 10.4103/0256-4947.81542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venmans A, et al. Percutaneous Vertebroplasty and Pulmonary Cement Embolism: Results from VERTOS II. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKiernan F, Faciszewski T, Jensen R. Latent mobility of osteoporotic vertebral compression fractures. J Vasc Interv Radiol. 2006;17(9):1479–87. doi: 10.1097/01.RVI.0000235742.26624.37. [DOI] [PubMed] [Google Scholar]
- 7.Tanigawa N, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: long-term evaluation of the technical and clinical outcomes. AJR Am J Roentgenol. 2011;196(6):1415–8. doi: 10.2214/AJR.10.5586. [DOI] [PubMed] [Google Scholar]
- 8.Dansie DM, et al. MRI findings after successful vertebroplasty. AJNR Am J Neuroradiol. 2005;26(6):1595–600. [PMC free article] [PubMed] [Google Scholar]
- 9.Blasco J, et al. Effect of vertebroplasty on pain relief, quality of life, and the incidence of new vertebral fractures: a 12-month randomized follow-up, controlled trial. J Bone Miner Res. 2012;27(5):1159–66. doi: 10.1002/jbmr.1564. [DOI] [PubMed] [Google Scholar]
- 10.Chosa K, Naito A, Awai K. Newly developed compression fractures after percutaneous vertebroplasty: comparison with conservative treatment. Jpn J Radiol. 2011;29(5):335–41. doi: 10.1007/s11604-011-0564-z. [DOI] [PubMed] [Google Scholar]
- 11.Hulme PA, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976) 2006;31(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 12.Uppin AA, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226(1):119–24. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 13.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27(1):217–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ, 3rd, Kallmes DF. Epidemiology of vertebral fractures: implications for vertebral augmentation. Acad Radiol. 2006;13(5):538–45. doi: 10.1016/j.acra.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Álvarez L, et al. Percutaneous vertebroplasty: Functional improvement in patients with osteoporotic compression fractures. Spine. 2006;31(10):1113–1118. doi: 10.1097/01.brs.0000216487.97965.38. [DOI] [PubMed] [Google Scholar]
- 16.Mudano AS, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporosis Int. 2009;20:819–826. doi: 10.1007/s00198-008-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang H, Zhao J, Hao C. Osteoporotic vertebral compression fractures: surgery versus non-operative management. J Int Med Res. 2011;39:1438–1447. doi: 10.1177/147323001103900432. [DOI] [PubMed] [Google Scholar]
- 18.Farrokhi M, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine. 2011;14(5):561–9. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 19.Staples MP, et al. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ. 2011;343:d3952. doi: 10.1136/bmj.d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroon F, et al. Two Year Results of a Randomised Placebo-Controlled Trial of Vertebroplasty for Acute Osteoporotic Vertebral Fractures. J Bone Mineral Res. 2014;29(6):1346–55. doi: 10.1002/jbmr.2157. [DOI] [PubMed] [Google Scholar]
- 21.Buchbinder R, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial [ACTRN012605000079640] BMC Musculoskelet Disord. 2008;9:156. doi: 10.1186/1471-2474-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genant HK, et al. Vertebral fracture assessment using a semiquantitative technique. Journal of Bone & Mineral Research. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 23.Rousing R, et al. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila Pa 1976) 2009;34(13):1349–54. doi: 10.1097/BRS.0b013e3181a4e628. [DOI] [PubMed] [Google Scholar]
- 24.Rousing R, et al. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine (Phila Pa 1976) 2010;35(5):478–82. doi: 10.1097/BRS.0b013e3181b71bd1. [DOI] [PubMed] [Google Scholar]
- 25.Klazen CA, et al. Percutaneous Vertebroplasty Is Not a Risk Factor for New Osteoporotic Compression Fractures: Results from VERTOS II. AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Quantitative measures of modic changes in lumbar spine magnetic resonance imaging: intra- and inter-rater reliability. Spine (Phila Pa 1976) 2011;36(15):1236–43. doi: 10.1097/BRS.0b013e3181ecf283. [DOI] [PubMed] [Google Scholar]
- 27.Hauptfleisch J, et al. Interobserver agreement of magnetic resonance imaging signs of osteomyelitis in pelvic pressure ulcers in patients with spinal cord injury. Arch Phys Med Rehabil. 2013;94(6):1107–11. doi: 10.1016/j.apmr.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Madsen KB, Jurik AG. MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol. 2010;65(1):6–14. doi: 10.1016/j.crad.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Delmas PD, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20(4):557–63. doi: 10.1359/JBMR.041214. Epub 2004 Dec 6. [DOI] [PubMed] [Google Scholar]
- 30.Cauley JA, et al. Risk of mortality following clinical fractures. Osteoporosis International. 2000;11(7):556–61. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 31.Center JR, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 32.Kado DM, et al. Rate of bone loss is associated with mortality in older women: a prospective study. Journal of Bone & Mineral Research. 2000;15(10):1974–80. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- 33.McCullough BJ, et al. Major Medical Outcomes With Spinal Augmentation vs Conservative Therapy. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]