Abstract
Purpose
To investigate the feasibility of correcting ocular higher order aberrations (HOA) in keratoconus (KC) using wavefront-guided optics in a scleral lens prosthetic device (SLPD).
Methods
Six advanced keratoconus patients (11 eyes) were fitted with a SLPD with conventional spherical optics. A custom-made Shack-Hartmann wavefront sensor was used to measure aberrations through a dilated pupil wearing the SLPD. The position of SLPD, i.e. horizontal and vertical decentration relative to the pupil and rotation were measured and incorporated into the design of the wavefront-guided optics for the customized SLPD. A submicron-precision lathe created the designed irregular profile on the front surface of the device. The residual aberrations of the same eyes wearing the SLPD with wavefront-guided optics were subsequently measured. Visual performance with natural mesopic pupil was compared between SLPDs having conventional spherical and wavefront-guided optics by measuring best-corrected high-contrast visual acuity and contrast sensitivity.
Results
Root-mean-square of HOA(RMS) in the 11 eyes wearing conventional SLPD with spherical optics was 1.17±0.57μm for a 6 mm pupil. HOA were effectively corrected by the customized SLPD with wavefront-guided optics and RMS was reduced 3.1 times on average to 0.37±0.19μm for the same pupil. This correction resulted in significant improvement of 1.9 lines in mean visual acuity (p<0.05). Contrast sensitivity was also significantly improved by a factor of 2.4, 1.8 and 1.4 on average for 4, 8 and 12 cycles/degree, respectively (p<0.05 for all frequencies). Although the residual aberration was comparable to that of normal eyes, the average visual acuity in logMAR with the customized SLPD was 0.21, substantially worse than normal acuity.
Conclusions
The customized SLPD with wavefront-guided optics corrected the HOA of advanced KC patients to normal levels and improved their vision significantly.
Keywords: keratoconus, higher order aberrations, contact lens, Boston scleral lens, wavefront-guided treatment, visual acuity, contrast sensitivity, neural adaptation and plasticity
In 1961, Smirnov suggested upon quantifying the optical imperfections of the eye that it was conceivable to make lenses to correct for them1. However, he also remarked it was highly impractical given the laborious nature of the aberration measurements. Recent technological advances have allowed the routine and accurate quantification of the ocular higher order aberrations (HOA) in normal eyes 2–4. Using these advanced methodologies, the abnormal corneal disorder of keratoconus (KC) has also been evaluated 5, 6. The main optical consequence of the corneal steepening and thinning in KC is the presence of large magnitude of HOA, around 5–6 times typically found in normal eyes, thus severely degrading retinal image quality. For a 5.7 mm pupil, Guirao et al. 7 theoretically demonstrated that an improvement by a factor of 12 in retinal image contrast at 16 c/deg could be achievable in KC compared to only 2.5-fold benefit in normals with the correction of HOA. Similarly for a 6 mm pupil, when computing the area under the modulation transfer function, Pantanelli et al.5 estimated a 4.4-fold improvement in retinal image quality in KC compared to only 2.1-fold improvement in normals with correction of HOA. Therefore, these KC patients stand to benefit to a great extent by correcting HOA. An important feature of ocular HOA in general, whether normal or highly aberrated eyes, is the inter-individual variability. Even though vertical coma and secondary astigmatism show a consistent trend across the KC population, being negative in sign, there is substantial variability in sign and magnitude of HOA. Therefore, any methodology proposed to correct for HOA must account for the particular aberration profile of the patient. Pupil size, receptoral sampling limits and post-receptoral neural factors are additional important factors to bear in mind while aiming at maximizing visual benefit with an optical correction8. In normal eyes, customized optical and surgical methods, such as adaptive optics9, 10, phase plates11 and customized laser refractive surgery12 have been proposed to compensate for HOA to provide improvement in vision. However, limited effort has been made towards developing such methodologies for KC.
Presently, rigid gas permeable (RGP) corneal and scleral lenses are considered the standard of correction in KC. These lenses achieve correction by masking corneal irregularities with the tear lens between the posterior lens surface and the anterior corneal surface. RGP corneal contact lenses are 8.5 to 10.5 mm in diameter and cover only 75–80 % of the cornea. Mini-scleral, corneo-scleral and scleral lenses which range in diameter from 13–24 mm, depending on type or fit, may rest partly on the cornea. A scleral lens prosthetic device (SLPD) with diameters ranging from 17.5–24 mm is designed and fit to vault the cornea entirely. A noteworthy difference between RGP corneal lens and SLPD is the dynamic movement of these corrective devices on the eye. A well-fitted corneal RGP lens slides with each blink to allow for tear exchange necessary for physiological tolerance at the corneal surfaces where contact is made. Optical correction in an RGP corneal lens is thus inherently unstable. A carefully fitted SLPD is expected to exhibit minimal movement because the bearing haptic aligns with a large area of conjunctiva overlying the sclera. Suction is avoided by precise alignment with the sclera or by creation of channels on the posterior surface13, 14. The devices used in this study were approved by the FDA in 1994 for daily wear in the treatment of irregular astigmatism and ocular surface disorders. Over the past 20 years, these devices have been referred to as the Boston Scleral Contact Lens, the Boston Scleral Lens, the Boston Scleral Lens Device, the Boston Scleral Lens Prosthetic Device, and the Boston Ocular Surface Prosthesis (BOS-P) manufactured by the Boston Foundation for Sight, Needham, MA. The clinical benefits of an SLPD in terms of improvement in visual acuity and visual function across a wide range of diagnoses including KC have been established15. By virtue of their larger diameter and broader bearing zone, these devices have been employed in the treatment of various other forms of corneal ectasia, corneal irregularity following transplant, ulcers, dry eye syndrome, ocular surface disease and others16, 17. Despite the large diameter and complexity of fitting and training in insertion and removal, high patient satisfaction in terms of wearing comfort has been observed with scleral lenses in the management of corneal abnormality18. Reduction of HOA has been reported in normal and KC eyes with corneal RGP lens19–22 and SLPD23. However, since these lenses minimize only anterior corneal aberrations, significant posterior corneal aberrations remain uncompensated24. In addition, aberrations such as coma and astigmatism are induced due to RGP corneal lens rotation and decentration, further degrading retinal image quality. The positional stability between blinks due to the large surface coverage in SLPD makes it an ideal platform for wavefront-guided correction of HOA in KC.
Soft contact lenses with wavefront-guided surface profiles have also been shown to have potential in correcting HOA and improving vision in KC 25–27. In such a scheme of correction in two eyes with KC shown by López-Gil et al., the lens was designed according to the aberration profile of the eye, but the reduction in higher order aberration and improvement in vision were relatively small25. One possible explanation for the small average reduction in higher order aberration could be the failure to account for the decentration and rotation of the contact lens on the eye. Static and dynamic contact lens movements critically affect correction performance of HOA, as the lens is not aligned to the center of the visual axis, especially in eyes with abnormal corneal surface profiles 11, 28. Sabesan et al. first demonstrated a scheme of correction in KC using soft contact lenses where the lens was designed by accounting for both the eye’s aberration profile and the static lens decentration and rotation on eye26. Using these wavefront-guided customized soft contact lenses, Sabesan et al. demonstrated an improvement in optical quality by a factor of 3 in HOA with respect to the conventional lens on average in 3 KC patients. The improved optics resulted in an average improvement of 2.1 lines in visual acuity over the conventional correction of defocus and astigmatism alone. However, the residual higher order wavefront error was still nearly double of what is observed in normal eyes. This residual error was explained to a reasonable extent by the manufacturing error and lens movement. To reduce the variability of lens position by conferring mechanical stability between blinks, Chen et al.29 employed back surface customized soft contact lenses whose posterior surface profiles were sculpted to match the anterior corneal surface in KC. On-eye performance of the back surface customized soft contact lens demonstrated that the lens stability was improved by a factor of 2 for horizontal and vertical decentration, and a factor of 5 in rotational orientation over conventional lens. However, significant residual HOA induced by internal optics, especially posterior corneal surface still degraded retinal image quality. Additional customization of the front surface of the lens thus has the potential to further correct these residual aberrations.
In this article, the feasibility of correcting HOA in eyes with advanced KC using SLPD with wavefront-guided optics was investigated, with the aim of reducing optical aberrations to normal levels. SLPD was chosen as the platform for customization due to their excellent positional stability on the eye. A custom-built Shack-Hartmann wavefront sensor equipped with the large dynamic range of wavefront measurement and real time pupil imaging capability was employed to overcome the limitations of clinically available aberrometers in evaluating severely aberrated KC. Optical and visual performance was measured with SLPD on eye with conventional spherical optics and wavefront-guided optics to determine the efficacy of customized treatment.
METHODS
Subjects
The New England Institutional Review Board approved this research and all patients signed an informed consent form before their participation in this study. All procedures involving human patients were in accordance with the tenets of the Declaration of Helsinki. Six patients with advanced KC (11 eyes) were enrolled in this study. Their average age and steep K reading was 41.2±10.6 years and 58.0±13.5 D, respectively. Other than KC, all eyes had otherwise normal eye exam and clear media with the exception of one eye that had history of hydrops with residual scar outside the visual axis and another eye that had an incidental finding of a small polar congenital cataract.
Design of SLPD with Wavefront-guided Optics
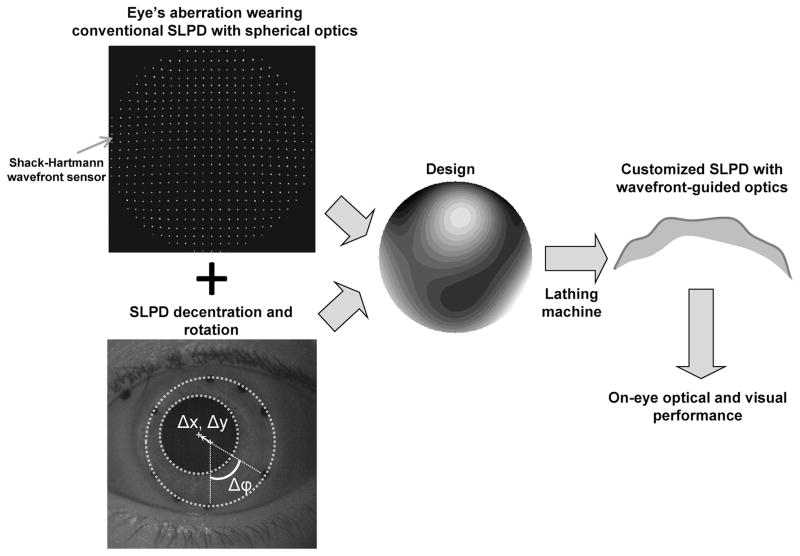
The patients were fitted first with conventional SLPD with spherical optics. Each conventional spherical optics device featured a central optic zone with a customized peripheral haptic that aligned with the sclera. By incorporating back surface toricity and quadrant specific adjustments, there was minimal to no compression nor impingement of the conjunctiva. The toricity was defined as an increase of sagittal depth at the edge from the flattest meridian, without any angular dependence. The meridians were 90 degrees apart and aligned to the scleral toricity as a bitoric or back surface toric corneal gas permeable lens would. Satisfactory fit was confirmed with the absence of rebound hyperemia (from compression) and the absence of conjunctival staining (from impingement) after removal of the device worn for six hours. Spline functions were used to create seamless transition zones, allowing control of vault above the cornea independent of lens base curve. Each device had varying amounts of back surface toricity that varied independently between four quadrants. Back surface toricity provided the alignment of the haptic to the scleral shape, but did not extend into the central optic zone of 10mm. This fitting process was repeated for every eye so that the fit of the conventional SLPD was customized in order to minimize its dynamic movement. In majority of the cases, one trial was sufficient to ensure a satisfactory device. Precise alignment points were lathe-cut around the edge of the conventional SLPD to determine its movement on the eye. A surgical marking pen was used to color the alignment marks with black ink under a microscope, to aid their visibility in pupil images. The schematic of the design of SLPD with wavefront-guided optics is shown in Figure 1. The pupil of each subject’s eye was dilated using 1% tropicamide ophthalmic solution, so that wavefront aberration measurement could be obtained over the largest measurable pupil. Aberrations of the KC eyes wearing conventional SLPD with spherical optics were first measured using a custom-made Shack-Hartmann wavefront sensor with simultaneous pupil imaging capability. The wavefront calculated from the Shack-Hartmann spot pattern served as the profile to be fabricated on the device. Using the device center as the coordinate system origin, the static horizontal (Δx), vertical (Δy) decentration and rotation (Δφ) of the device with respect to the pupil center was quantified from the pupil images using custom-built software in MATLAB (Mathworks Inc., Natick, MA). A sub-micron precision, computerized lathe (Precitech Nanoform 250, AMETEK, Precitech, Inc., Keene, NH) was used to diamond-turn the irregular profile on the front surface of the device. The optic zone diameter for manufacturing the optical correction corresponded to the maximum measurable wavefront diameter through the pharmacologically dilated pupil ranging from 7–8.5mm in our subject group. The customized SLPD with wavefront-guided optics, thus manufactured, was first evaluated using optical metrology described in the following section to determine the precision of its fabrication. This was followed by a comparison of its optical and visual performance with the conventional SLPD with spherical optics when placed on the same eye.
Figure 1.
Schematic of the design of SLPD with wavefront-guided optics. The eye’s aberration wearing the conventional SLPD with spherical optics, in addition to the static device decentration and rotation was combined to yield the surface profile to be lathed. The manufactured customized SLPD with wavefront-guided optics was evaluated by comparing its optical and visual performance with the conventional SLPD when placed on the same KC eye. A color version of this figure is available online at www.optvissci.com.
Optical and Visual Performance Evaluation
Optical metrology to measure the precision of fabrication was performed with a custom-developed Shack-Hartmann wavefront sensor. This sensor was specifically designed to measure the optical aberrations of contact and scleral lenses in vitro, whether in their dry or hydrated state. The fidelity of measurement has been established previously and described elsewhere30 in the case of soft contact lenses. For this study, aberration measurements were performed for the customized SLPD with wavefront-guided optics and compared with its corresponding design parameters.
On-eye performance was evaluated by another custom-developed Shack-Hartmann wavefront sensor by measuring optical aberrations with the conventional and customized SLPD in situ. The pupil of the eye was optically conjugated to the Shack-Hartmann microlens array after demagnifying it by 33%. The Shack-Hartmann microlens array had a spacing of 150 μm and a focal length of 3.76 mm. The spot pattern formed by the microlens array was imaged on a charge coupled device camera with 6.45 μm pixel size. Wavefront aberrations were calculated from this spot array pattern and decomposed into coefficients of Zernike polynomials up to the 6th order. Zernike coefficients were expressed over a 6 mm pupil across all eyes for comparison between the conventional and customized SLPD. The eye’s pupil was imaged using another camera focused at the pupil plane under infra-red light emitting diode illumination, simultaneously with the wavefront measurement. These pupil images allowed the quantification of x,y decentration and rotation of the SLPD with respect to the pupil center.
Visual performance was evaluated monocularly under natural mesopic pupil condition by measuring high contrast tumbling ‘E’ visual acuity and contrast sensitivity using a calibrated cathode ray tube display. The display was placed 10 feet from the patient in a dark room. The untested eye was occluded. The tumbling ‘E’ test used the four-alternate forced-choice method where the illiterate letter ‘E’ was presented to the observer in one of four orientations, 0, 90, 180 or 270 deg and the observer’s task was to respond to the orientation of the letter by pressing the appropriate button. A psychometric function based on 30 trials was derived by using the QUEST paradigm31. Visual acuity was determined as the line thickness of the letter for which at least 62.5% of the observer’s responses were correct. Contrast sensitivity was measured similarly using the two-alternate forced-choice method where the observer’s task was to distinguish the orientation of 2-D Gabor functions, shown either vertically or horizontally. A 2-D Gabor function is a sinusoidal luminance distribution overlaid with a Gaussian envelope and is routinely used in several psychophysical experiments. Contrast threshold at 4, 8 and 12 c/deg was determined by the contrast at the respective spatial frequency for which at least 75% of the observer’s responses were correct. The size of the visual field on the retina was 3 deg. These visual performance measurements were performed after spherocylindrical refractive error was optimized with trial lenses to assess the visual benefit of correcting only HOAs.
RESULTS
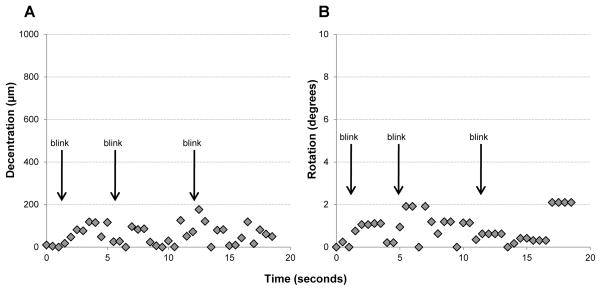
Table 1 shows the horizontal, vertical decentration and rotation for all the patients with the conventional and customized SLPD. For 2 of the eyes, these measures were not obtainable because of insufficient contrast in pupil camera images. From the designed (or measured) x and y decentration and rotation with the conventional lens, the customized SLPD with wavefront-guided optics deviated by 63.4μm, 136.9μm and 6.9 degrees, respectively on average, between the eyes. In addition, the SLPD exhibited good temporal stability on the eye between blinks. Figure 2 shows the magnitude of vector decentration (A) and rotation (B) with time over 3 natural blinks, when the conventional SLPD was placed on an advanced KC eye. The offset from the designed decentration and rotation averaged over 20 secs was 67.3±54.5 μm and 0.94±0.58 deg respectively.
Table 1.
Horizontal, vertical decentration and rotation with the conventional and customized SLPD for the KC patients.
| Patient # | Conventional SLPD | Customized SLPD | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| X Decentration (μm) | Y Decentration (μm) | Rotation (deg) | X Decentration (μm) | Y Decentration (μm) | Rotation (deg) | ||
| 1 | OD | 465 | 766 | −11 | Not available | ||
| OS | −255 | 443 | 20 | −230 | 420 | 16 | |
|
| |||||||
| 2 | OD | 327 | 1127 | −54 | Not available | ||
| OS | 32 | 1008 | 51 | 30 | 1140 | 58 | |
|
| |||||||
| 3 | OD | 266 | 731 | −16 | 330 | 450 | −6 |
| OS | 100 | 731 | 32 | 40 | 700 | 33 | |
|
| |||||||
| 4 | OD | 188 | 1205 | −19 | 90 | 1320 | −19 |
| OS | −240 | 1300 | 19 | −190 | 1240 | 19 | |
|
| |||||||
| 5 | OD | 596 | 740 | 2 | 690 | 1050 | 5 |
| OS | −323 | 740 | 1 | −490 | 980 | 20 | |
|
| |||||||
| 6 | OS | −130 | 920 | 16 | 120 | 960 | −1 |
Figure 2.
Magnitude of vector decentration (A) and rotation (B) with time over 3 natural blinks, when the SLPD was placed on an advanced KC eye.
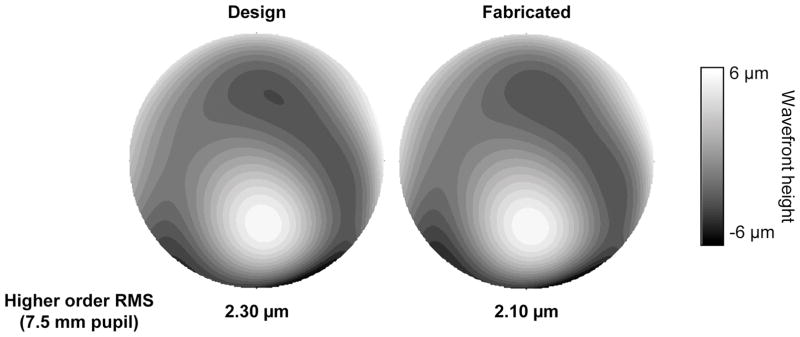
Figure 3 shows the higher order wavefront maps for the designed and the fabricated customized SLPD with wavefront-guided optics for one eye. The higher order RMS for the designed SLPD was 2.30μm over 7.5mm pupil. The error in fabrication, defined as the higher order RMS difference between the designed and fabricated customized SLPD, was 0.2μm.
Figure 3.
Higher order wavefront map for the designed and the fabricated customized SLPD with wavefront-guided optics over a 7.5mm pupil. A color version of this figure is available online at www.optvissci.com.
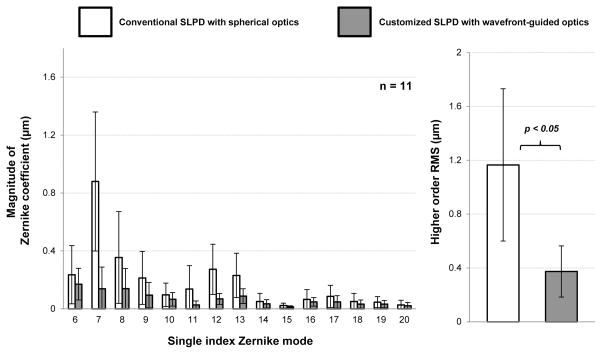
Table 2 shows the keratometric readings in the patients, in addition to the lower and higher order RMS with the conventional and customized SLPD. Figure 4 shows the wavefront aberration in terms of the magnitude of Zernike polynomial coefficients with the conventional spherical and customized wavefront-guided optics SLPD in 11 KC eyes over a 6mm pupil. Also shown is the higher order root-mean-square (RMS) of the aberrations in both cases. Mean ± standard deviation of higher order RMS of the eyes with the conventional SLPD was 1.17 ± 0.57 μm for a 6 mm pupil. The most dominant higher order aberration was positive vertical coma that accounted for 79% of the total higher order aberration. Vertical coma and secondary astigmatism (Zernike single mode numbers 7 and 13 respectively) were the only two aberrations which were consistently positive across all patients with the conventional SLPD. Almost all the HOAs were effectively corrected by the customized SLPD and higher order RMS was reduced 3.1 times on average to 0.37 ± 0.19 μm for the same 6 mm pupil. Thus, a level of aberration similar to that observed in a normal population was achieved.
Table 2.
Keratometric readings & lower (LO) and higher( order(HO) RMS with the conventional and customized SLPD for the KC patients.
| Patient # | Keratometric values | Conventional SLPD (6 mm pupil) | Customized SLPD (6 mm pupil) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| K flat (D) | K steep (D) | LO RMS (μm) | HO RMS (μm) | LO RMS (μm) | HO RMS (μm) | ||
| 1 | OD | 47.8 | 50.8 | 0.64 | 0.32 | 0.18 | 0.28 |
| OS | 48.9 | 50.1 | 1.24 | 0.43 | 0.41 | 0.22 | |
|
| |||||||
| 2 | OD | 69.9 | 80.9 | 1.51 | 1.95 | 0.19 | 0.67 |
| OS | 55.9 | 69.1 | 1.18 | 1.50 | 0.14 | 0.71 | |
|
| |||||||
| 3 | OD | 46.9 | 52.8 | 2.82 | 1.49 | 0.49 | 0.37 |
| OS | 50.3 | 57.4 | 2.66 | 1.74 | 0.48 | 0.35 | |
|
| |||||||
| 4 | OD | 43.1 | 44.4 | 1.26 | 0.83 | 0.78 | 0.20 |
| OS | 51.4 | 67.5 | 1.10 | 1.35 | 1.04 | 0.21 | |
|
| |||||||
| 5 | OD | 71.3 | 88.3 | 3.22 | 1.67 | 1.65 | 0.22 |
| OS | 57.5 | 81.4 | 2.53 | 0.95 | 0.80 | 0.56 | |
|
| |||||||
| 6 | OS | 42.5 | 48.4 | 0.60 | 0.59 | 0.18 | 0.31 |
Figure 4.
Average magnitude of Zernike coefficient with the conventional spherical and customized wavefront-guided optics SLPD in 11 eyes with advanced KC over a 6mm pupil. The Zernike coefficients are expressed according to the single value modes suggested in the ANSI Z80.28-2004 standard. The average higher order root-mean-square with the conventional and customized SLPD is also shown for the 11 KC eyes. The result of two-tailed, paired student’s t-test of statistical significance is indicated.
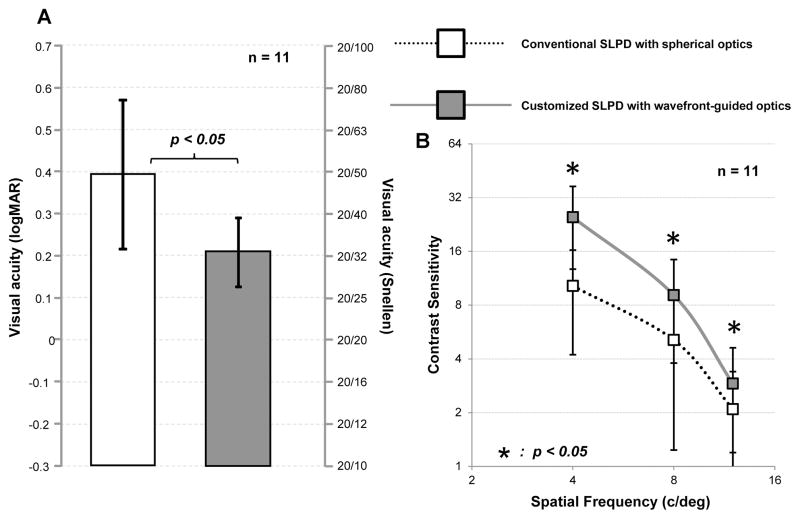
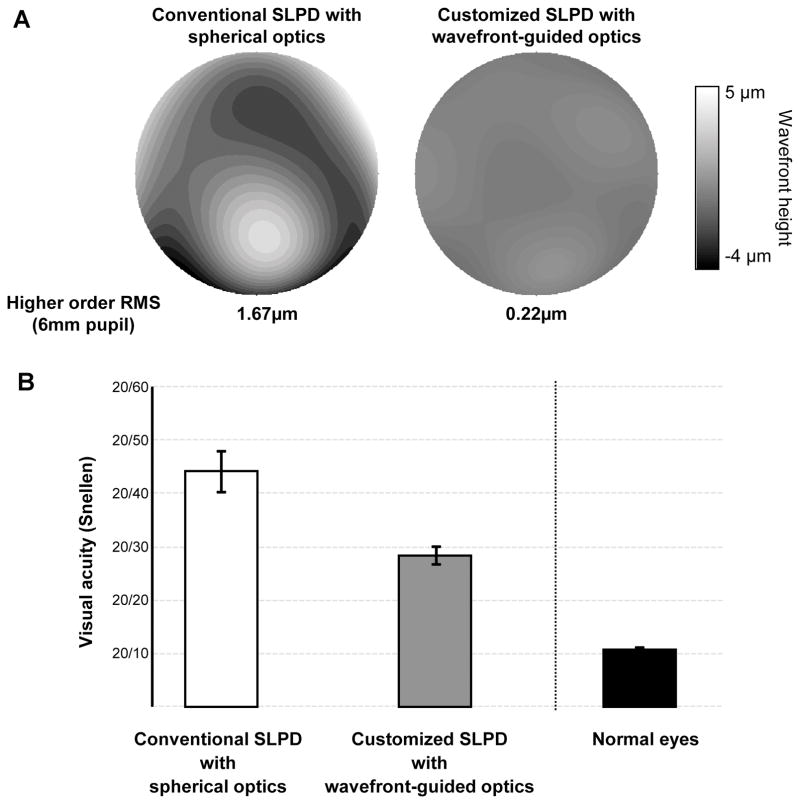
Figures 5 (a) and (b) show the average visual acuity and contrast sensitivity respectively in the 11 KC eyes with the conventional spherical and customized wavefront-guided optics SLPD. The wavefront-guided optical correction resulted in significant improvement of 1.9 lines on average in visual acuity (p<0.05). Contrast sensitivity was also significantly improved by a factor of 2.4, 1.8 and 1.4 on average for 4, 8 and 12 c/deg respectively (p<0.05 for all frequencies). All patients reported a considerable improvement in subjective image quality. Although the magnitude of the residual aberration was comparable with the normal eye, the average visual acuity in Snellen equivalent with the customized SLPD with wavefront-guided optics was 20/32, significantly worse than normal acuity. Figure 6 presents the case of the advanced KC patient (age: 37yo) whose optical quality with the customized SLPD was the best among the 11 eyes. In this eye over 6 mm pupil, the higher order RMS of 1.67 μm with the conventional spherical optics SLPD was reduced to a value of 0.22μm with the customized wavefront-guided optics SLPD (figure 6A). With the wavefront-guided correction, the high contrast visual acuity was improved by 2 lines (figure 6B) while the contrast sensitivity improved by a factor 5.9, 4.8 and 3.8 for 4, 8 and 12 c/deg respectively, over the spherical optics correction. However, even with substantial optical correction, the Snellen equivalent visual acuity was still 20/28.4, significantly worse than normals. The Snellen equivalent visual acuity in 4 normal eyes (average age: 25 ± 4.6yo) with comparable native higher order RMS of 0.25 ± 0.04 μm over the same 6 mm pupil was also measured and is shown in figure 6B32. With similar level of wavefront error, these normal eyes obtained Snellen equivalent visual acuity of 10.7 ± 0.3, close to the approximate upper-bound sampling-limited visual acuity of 20/10.
Figure 5.
(A) Mean visual acuity and (B) mean contrast sensitivity at 4, 8 and 12 c/deg with conventional spherical and customized wavefront-guided optics SLPD in 11 eyes with advanced KC eyes viewing through natural mesopic pupil. The result of two-tailed, paired student’s t-test of statistical significance is indicated for visual acuity and at all spatial frequencies for contrast sensitivity.
Figure 6.
Optical and visual performance of the eye with advanced KC which had the best correction among the 11 eyes. The wavefront maps (A) and visual acuity (B) with the conventional spherical optics and customized wavefront-guided optics SLPD is shown. The average measured visual acuity of 4 normal eyes with comparable native optical quality is also shown in figure 6B. A color version of this figure is available online at www.optvissci.com.
DISCUSSION
We have demonstrated the significant reduction of HOA by incorporating wavefront-guided optics with SLPD in advanced KC patients resulting in a substantial improvement in visual performance. This establishes the possibility of providing abnormal corneal patients with nearly a normal level of optical and visual quality using wavefront-guided corrections. In addition, the better subjective preference and corneal health with these devices make them an excellent candidate for habitual wear33, 34
Although, the neutralization of HOA to improve vision using ophthalmic lenses was first proposed by Smirnov in 19611, it has taken several decades to realize such corrections which can be clinically prescribed for treatment. Methodologies such as the use of phase plates for the correction of HOA have been developed, but face inherent practical limitations for routine use, especially with respect to optical alignment with the pupil. The growth in wavefront sensing and sub-micron precision lathing technology have contributed significantly to this endeavor by allowing for accurate measurement and correction of the wave aberrations in highly aberrated eyes. Significant visual benefit upon observing the large magnitudes of HOA in KC was predicted 5, 7. To translate the theoretical visual benefit into practical improvement in optical and visual performance, some key factors were important. Incorporating the centration information of the corrective optic for each SLPD on eye was indispensable in achieving effective neutralization of aberrations. The effect of the interaction of the SLPD with the ocular surface was also important to account for by first undertaking the measurements with the conventional SLPD on the eye. Both these factors have been employed previously in the design of wavefront-guided treatments for KC26, 27. Sabesan et al.26 achieved similar factors of improvement in optical and visual acuity with customized soft contact lenses in 3 KC eyes. However, the residual higher order wavefront error was 0.93 ± 0.19 μm for a 6mm pupil in their study, nearly 3 times larger than the residual RMS of 0.37 ± 0.19 μm obtained with customized SLPD with wavefront-guided optics in this study for the same pupil size. Similarly, with customized soft contact lenses Marsack et al.27 obtained residual higher order RMS of 0.31 and 0.38μm for 4.25mm pupil in 2 KC patients and 0.76μm for 4.5mm pupil in the third KC patient. For comparison, the pupil size was re-normalized from 6 mm to 4.5 mm in the present study and a lower residual higher order RMS with the customized SLPD in the 11 KC eyes, equal to 0.22 ± 0.11μm, was obtained.
Both these previous studies employed soft contact lens as the vehicle for customization which faces additional limitations such as the effect of variable and dynamic lens movement and lens flexure when it conforms to the cornea. In addition, the masking of the anterior corneal aberrations with the tear lens due to refractive index matching is minimal with soft lenses compared to RGP lenses. The latter maintains its physical attributes when placed on the eye and partially compensates the anterior corneal irregularities by the aforementioned tear film masking. However, with conventional corneal RGP lens, scleral lens and SLPD, an overcorrection of aberrations can be expected due to two factors. Firstly, the aberrations arising from the posterior cornea and crystalline lens, which partially compensate for the anterior corneal aberrations in the naked eye, remain uncorrected with these lenses. Chen et al. showed that the resultant vertical coma induced by the internal optics is positive in sign24. Secondly, aberrations induced due to the static decentration of the corneal RGP lens, scleral lens and SLPD affects retinal image quality significantly. Spherical surfaces of these lenses induce positive spherical aberration. This combined with the decentration with respect to the pupil gives rise to coma and astigmatism. The vertical coma induced due to static decentration of corneal RGP lens and SLPD is also positive in sign, since they normally rest inferiorly to the pupil by about 1mm. Therefore, the aberrations arising from internal optics and inferior decentration of spherical optics account for the positive sign of vertical coma with the conventional SLPD shown in figure 4, which is otherwise negative in sign for an uncorrected eye with keratoconus 5. Overcorrection of negative vertical coma was also observed with RGP corneal lens correction previously35. To overcome these additional residual aberrations, the front surface of the conventional SLPD was sculpted in accordance with both the measured wavefront aberration with the SLPD on eye and its static movement. The custom-developed high-dynamic range Shack Hartmann wavefront sensor with pupil imaging capability ensured rapid and accurate quantification of these parameters. It is important, however, to quantify the movement of these devices across a longer time period to investigate their dynamic stability. Unlike conventional corneal RGP and soft contact lenses, we found that the static deviation of the optic zone from the designed position was minimal in the SLPD. This combined with the sub-micron precision lathing technology facilitated an excellent optical correction by 3.1 times in higher order RMS leading to a substantial improvement in visual performance.
Wavefront-guided SLPD thus offer for the first time a superior optical correction of highly aberrated eyes to around what is typically observed in normals. Interestingly, the visual acuity was still significantly poorer than what is typically observed in the normal population over the same pupil size. For instance, in the case of the patient shown in Figure 6, the residual higher order RMS was as low as 0.22μm over a 6mm pupil, but the Snellen visual acuity was still significantly worse than normals at 20/28.4. For the same level of higher order RMS in normal eyes, visual acuity better than 20/15 can be expected. Inexplicable by optical factors, this discrepancy in visual performance might be attributed to post-receptoral neural factors. In particular, long-term visual experience with poor retinal image quality in KC may restrict the visual benefit achievable immediately after the customized correction. Isolation of the neural factors from optical factors in determining the causes for restricted visual performance is tedious with such customized ophthalmic lenses due to practical difficulties and relatively higher residual aberrations. By surpassing the limit imposed by the optics of the eye using adaptive optics, Sabesan and Yoon36 recently compared the post-receptoral neural factors in normals and in patients with KC. Visual acuity was significantly worse in KC compared to normal eyes even after completely correcting aberrations to similar near-diffraction limited retinal image quality in both groups. As a consequence of chronic exposure to blur in KC patients, there might be a loss in sensitivity to fine spatial detail as present in a perfect retinal image, thus limiting the visual performance when correcting the ocular optics completely. Such neural deficit in the presence of HOA correction observed in KC might be considered analogous to meridional amblyopia observed in astigmats37 and to sub-normal visual performance in low myopes after HOA correction38. It is important to note that such neural deficit in KC arises long after visual development is completed while meridional amblyopia occurs during visual development. Moving forward, it is of both scientific and clinical interest to investigate the time taken for the visual system to re-adapt to the improved ocular optics, as provided by wavefront-guided SLPD, in order to achieve maximum visual performance.
In summary, wavefront-guided customized SLPD provided substantial reduction in HOA in advanced KC, thus providing them for the first time with a normal level of ocular optics. The corrected optics led to a substantial benefit in visual acuity and contrast sensitivity. However, chronic exposure to poor retinal image quality restricted the visual benefit achievable immediately after wavefront-guided customized treatment in these eyes. Nevertheless, the utility of customized ophthalmic lenses is not only limited to provide normal level of vision for KC and abnormal corneal patients, but can be extended to the design of any optical treatment designated for aberration manipulation.
Acknowledgments
This research was funded by the NIH/NEI grant RO1EY 014999 and Research to Prevent Blindness. None of the authors have any commercial interest in the matter presented in this article. This research was presented at the American Academy of Optometry annual meeting in Boston, MA on 12th October 2011 and at the Association for Research in Vision and Ophthalmology meeting at Fort Lauderdale, FL on May 7th 2012.
References
- 1.Smirnov MS. Measurement of the wave aberration of the human eye. Biofizika. 1961;6:776–95. [PubMed] [Google Scholar]
- 2.Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am (A) 2001;18:1793–803. doi: 10.1364/josaa.18.001793. [DOI] [PubMed] [Google Scholar]
- 3.Thibos LN, Hong X, Bradley A, Cheng X. Statistical variation of aberration structure and image quality in a normal population of healthy eyes. J Opt Soc Am A Opt Image Sci Vis. 2002;19:2329–48. doi: 10.1364/josaa.19.002329. [DOI] [PubMed] [Google Scholar]
- 4.Liang J, Grimm B, Goelz S, Bille JF. Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J Opt Soc Am (A) 1994;11:1949–57. doi: 10.1364/josaa.11.001949. [DOI] [PubMed] [Google Scholar]
- 5.Pantanelli S, MacRae S, Jeong TM, Yoon G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology. 2007;114:2013–21. doi: 10.1016/j.ophtha.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Maeda N, Fujikado T, Kuroda T, Mihashi T, Hirohara Y, Nishida K, Watanabe H, Tano Y. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology. 2002;109:1996–2003. doi: 10.1016/s0161-6420(02)01279-4. [DOI] [PubMed] [Google Scholar]
- 7.Guirao A, Porter J, Williams DR, Cox IG. Calculated impact of higher-order monochromatic aberrations on retinal image quality in a population of human eyes. J Opt Soc Am (A) 2002;19:1–9. doi: 10.1364/josaa.19.000001. [DOI] [PubMed] [Google Scholar]
- 8.Williams DR, Porter J, Yoon G, Guirao A, Hofer H, Chen L, Cox I, MacRae SM. How far can we extend the limits of human vision? In: Krueger RR, Applegate RA, editors. Wavefront Customized Visual Corrections: The Quest for Super Vision II. Thorofare, NJ: SLACK Inc; 2004. pp. 19–38. [Google Scholar]
- 9.Yoon GY, Williams DR. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J Opt Soc Am (A) 2002;19:266–75. doi: 10.1364/josaa.19.000266. [DOI] [PubMed] [Google Scholar]
- 10.Fernández EJ, Iglesias I, Artal P. Closed-loop adaptive optics in the human eye. Opt Lett. 2001;26:746–8. doi: 10.1364/ol.26.000746. [DOI] [PubMed] [Google Scholar]
- 11.Navarro R, Moreno-Barriuso E, Bara S, Mancebo T. Phase plates for wave-aberration compensation in the human eye. Opt Lett. 2000;25:236–8. doi: 10.1364/ol.25.000236. [DOI] [PubMed] [Google Scholar]
- 12.MacRae SM, Schwiegerling J, Snyder R. Customized corneal ablation and super vision. Journal of Refractive Surgery. 2000;16:S230–S5. doi: 10.3928/1081-597X-20000302-06. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal P. Scleral lens with scalloped channels or circumferential fenestrated channels. 7,695,135. US Patent. 2010 Apr 13;
- 14.Rosenthal P. Scleral contact lens with grooves and method of making lens. 7,591,556. US Patent. 2009 Sep 22;
- 15.Stason WB, Razavi M, Jacobs DS, Shepard DS, Suaya JA, Johns L, Rosenthal P. Clinical benefits of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2010;149:54–61. doi: 10.1016/j.ajo.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Pullum KW, Whiting MA, Buckley RJ. Scleral contact lenses: the expanding role. Cornea. 2005;24:269–77. doi: 10.1097/01.ico.0000148311.94180.6b. [DOI] [PubMed] [Google Scholar]
- 17.Segal O, Barkana Y, Hourovitz D, Behrman S, Kamun Y, Avni I, Zadok D. Scleral contact lenses may help where other modalities fail. Cornea. 2003;22:308–10. doi: 10.1097/00003226-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Visser ES, Visser R, van Lier HJ, Otten HM. Modern scleral lenses part II: patient satisfaction. Eye Contact Lens. 2007;33:21–5. doi: 10.1097/01.icl.0000228964.74647.25. [DOI] [PubMed] [Google Scholar]
- 19.Hong X, Himebaugh N, Thibos LN. On-eye evaluation of optical performance of rigid and soft contact lenses. Optom Vis Sci. 2001;78:872–80. doi: 10.1097/00006324-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Dorronsoro C, Barbero S, Llorente L, Marcos S. On-eye measurement of optical performance of rigid gas permeable contact lenses based on ocular and corneal aberrometry. Optom Vis Sci. 2003;80:115–25. doi: 10.1097/00006324-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Marsack JD, Parker KE, Pesudovs K, Donnelly WJ, 3rd, Applegate RA. Uncorrected wavefront error and visual performance during RGP wear in keratoconus. Optom Vis Sci. 2007;84:463–70. doi: 10.1097/OPX.0b013e31802e64f0. [DOI] [PubMed] [Google Scholar]
- 22.Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of higher-order aberrations on visual function in keratoconic eyes with a rigid gas permeable contact lens. Am J Ophthalmol. 2007;144:924–9. doi: 10.1016/j.ajo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Gumus K, Gire A, Pflugfelder SC. The impact of the Boston ocular surface prosthesis on wavefront higher-order aberrations. Am J Ophthalmol. 2011;151:682–90. doi: 10.1016/j.ajo.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Yoon G. Posterior corneal aberrations and their compensation effects on anterior corneal aberrations in keratoconic eyes. Invest Ophthalmol Vis Sci. 2008;49:5645–52. doi: 10.1167/iovs.08-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Gil N, Chateau N, Castejón-Monchón J, Benito A. Correcting ocular aberrations by soft contact lenses. S Afr Optom. 2003;62:173–7. [Google Scholar]
- 26.Sabesan R, Jeong TM, Carvalho L, Cox IG, Williams DR, Yoon G. Vision improvement by correcting higher-order aberrations with customized soft contact lenses in keratoconic eyes. Opt Lett. 2007;32:1000–2. doi: 10.1364/ol.32.001000. [DOI] [PubMed] [Google Scholar]
- 27.Marsack JD, Parker KE, Applegate RA. Performance of wavefront-guided soft lenses in three keratoconus subjects. Optom Vis Sci. 2008;85:1172–8. doi: 10.1097/OPX.0b013e31818e8eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guirao A, Williams DR, Cox IG. Effect of rotation and translation on the expected benefit of an ideal method to correct the eye’s higher-order aberrations. J Opt Soc Am (A) 2001;18:1003–15. doi: 10.1364/josaa.18.001003. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Sabesan R, Ahmad K, Yoon G. Correcting anterior corneal aberration and variability of lens movements in keratoconic eyes with back-surface customized soft contact lenses. Opt Lett. 2007;32:3203–5. doi: 10.1364/ol.32.003203. [DOI] [PubMed] [Google Scholar]
- 30.Jeong TM, Menon M, Yoon G. Measurement of wave-front aberration in soft contact lenses by use of a Shack-Hartmann wave-front sensor. Appl Opt. 2005;44:4523–7. doi: 10.1364/ao.44.004523. [DOI] [PubMed] [Google Scholar]
- 31.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–20. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 32.Sabesan R, Zheleznyak L, Yoon G. Binocular visual performance and summation after correcting higher order aberrations. Biomed Opt Express. 2012;3:3176–89. doi: 10.1364/BOE.3.003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser ES, Visser R, Van Lier HJ. Advantages of toric scleral lenses. Optom Vis Sci. 2006;83:233–6. doi: 10.1097/01.opx.0000214297.38421.15. [DOI] [PubMed] [Google Scholar]
- 34.Segal O, Barkana Y, Hourovitz D, Behrman S, Kamun Y, Avni I, Zadok D. Scleral contact lenses may help where other modalities fail. Cornea. 2003;22:308–10. doi: 10.1097/00003226-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Kosaki R, Maeda N, Bessho K, Hori Y, Nishida K, Suzaki A, Hirohara Y, Mihashi T, Fujikado T, Tano Y. Magnitude and orientation of Zernike terms in patients with keratoconus. Invest Ophthalmol Vis Sci. 2007;48:3062–8. doi: 10.1167/iovs.06-1285. [DOI] [PubMed] [Google Scholar]
- 36.Sabesan R, Yoon G. Visual performance after correcting higher order aberrations in keratoconic eyes. J Vis. 2009;9(6):1–10. doi: 10.1167/9.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell DE, Freeman RD, Millodot M, Haegerstrom G. Meridional amblyopia: evidence for modification of the human visual system by early visual experience. Vision Res. 1973;13:535–58. doi: 10.1016/0042-6989(73)90023-0. [DOI] [PubMed] [Google Scholar]
- 38.Rossi EA, Weiser P, Tarrant J, Roorda A. Visual performance in emmetropia and low myopia after correction of high-order aberrations. J Vis. 2007;7:14. doi: 10.1167/7.8.14. [DOI] [PubMed] [Google Scholar]