Abstract
Context
Toddlers with autism spectrum disorders (ASD) exhibit poor face recognition and atypical scanning patterns in response to faces. It is not clear if face-processing deficits are also expressed on an attentional level. Typical individuals require more effort to shift their attention from faces compared with other objects. This increased disengagement cost is thought to reflect deeper processing of these socially relevant stimuli.
Objective
To examine if attention disengagement from faces is atypical in the early stages of ASD.
Design
Attention disengagement was tested in a variation of the cued attention task in which participants were required to move their visual attention from face or non-face central fixation stimuli and make a reactive saccade to a peripheral target. The design involved diagnosis as a between-group factor and central fixation stimuli type as a within-group factor.
Setting
Participants were taken from a cohort of patients at a university-based specialized clinic or from a pool of subjects participating in a prospective study of social cognition in ASD.
Participants
Toddlers with ASD (mean age, 32 months [n=42]) were compared with toddlers with nonautistic developmental delays (mean age, 29 months [n=31]) and with typically developing toddlers (mean age, 29 months [n=46]).
Main Outcome Measure
Saccadic reaction time.
Results
Developmentally delayed and typically developing toddlers had more difficulties disengaging visual attention from faces than toddlers with ASD. This effect was not present in response to nonfacial stimuli. These results suggest that toddlers with ASD are not captivated by faces to the same extent as toddlers without ASD and that this effect is not driven by a generalized impairment in disengagement of attention.
Conclusion
The results suggest that face-processing difficulties in toddlers with ASD involve disruption of an attentional mechanism that typically supports deeper processing of these highly socially relevant stimuli.
Autism spectrum disorders (ASD) represent a class of complex, neurodevelopmental disorders that share marked impairments in social interactions and communication as well as the presence of repetitive behaviors and restricted interests.1 Impairments in the social domain include difficulties in deriving and processing socially relevant information from faces.2–7 These deficits are already apparent at early stages of the disorder and include difficulties in selecting faces among other stimuli in the environment, failure to seek affective cues from faces in uncertain situations (social referencing),8–11 failure to monitor the gaze direction of others to gauge their attentional focus (joint attention),12 use of atypical face-scanning strategies,13 and impairments in face recognition.13,14
In typically developing individuals, faces belong to a class of biologically relevant stimuli that enjoy a privileged status as potential targets for attentional focus: they are detected faster than other classes of stimuli15–19 and engage and hold attention to a greater extent than other objects, even if their presence in the visual field is clearly task-irrelevant.17,20,21 The enhanced attentional engagement is expressed either in shortening of reaction time to targets that appear in the same spatial location as the face22 or in increased disengagement cost expressed in lengthening of reaction time to targets presented in other locations.17,23,24 Under certain experimental conditions, faces with direct gaze command greater attentional capture and engender increased dwell time than those with averted gaze.25
Direct evidence that suggests limited attentional engagement by faces has been reported as early as in the third year in children with ASD.26 Tested in a variation of the cued attention task27 and compared with chronologic age (CA)–matched typical controls, toddlers with ASD required less time to initiate visually guided saccades to peripheral targets when they had to look away from a face (experiment 1). However, in the nonface condition, the reaction time in typical and ASD groups was comparable (experiment 2). The study suggested that in toddlers with ASD, the disengagement of attention was not encumbered by the social content of the stimulus. The study26 used a null (no gap) version of the cued attention task in which the disappearance of the central cue (eg, face) coincided with the onset of the peripheral target. In real life, however, faces do not vanish suddenly, and attentional shifts often occur away from an ongoing stimulus (eg, looking away from a mother’s face) toward a peripheral target (eg, a cat that walked into the room). Thus, it was not clear if the limited dwell time or engagement with faces generalizes to more ecologically valid attention cuing conditions and whether it is specific to ASD or is shared amongst children with a variety of developmental disabilities, including ASD.
METHODS
The present study examined attentional bias associated with faces and nonfacial stimuli in a large cohort of toddlers with and without social disability (N=119). Children with ASD, developmental delay (DD), and typical development (TD) were tested in the overlap cued attention task in which the saccadic reaction time to peripheral targets was examined as a function of the central fixation stimulus characteristics. The central fixation stimulus remained displayed on the screen at the time of target onset, allowing us to study disengagement of attention when competing stimuli are displayed simultaneously. The central fixation stimuli included social (face) and nonsocial (mosaic) displays; each participant was exposed to both. Considering reports on the effect of direct gaze on attention capture and engagement,25 we used 2 types of social displays: one containing a direct gaze and another in which only a face with an averted gaze was present. Based on previous findings regarding attention bias in toddlers,26 we hypothesized that in the social conditions, toddlers with ASD would require less time to initiate reactive saccades to peripheral targets compared with toddlers with TD and DD. However, in the nonsocial condition, we anticipated that children with ASD would exhibit saccadic reaction times comparable with those observed in TD and DD controls. Thus, planned contrasts involved comparisons between ASD and TD as well as ASD and DD groups. With regard to the gaze direction, we anticipated that presence of direct gaze would accentuate the magnitude of the attentional bias for faces in the TD and DD groups, but not in toddlers with ASD owing to their decreased sensitivity to gaze direction.
PARTICIPANTS
Forty-two toddlers with a mean age of 32 months (SD, 10 months) with ASD (24 with autism and 18 with pervasive developmental disorder not otherwise specified) and 31 toddlers with a mean age of 29 months (SD, 10 months) with DD (22 with global delay and 9 with language delay) were recruited from a cohort of patients at a university-based specialized clinic or from a pool of subjects participating in a prospective study of social cognition in ASD. Each child was assessed with the Mullen Scale of Early Learning28 and the Autism Diagnostic Observation Scale–Generic, module 1.29 We report updated Autism Diagnostic Observation Scale–Generic algorithm scores based on the recent revision aimed at improving their sensitivity and specificity in young verbal and nonverbal children.30 Parents were interviewed with the Vineland Adaptive Behaviors Scales–Expanded Form31 and the Autism Diagnostic Interview–Toddler Version.32 Two expert clinicians made the diagnoses independently based on all relevant background and evaluation data. Because the toddlers participated in a prospective study, the ASD diagnosis was based in all but 1 case (lost to follow-up) on their reassessment between 3 and 5 years of age. Forty-six toddlers with TD with a mean age of 29 months (SD, 10 months) were recruited through advertisements in a local newspaper. Their developmental status was confirmed through parent interview and standard assessment of verbal and nonverbal skills. Participants with major comorbid health problems (eg, seizure disorder or uncorrected visual or auditory abnormalities) or known genetic abnormalities and toddlers with a gestational age below 32 weeks were not included in the study.
The 2 clinical groups were matched on both CA and verbal and nonverbal developmental quotient (DQ), but as expected, the ASD group had higher scores on the Autism Diagnostic Observation Scale–Generic (Table). Both clinical groups were also matched with controls with TD on CA. The decision to enroll TD participants with the same range of CA rather than levels of cognitive functioning was driven by the fact that the task required a simple reflexive oculomotor response and posited a very low cognitive demand on the participants. This approach minimized the impact of maturation- and experience-related changes in the visual system on saccadic reaction time. Considering a relatively wide age range represented in all 3 samples (15–59 months), we entered CA as a covariate in all analyses.
Table.
Sample Characterization Data
| Characteristic | Mean (SD)
|
P Value | ||
|---|---|---|---|---|
| ASD (n = 42) | DD (n = 31) | TD (n = 46) | ||
| Age, mo | 32 (10) | 29 (10) | 29 (10) | .24 |
| Male sex, % | 81 | 77 | 63 | .14 |
| Mullen Verbal DQa | 60 (27) | 63 (23) | 105 (16) | .001b |
| Mullen Nonverbal DQc | 79 (16) | 83 (18) | 102 (13) | .001b |
| ADOS-G Social Affect scored | 13.6 (4.3) | 6.1 (5.1) | .001 | |
| ADOS-G Restricted and Repetitive Behaviors scored | 4.2 (2.0) | 1.4 (1.9) | .001 | |
Abbreviations: ADOS-G, Autism Diagnostic Observation Scale–Generic; ASD, autism spectrum disorder; DD, developmental delays; DQ, developmental quotient; TD, typical development.
Verbal DQ score based on the average DQ of the Expressive and Receptive subscales of the Mullen Scales.
ASD = DD < TD.
Nonverbal DQ based on the average DQ on the Visual Reception and Fine Motor subscales of the Mullen Scales.
The ADOS-G algorithms were computed based on calculations by Gotham et al30; higher scores index greater degree of pathology.
The experimental protocol was approved by the Human Investigations Committee of Yale University School of Medicine. Informed written consent was obtained from all parents prior to the testing.
EXPERIMENTAL PROCEDURE AND SETTING
During the experiment, toddlers sat in a car seat approximately 80 cm in front of a computer monitor with their eyes level with the center of the monitor. Two speakers, connected to the computer, were located on the left and right side of the screen. Four infrared light sources were positioned 25 cm from the outside edge of the monitor to provide illumination for an infrared-sensitive camera (spectral sensitivity of 400–1300 nm) located above the screen. A 48-mm lens provided adequate resolution of the eyes and much of the subject’s face. The computer monitor, speakers, and camera were mounted behind a black screen in a room that was curtained and dimly lit, such that only the front of the monitor screen was visible to the child. The image of the child’s face was recorded on a videotape along with a stopwatch running at increments of 10 milliseconds. The recorded image also contained an insert screen with a view of the computer monitor to allow for coding of the timing and location of each stimulus presentation.
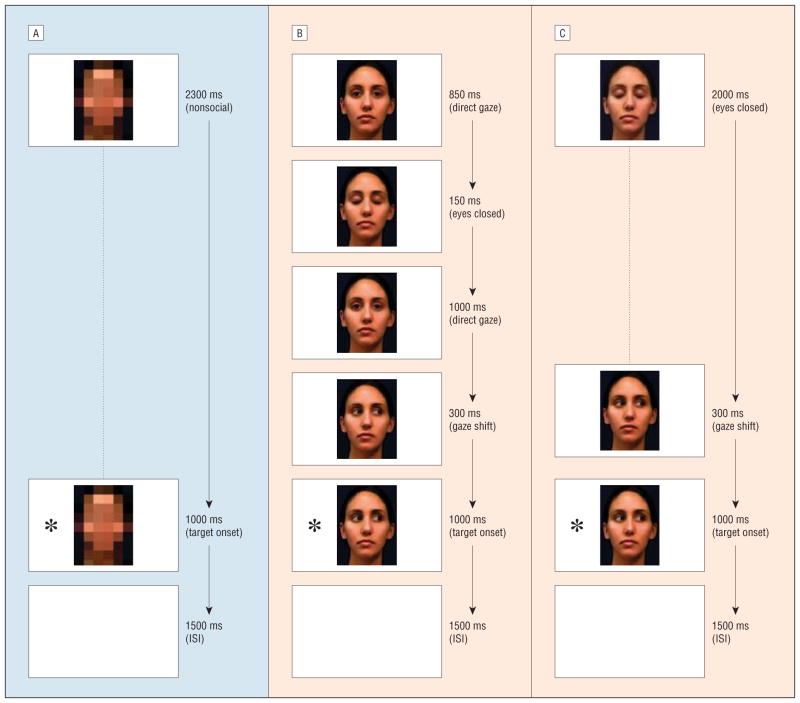
Three types of central fixation stimuli were prepared. For the nonsocial trials (Figure 1A), the central fixation stimulus consisted of a facial image that was digitally transformed into a mosaic to eliminate its social content while maintaining comparable luminance. For the direct gaze and averted gaze trials, the central fixation stimuli consisted of brief presentations constructed by a succession of static images of a woman’s face processed using Adobe software. A single direct gaze trial consisted of a movielike display in which the face looked directly at the viewer, blinked, looked at the viewer again, and then looked to the left or right (Figure 1B). In the averted gaze trial, the participants saw the woman with her eyes closed followed by her opening her eyes while looking to either side (Figure 1C). The social and nonsocial central fixation stimuli subtended 10° of visual angle. Target stimuli consisted of age-appropriate images of toys and cartoon characters in color; they were presented at an eccentricity of 15° either to the left or right of center and subtended 8° of visual angle.
Figure 1.
Examples of a nonsocial trial and direct and averted gaze trials. All participants were first exposed to 10 nonsocial trials (A), then half of the sample viewed 64 direct gaze trials (B), while the other half watched 64 averted gaze trials (C). Trials were equated for total duration across conditions. The asterisks represent a peripheral target; ISI, interstimulus interval (a blank screen).
On each trial, the target appeared while the central fixation stimulus was still being presented and both the target and the central fixation stimulus disappeared simultaneously at the end of the trial. The children were assigned randomly to 1 of 2 conditions: 10 nonsocial trials followed by 64 direct gaze trials or 10 nonsocial trials followed by 64 averted gaze trials. Presenting the nonsocial condition first guaranteed that the participants would not enter the block with any type of expectations associated with faces, which allowed us to measure their basic reaction time. Trials in the direct and averted gaze conditions were matched for overall duration with the nonsocial trials and were accompanied by a beeping sound (440 milliseconds) during the initial presentation of central fixation stimuli to enhance the likelihood that the child would orient to the screen.33 Direction of gaze did not predict target location, and targets appeared randomly with equal frequency on the left and right of the screen.
DATA REDUCTION
The image of the child’s face combined with an insert showing a location of the target was video-recorded at 30 Hz and subsequently scanned field by field and coded by 5 experienced coders masked to the hypotheses of the study and diagnostic status of the participants. Using the timer recorded on the tape, the coders recorded the onset time of the target as well as the direction and time of the initiation of a saccade by the child. Based on 15% of trials, the intraclass r reliability between all 5 coders for saccadic reaction time was 0.974 and the Cohen κ for direction of gaze was 0.823.
DEPENDENT VARIABLES
Two dependent variables were analyzed: saccadic reaction time and the proportion of trials in which the participants failed to disengage from the central fixation stimulus. The saccadic reaction time reflects the time necessary for disengagement or decoupling of attention from the central fixation point, calculation of the saccadic parameters needed to fixate the stimulus, and initiation of an eye movement34,35 and was operationalized as the interval between target and saccade onset. Target onset was determined by identifying the first frame when the target appeared on the screen. Saccade onset was identified by the first frame with a noticeable deviation of the infant’s eyes toward the target. Considering that the overlap paradigm introduces strong perceptual competition between the centrally located fixation stimulus and a peripheral target, we computed the mean proportion of valid trials on which the participants failed to disengage attention from the central fixation stimulus throughout the time when the target was presented. High failure-to-disengage values were expected in those with extreme difficulties disengaging and shifting their attention away from the central fixation stimulus.
VALID TRIALS
A trial was regarded valid if the child fixated on the central fixation stimulus throughout its presentation and subsequently made a saccade toward the peripheral target (left or right). Trials in which the child remained fixated on the central cue throughout the trial and failed to make a saccadic shift to the target were retained for the failure-to-disengage analysis. Trials in which the child was inattentive or the child’s eyes were invisible (eg, obscured by eyelids) were excluded from the analysis. On average out of 74 total trials, 39 trials (SD, 13) in the ASD group, 34 (SD, 14) in the DD group, and 37 (SD, 11) in the TD group were considered valid. A diagnosis×condition analysis of variance (ANOVA) on the mean number of valid trials with CA as a covariate revealed no significant effects of diagnosis (P=.33), condition (P=.64), or age (P=.17). Thus, there was no differential loss of trials due to diagnosis, condition, or age of the participants.
STATISTICAL ANALYSIS
In both nonsocial and social conditions, we identified 3 cases with extremely high saccadic reaction time values (1 from each diagnostic group); the cases were excluded from the subsequent analysis. Considering the study design and its primary hypotheses, we analyzed the social and nonsocial conditions separately, though in the secondary analysis, we also compared overall saccadic reaction time in social and nonsocial conditions. Saccadic reaction time in the nonsocial condition and the proportion of failure-to-disengage trials were analyzed using ANOVA, with diagnosis as a between-group factor. In the analysis of saccadic reaction time in the social conditions, we considered between-group factors of diagnosis and gaze condition (direct vs averted gaze) and a within-group factor or type of trial (congruent vs incongruent). Given the design of the cuing task (ie, overlap between central fixation and target and lack of a predictive relationship between gaze direction and target location), in trials in which the gaze direction in the central fixation stimulus was congruent with target location, we did not anticipate that saccadic reaction time would differ from those in which the gaze direction was incongruent. Significant effects were followed by planned contrasts comparing ASD group with DD and TD contrast groups. Effect of CA was evaluated by using it as a covariate in all analyses. Whenever appropriate, the CA effect was assessed using Pearson product-moment correlation coefficient analysis.
RESULTS
FAILURE TO DISENGAGE ATTENTION
We expected that if the ASD toddlers had marked difficulties in disengagement of attention, they would remain fixated on the central cue and consequently fail to make a saccade to the target more frequently than controls. To test this hypothesis, we compared the proportion of failure-to-disengage trials between groups and conditions. On average, toddlers with ASD failed to disengage in 2.9% (SD, 4.9%) of trials compared with 12.4% (SD, 18.2%) in the DD and 5.6% (SD, 7.0%) in the TD group. A diagnosis×condition ANOVA on mean failure to disengage with CA as a covariate indicated only a significant effect of diagnosis (F2,118=6.39, P =.003). Planned contrast revealed that ASD group showed failure to disengage on fewer trials than the DD group (P<.001) but that the ASD and TD groups were comparable (P=.23). The high percentage of trials in which the child failed to disengage in the DD group was driven primarily by those with global delays (mean, 14.0%; SD, 18%) rather than language delays (mean, 8.5%; SD, 17%). Thus, toddlers with ASD did not exhibit difficulties in disengagement of attention that would prevent them from orienting to a peripheral target more frequently than typical controls. Increased frequency of failures to disengage was associated primarily with global DD without autistic features.
SACCADIC REACTION TIME IN NONSOCIAL AND SOCIAL CONDITIONS
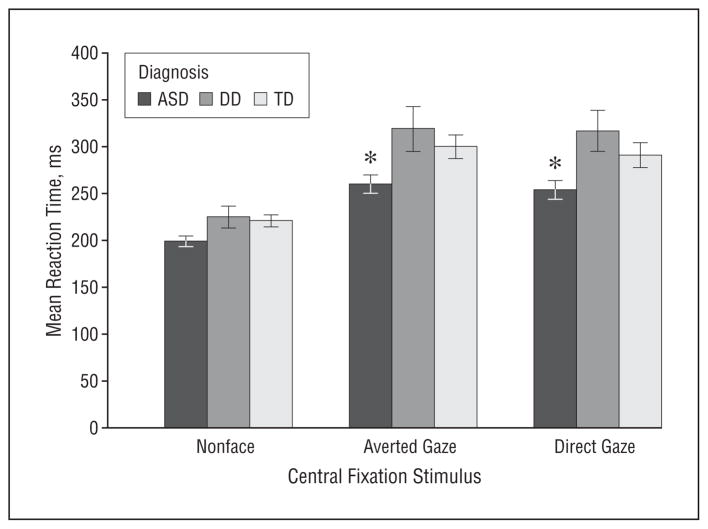
Responses faster than 100 milliseconds, ie, anticipatory saccades (3.4%), or slower than 1000 milliseconds (0.7%) and responses that were slower by more than 2 SDs from each subject’s mean (4.5%) were excluded from the saccadic reaction time analysis. A between-group ANOVA on the individual saccadic reaction time in the nonsocial condition revealed no significant effect of diagnosis (P=.10) but a significant effect of CA (F1,115=7.22, P =.008) (Figure 2). Analysis of the age effect indicated that an increase in age was associated with shortening of the reaction time (r116=−0.28, P =.003). In the social conditions, a 3 (diagnosis)×2 (condition: direct gaze vs averted gaze)×2 (type of trial: congruent vs incongruent) ANOVA with repeated measures on the last factor revealed a significant effect of diagnosis (F2,110=5.72, P =.004), no effect of condition (P=.36), condition×diagnosis interaction (P=.67), trial type (P=.26), or any higher-order interaction but a significant effect of CA (F1,110=14.18, P <.001) (Figure 2). Planned contrasts revealed that when the central fixation stimulus consisted of a face, regardless of whether direct gaze was present in the display, toddlers with ASD had shorter saccadic reaction time than their typical peers (P=.02) and DD controls (P<.001). Similar to the nonsocial condition, an older age was associated with shorter reaction time (r117=−0.36, P <.001). Thus, while the time necessary for initiation of a saccade from a mosaic stimulus was similar across all groups, cost of disengagement of attention from a face was higher in TD and DD groups than in toddlers with ASD. As could be expected, reaction time decreased with age. To examine potential contribution of cognitive levels of functioning to the observed differences in the ASD and DD groups, we compared their saccadic reaction time, including in the model additional covariates of verbal and nonverbal DQ. The DQ scores did not contribute significantly to the model (P = .83, and P = .29, respectively), and the diagnosis effect remained significant (P=.001).
Figure 2.
Mean saccadic reaction time in toddlers with autism spectrum disorder (ASD), typical development (TD), and developmental delay (DD) in the nonsocial, direct gaze, and averted gaze conditions. Error bars represent standard error; *mean difference of at least P<.05.
As can be observed in Figure 2, reaction time increased in all groups, following a shift from the nonsocial to social trials. Considering the nonsignificant effects of the type of the social display and type of trial, we collapsed the data in the social conditions across the 2 factors and conducted a diagnosis×condition (nonsocial vs social) ANOVA, with condition as a within-group factor. The analysis indicated a significant effect of condition (F1,110=182.37, P<.001) as well as the effects of diagnosis (F2,110=7.11, P<.001) and diagnosis×condition interaction (F2,110=3.42, P=.04), such that in nonsocial conditions, saccadic reaction time was similar in all groups, and in the social conditions, toddlers with ASD showed the shortest reaction time. Thus, as their non-ASD controls, toddlers with ASD showed similar lengthening of reaction time after the shift from nonsocial to the social conditions, though it is not clear to what extent this increase was driven by a change in perceptual (eg, complexity) or semantic stimulus characteristics, as the 2 were confounded. Regardless of this effect, in the social conditions children with ASD showed consistently shorter reaction time than the 2 comparison groups.
COMMENT
The present study replicates and extends the earlier study on limited attentional bias for faces in toddlers with ASD.26 The results suggest that faces have a lower capacity to engage toddlers with ASD compared with toddlers with DD or TD, as indexed by a shorter reaction time necessary to disengage their attention to fixate on a suddenly appearing peripheral target. This phenomenon cannot be attributed to the generalized difference in reaction time in response to other classes of stimuli. Moreover, in young children with ASD, the limited attentional bias for faces is not associated with the degree of verbal or nonverbal cognitive impairments.
While it is not entirely clear what drives the enhanced attentional engagement in TD and DD toddlers, the effect can be attributed to deeper obligatory processing triggered by faces. Research on typical adults36,37 suggests that depth-of-face processing and consequently the quality of its representation (measured by accuracy of recognition of the given face) can be manipulated experimentally. That is, typical adults set to judge a semantic property of a face (eg, honesty) tend to remember the face better than those whose goal is to judge its physical characteristics (eg, sex). Toddlers in our study were not given any explicit instruction other than to look at the screen, though one can safely assume that each of them entered the task with a set of implicit goals and expectations formed over their lifetime experience with the social environment. While, to our best knowledge, the relationship between depth of processing and disengagement time has not been directly studied, research on typical adults suggests that internal states (eg, affect) can delay disengagement from faces.24 Thus, we hypothesize that when toddlers without social disability examine a novel face, they spontaneously conduct a range of computations, including classification (eg, Is it a face or something else? Is it my parent or someone else? Is this person nice or mean?) and encoding of invariant features for later comparisons. In the same vein, when a toddler with ASD looks at a novel face, the number of questions on his or her mind might be more limited, or he or she might be restricted to focus on physical facial characteristics (eg, face/no face or male/female). If so, based on the research on depth-of-face processing in adults, one would expect less effective face recognition skills in toddlers with ASD. Albeit limited, the extant evidence suggests that compared with TD controls, toddlers with ASD indeed have greater difficulties in face recognition, despite seemingly intensive examination of the novel faces,13 which provides preliminary support for the depth-of-processing hypothesis.
Neuroimaging studies suggest that in response to novel faces, both adults4,38–41 and children42 with ASD show hypoactivation in the fusiform gyrus, a cortical area specialized for face processing. The deficit appears to be associated to some extent with atypical modulatory activity of the amygdala,40,42–44 a subcortical structure involved in assessing saliency and biological relevance of stimuli as well as emotional learning and memory.45–47 While rapid detection of faces observed in infants is supported by subcortical structures, including the superior colliculus, pulvinar, and amygdala,48 disengagement of attention involves cortical structures like the posterior parietal cortex,35,49 which, in the case of faces, is likely to be further influenced by face-specific areas and the limbic system. It has been hypothesized that the deficits in face processing observed in adults with ASD and expressed in disruption of the cortically mediated functions (eg, recognition) might reflect an outcome of an early impairment of the subcortical system in infancy.48 This impairment might then disrupt the process of cortical specialization by preventing development of appropriate patterns of regional enhancement of cortical activity in response to faces in ASD. It is not clear whether the deficit in attentional engagement with faces observed in the present study is primary and present at the earliest stages of ASD or whether it evolves within the first years of life secondary to other pathogenic factors. This important issue would be best addressed through a prospective study of infants who, owing to genetic factors, are at risk of developing ASD.
Consistent with studies of adults50,51 and children51–53 with ASD, our results suggest that young children with ASD do not exhibit generalized abnormalities in generation of reactive saccades both in the presence (present study) and absence of competing nonsocial stimuli.26 There was also no evidence for an ASD-specific attention-disengagement deficit when a total failure to shift attention away from an ongoing stimulus was considered. Inconsistent with our findings are those in the study by Landry and Bryson,54 who described a marked deficit in disengagement of attention in young children with ASD but not in those with Down syndrome or typical controls. The differences between studies might be driven by certain design features. In the Bryson and Landry study, the central fixation point as well as the targets consisted of a dynamic display of shapes that “were falling on each other,” which might have been particularly salient for children with ASD, just as faces were salient in non-ASD samples in our studies, making disengagement of attention more difficult. While this interpretation would suggest that deficits in disengagement of attention in ASD might be associated with specific stimulus features, important methodological differences between the 2 studies preclude direct comparisons. In the Landry and Bryson study, trials in which the participant failed to disengage (ie, made no saccades) were included in computing the mean reaction time. This approach skewed the distribution of the mean reaction time, such that even in the TD group, saccadic reaction time exceeded the physiological range seen in individuals older than 6 months.52,55 Consequently, while some propose that presence of difficulties in disengagement of attention might represent an early behavioral marker for autism,56,57 we would like to argue that such an assertion might be premature, at least until the extent of the effect and its moderating factors are fully understood. Thus far, the evidence suggests that such deficits are more likely to be associated with either idiopathic global DD (present study) or genetic syndromes such as Williams syndrome.58 Although children with ASD in naturalistic contexts often display examples of overfocused attention, this phenomenon more likely reflects selection of objects that afford access to certain types of preferred experiences, such as motion (eg, fan or car wheels), perceptual features (eg, high-contrast areas),59,60 or perfect contingencies.61 The apparent difficulty with disengagement of attention might also be associated with marked underresponsiveness to certain classes of stimuli typically used to elicit reorienting (eg, their name being called or speech in general) that otherwise capture attention rapidly in typically developing individuals,62–65 rather than representing a generalized deficit in the ability to disengage attention from ongoing visual stimuli. With regard to the importance of regulating visual attention for development of learning and cognition, clarifying conditions (eg, types of orienting and stimuli involved) under which abnormalities in disengagement of visual attention are present in autism warrants further investigation.
Taken together, our results suggest that in addition to atypical scanning patterns and deficits in recognition, impairments in face processing in toddlers with ASD involve limited attentional bias for these highly socially relevant stimuli. The attention disengagement task should thus be explored as a potential behavioral marker of underactivation of the brain areas involved in social cognition in ASD. With further work on establishing sensitivity and specificity of these findings in ASD at younger ages, a task targeting deficits in attentional bias for faces might become a useful tool for identifying infants at risk for ASD. In this context, however, further investigation is necessary to explore whether the limited attentional bias for faces might also extend to other biologically relevant stimuli and whether it is present in response to both familiar and novel faces.42 Furthermore, it will be important to examine if the attentional bias in ASD could be manipulated by varying certain perceptual properties (saliency) of nonsocial stimuli.
Acknowledgments
Funding/Support: This study was supported by the National Alliance for Autism Research foundation and grant I54 MH66494 from the National Institute of Mental Health’s Studies to Advance Autism Research and Treatment.
Footnotes
Author Contributions: Dr Chawarska had access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: We thank the families of the children included in this study for their time and participation. Jessica Bradshaw, BS, Jennifer Buchanan, BA, Brittany Butler, BA, Joslin Latz, BA, Paula Ogston, BA, and Jessica Reed, BA, collected and coded data; Frederick Shic, PhD, and Suzanne Macari, PhD, provided helpful comments on the earlier versions of the manuscript; and Celine Saulnier, PhD, and Rhea Paul, PhD, contributed to the sample characterization.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. J Child Psychol Psychiatry. 2003;44(4):529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- 3.López B, Donnelly N, Hadwin JA, Leekam SR. Face processing in high-functioning adolescents with autism: evidence for weak central coherence. Vis Cogn. 2004;11(6):673–688. [Google Scholar]
- 4.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 5.Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 6.Scherf KS, Behrmann M, Minshew N, Luna B. Atypical development of face and greeble recognition in autism. J Child Psychol Psychiatry. 2008;49(8):838–847. doi: 10.1111/j.1469-7610.2008.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, Minshew N. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14(2):239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- 9.Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, Palacio-Espasa F. Attentional skills during the first 6 months of age in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Rees L, Wheelwright S. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. J Child Psychol Psychiatry. 1998;39(5):747–753. [PubMed] [Google Scholar]
- 11.Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- 12.Mundy P, Burnette C. Joint attention and neurodevelopmental models of autism. In: Volkmar F, Paul R, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders: Diagnosis, Development, Neurobiology, and Behavior. 3. Vol. 1. Hoboken, NJ: John Wiley & Sons; 2005. p. 703. [Google Scholar]
- 13.Chawarska K, Shic F. Looking but not seeing: atypical visual face scanning and recognition of faces 2 and 4-year-old children with autism spectrum disorder [published online ahead of print July 10, 2009] J Autism Dev Disord. 2009;39(12):1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawarska K, Volkmar F. Impairments in monkey and human face recognition in 2-year-old toddlers with autism spectrum disorder and developmental delay. Dev Sci. 2007;10(2):266–279. doi: 10.1111/j.1467-7687.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher-Watson S, Findlay JM, Leekam SR, Benson V. Rapid detection of person information in a naturalistic scene. Perception. 2008;37(4):571–583. doi: 10.1068/p5705. [DOI] [PubMed] [Google Scholar]
- 16.Hershler O, Hochstein S. At first sight: a high-level pop out effect for faces. Vision Res. 2005;45(13):1707–1724. doi: 10.1016/j.visres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Ro T, Friggel A, Lavie N. Attentional biases for faces and body parts. Vis Cogn. 2007;15(3):322–348. [Google Scholar]
- 18.Eastwood JD, Frischen A, Reynolds M, Gerritsen C, Dubins M, Smilek D. Do emotionally expressive faces automatically capture attention? evidence from global-local interference. Vis Cogn. 2008;16(2–3):248–261. [Google Scholar]
- 19.Frischen A, Eastwood JD, Smilek D. Visual search for faces with emotional expressions. Psychol Bull. 2008;134(5):662–676. doi: 10.1037/0033-2909.134.5.662. [DOI] [PubMed] [Google Scholar]
- 20.Lavie N, Ro T, Russell C. The role of perceptual load in processing distractor faces. Psychol Sci. 2003;14(5):510–515. doi: 10.1111/1467-9280.03453. [DOI] [PubMed] [Google Scholar]
- 21.Langton SR, Law AS, Burton A, Schweinberger SR. Attention capture by faces. Cognition. 2008;107(1):330–342. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Brosch T, Sander D, Scherer KR. That baby caught my eye . . . attention capture by infant faces. Emotion. 2007;7(3):685–689. doi: 10.1037/1528-3542.7.3.685. [DOI] [PubMed] [Google Scholar]
- 23.Fox E, Russo R, Dutton K. Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cogn Emotion. 2002;16(3):355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiou GA, Bleakley C, Hayward J, Russo R, Dutton K, Eltiti S, Fox E. Focusing on fear: attentional disengagement from emotional faces. Vis Cogn. 2005;12(1):145–158. doi: 10.1080/13506280444000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senju A, Hasegawa T. Direct gaze captures visuospatial attention. Vis Cogn. 2005;12(1):127–144. [Google Scholar]
- 26.Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Dev. 2003;74(4):1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- 27.Hood BM, Willen JD, Driver J. Adult’s eyes trigger shifts of visual attention in human infants. Psychol Sci. 1998;9(2):131–134. [Google Scholar]
- 28.Mullen E. Mullen Scales of Early Learning. AGS Edition. Circle Pines, MN: American Guidance Serivce, Inc; 1995. [Google Scholar]
- 29.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 30.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 31.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition, Survey Form Manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- 32.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 33.Hainline L. Developmental changes in visual scanning of face and nonface patterns by infants. J Exp Child Psychol. 1978;25(1):90–115. doi: 10.1016/0022-0965(78)90041-3. [DOI] [PubMed] [Google Scholar]
- 34.Fischer B. The role of attention in the preparation of visually guided eye movements in monkey and man. Psychol Res. 1986;48(4):251–257. doi: 10.1007/BF00309089. [DOI] [PubMed] [Google Scholar]
- 35.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 36.Coin C, Tiberghien G. Encoding activity and face recognition. Memory. 1997;5(5):545–568. doi: 10.1080/741941479. [DOI] [PubMed] [Google Scholar]
- 37.Bloom LC, Mudd SA. Depth of processing approach to face recognition: a test of two theories. J Exp Psychol Learn Mem Cogn. 1991;17(3):556–565. [Google Scholar]
- 38.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(12):2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 39.Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(pt 10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 40.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith H, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Critchley HD, Daly E, Bullmore E, Williams S, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DGM. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 42.Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson L, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 44.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61(4):512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc Cogn Affect Neurosci. 2008;3(4):303–312. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 49.Yantis S. The neural basis of selective attention: cortical sources and targets of attentional modulation. Curr Dir Psychol Sci. 2008;17(2):86–90. doi: 10.1111/j.1467-8721.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52(5):917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 52.van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biol Psychiatry. 2001;50(8):614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- 53.Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Dev Psychol. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- 54.Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 55.Blaga OM, Colombo J. Visual processing and infant ocular latencies in the overlap paradigm. Dev Psychol. 2006;42(6):1069–1076. doi: 10.1037/0012-1649.42.6.1069. [DOI] [PubMed] [Google Scholar]
- 56.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Visual orienting in the early broader autism phenotype: disengagement and facilitation. J Child Psychol Psychiatry. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown JH, Johnson MH, Paterson SJ, Gilmore R, Longhi E, Karmiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia. 2003;41(8):1037–1046. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 59.Shic F, Chawarska K, Lin D, Scassellati B. Measuring context: the gaze patterns of children with autism evaluated from the bottom-up. Paper presented at: The IEEE 6th International Conference on Development and Learning; July 12, 2007; London, England. [Google Scholar]
- 60.Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuospatial information processing in autism depends on stimulus complexity. Brain. 2005;128(pt 10):2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- 61.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Dev. 1998;69(5):1276–1285. [PMC free article] [PubMed] [Google Scholar]
- 63.Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med. 2007;161(4):378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- 64.Klin A. Young autistic children’s listening preferences in regard to speech: a possible characterization of the symptom of social withdrawal. J Autism Dev Disord. 1991;21(1):29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- 65.Paul R, Chawarska K, Fowler C, Cicchetti D, Volkmar F. “Listen my children and thou shall hear”: auditory preferences in toddlers with autism spectrum disorders. J Speech Lang Hear Res. 2007;50(5):1350–1364. doi: 10.1044/1092-4388(2007/094). [DOI] [PubMed] [Google Scholar]