Abstract
Lesch-Nyhan disease (LND), a genetic disorder associated with motor and psychiatric disturbance and self-injurious behaviour (SIB) is caused by a complete deficiency of hypoxanthine-guanine phosphoribosyltransferase (HPRT). The connection between enzyme deficiency and neurological involvement is still unclear. Evidence exists for a role of basal ganglia dysfunction with decreased dopamine and excess serotonin striatal content. In this study, we investigate the role of serotonin receptor 2C (HTR2C) in the brains of HPRT gene knock-out mice, a model of LND. HTR2C expression is analyzed by real-time polymerase chain reaction (PCR) using SYBR-green detection methods. The percentage of edited HTR2C mRNA was determined by direct sequencing of amplification products of the region containing the editing sites. We found a 55% increase in the expression of HTR2C gene but no significant difference in mRNA editing levels between knock-out and control mice. The above alteration found in HPRT-deficient mice is similar to those found in other animal models used to study aggressive and self-injurious behaviour.
Keywords: Lesch-Nyhan disease, Self-injurious behaviour, Serotonin receptor 2C
1. Introduction
Lesch-Nyhan disease (LND) is an X-linked genetic disease caused by complete absence of the activity of hypoxanthine-guanine phosphoribosyltransferase (HPRT), a purine salvage enzyme. The disease is characterized by overproduction of uric acid and severe neurological involvement with motor disability and self-injurious behaviour (SIB).1,2 The connection between HPRT deficiency and the neurological manifestations of LND remains unclear, although dysfunction of basal ganglia,3 a brain region involved in movement control, is suggested.
HPRT knock-out mice have been used as a model of the disorder. Although the model does not fully emulate the human disease, it is useful to study the pathogenesis of LND.
Several studies have stated the implication of the serotonergic system in behavioural disturbances associated with aggression. A role of the serotonergic system in HPRT knock-out mice is supported by the finding of altered serotonin levels in the striatum,4 recently implicated in the regulation of behaviour.5
The neonatal 6-hydroxydopamine (6-OHDA)-treated rat model of LND is characterised by increased serotonin activity in the striatum,6 where the serotonin receptor 2C (HTR2C) becomes sensitised and administration of agonists induces self-injurious behaviour (SIB).
Recent evidence reports the role of alterations in mRNA editing of the HTR2C gene in the regulation of mood and behaviour. HTR2C mRNA is modified by RNA editing, which changes up to three genomically encoded amino acids in the second intracellular loop of this G-protein-coupled receptor. Extensively edited receptor isoforms activate G protein less efficiently than non-edited receptors.
We aimed to investigate the role of the HTR2C in the mouse model of LND by exploring the patterns of HTR2C gene expression and abnormalities in the mRNA editing process.
2. Materials and methods
The study was performed on brains obtained by decapitation of seven HPRT knock-out mice and six normal littermate controls and immediately frozen in liquid nitrogen.
To check expression of HTR2C, semi-quantitative estimates of real-time polymerase chain reaction (RT-PCR) products were obtained after electrophoresis on ethidium bromide-stained agarose gel. Gel images were photographed and the bands were quantified in relative absorbance units using image analysis software. A standard curve used for normalization was obtained for the housekeeping gene GAPDH. Samples were subsequently analyzed by RT-PCR (ABI PRISM 7000, Applied Biosystems; Foster City, CA, USA) using SYBR-green (Applied Biosystems). All PCR reactions were performed in triplicate. Relative quantification of gene expression was carried out using standard curves for the target genes and for a reference endogenous gene (b-actin). The 2−ΔΔCT method was used to determine the relative expression of genes analyzed in knock-out mice with respect to the control. The percentage of edited HTR2C mRNA was determined by direct sequencing of amplification products of the region containing the editing sites (indicated as A, B, C′, C, D). The edited nucleotide appeared as overlapping A/G peaks and the ratio of peak area of the edited nucleotide (G) to the sum of G and A peak areas represented the level of editing at any site. Statistical analysis (analysis of variance [ANOVA]) was performed to test the significance of differences found in each experiment.
3. Results and discussion
The expression of the HTR2C gene tested by semi-quantitative RT-PCR differed between knock-out and control mice, and RT-PCR confirmed a 55% increase in expression in knock-out mice brains compared to controls (Figs. 1 and 2). Editing could be detected in 3 (A, B, D) out of 5 possible sites in HTR2C mRNA. There was no significant difference in editing levels between knock-out and control mice (Fig. 3).
Fig. 1.
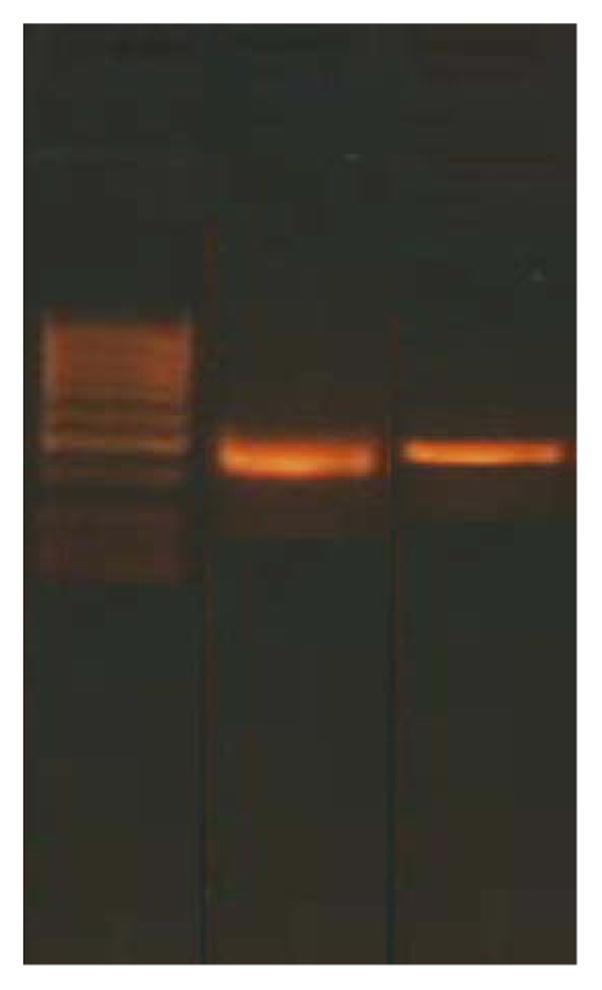
Serotonin receptor 2C (HTR2C) gene amplified by real-time polymerase chain reaction (PCR) from knock-out mice (lane 2) and controls (lane 3). Lane 1 shows molecular weight markers. Contemporary amplification of the housekeeping GAPDH gene was also performed (data not shown). Amplification kinetics, which showed an increase in expression in the hypoxanthine-guanine phosphoribosyl-transferase gene (HPRT) knock-out mice brains compared to controls, were obtained by analysing PCR samples at different steps (25, 27, 29, 30, 33 cycles).
Fig. 2.
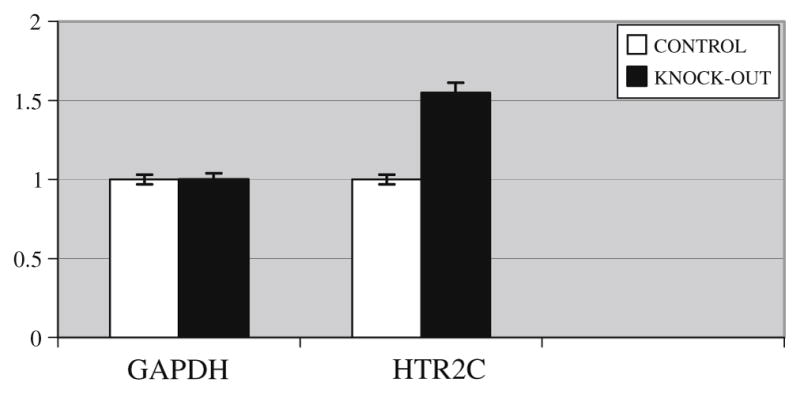
The expression level of the serotonin receptor 2C gene (HTR2C) analysed by real time-polymerase chain reaction in control and knock-out mice showing a 55% increase in expression in hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene knock-out mice brains compared to control mice. Expression of the GAPDH housekeeping gene was not different.
Fig. 3.
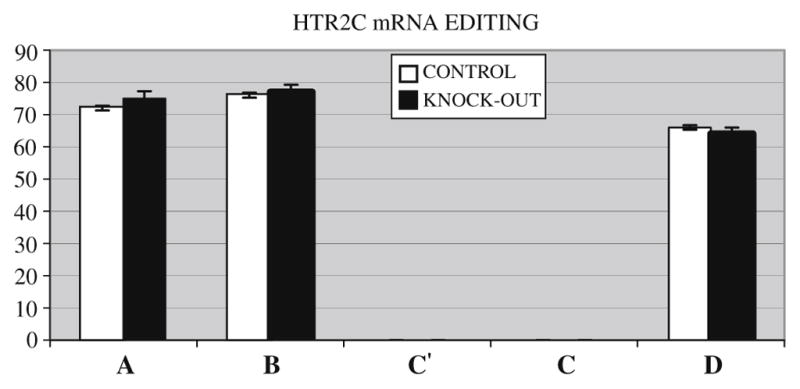
Messenger RNA editing level in the five sites (A,B,C′,C,D) of the serotonin receptor 2C gene (HTR2C) showing no difference in editing levels between the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene knock-out and control mice.
Thus, the connection between HPRT deficiency and the neurological manifestations of LND still remains unclear, although dysfunction of basal ganglia,3 a brain region involved in movement control, is suggested. This region is also involved in the regulation of behaviour,5 which has induced some researchers to classify LND as a genetic movement disorder associated with psychiatric disturbance.7
Several studies on the neurobiological basis of aggressive behaviour in different animal models have shown that alterations involving the serotonergic system may underlie the manifestation of symptoms.8,9 A link between HPRT deficiency and serotonergic dysfunction has been shown by experiments involving intrastriatal injection of hypoxanthine in rats and oral administration of hypoxanthine to healthy human volunteers showing a reduction in striatal serotonin and in urinary excretion respectively.10,11
However, there is no further evidence suggesting that HTR2C is critical for SIB. Data in the literature indicate that HTR2C is implicated in the regulation of mood and behaviour by specific alteration of mRNA editing; indeed several recent studies in humans have reported changes in HTR2C editing in brains of suicide victims.12 The process of mRNA editing constitutes a regulation system that recodes genomic information in a systematic and regulated manner. It is performed by a specific adenosine deaminase that converts adenosine to inosine (where inosine nucleotides are recognized functionally as guanosine nucleotides) and selectively changes the read-out of a gene in the primary RNA transcript, which generates single amino acid changes in the resulting protein, often with significant consequences for its function.
In this study, we investigated the role of HTR2C and mRNA editing of the HTR2C gene in brains of HPRT gene knock-out mice, a model of LND. As far as we know, this is the first report of a 55% increase in the expression of the HTR2C gene. The limitation of using the whole brain rather than isolated basal ganglia can be avoided by protein analysis.
The higher expression of HTR2C in this preliminary study is a new finding that may open pathways into investigating the molecular bases of neurobehavioural abnormalities in several aggression-related disorders.
Acknowledgments
We are grateful to Anna and Giuseppe Baschirotto for introducing me to the study of Lesch-Nyhan syndrome. Their institute [BIRD Foundation] is the best reference for Italian Lesch-Nyhan patients.
References
- 1.Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med. 1964;36:561–70. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- 2.Bertelli M, Randi D, Micheli V, et al. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in Italian Lesch-Nyhan patients: identification of nine novel mutations. J Inherit Metab Dis. 2004;27:767–73. doi: 10.1023/B:BOLI.0000045799.78633.23. [DOI] [PubMed] [Google Scholar]
- 3.Visser JE, Bar PR, Jinnah HA. Lesch-Nyhan disease and the basal ganglia. Brain Res Rev. 2000;32:449–75. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain Dev. 2000;22(Suppl 1):S122–31. doi: 10.1016/s0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 5.Trimble MR, Van Elst LT. On some clinical implications of the ventral striatum and the extended amygdala. Investigations of aggression. Ann N Y Acad Sci. 1999;877:638–44. doi: 10.1111/j.1749-6632.1999.tb09293.x. [DOI] [PubMed] [Google Scholar]
- 6.Allen SM, Davis WM. Relationship of dopamine to serotonin in the neonatal 6- OHDA rat model of Lesch-Nyhan syndrome. Behav Pharmacol. 1999;10:467–74. doi: 10.1097/00008877-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Jinnah HA, Visser JE, Harris JC, et al. Delineation of the motor disorder of Lesch- Nyhan disease. Brain. 2006;129:1201–17. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miczek KA, de Almeida RM, Kravitz EA, et al. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–6. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Praag HM, Plutchik R, Conte H. The serotonin hypothesis of (auto)aggression. Critical appraisal of the evidence. Ann N Y Acad Sci. 1986;487:150–67. doi: 10.1111/j.1749-6632.1986.tb27895.x. [DOI] [PubMed] [Google Scholar]
- 10.Bavaresco CS, Chiarani F, Duringon E, et al. Intrastriatal injection of hypoxanthine reduces striatal serotonin content and impairs spatial memory performance in rats. Metab Brain Dis. 2007;22:67–76. doi: 10.1007/s11011-006-9038-x. [DOI] [PubMed] [Google Scholar]
- 11.Manzke H, Gustmann H. Reduced urinary serotonin excretion after intake of high doses of hypoxanthine. Eur J Pediatr. 1989;148:337–40. doi: 10.1007/BF00444129. [DOI] [PubMed] [Google Scholar]
- 12.Niswender CM, Herrick-Davis K, Dilley GE, et al. RNA editing of the human serotonin 5-HT2C receptor. Alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–91. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]


