Abstract
This study used eyetracking to investigate the ability of young children with autism spectrum disorders (ASD) to recognize social (faces) and nonsocial (simple objects and complex block patterns) stimuli using the visual paired comparison (VPC) paradigm. Typically developing (TD) children showed evidence for recognition of faces and simple objects, but not complex block patterns. Children with ASD were successful at recognizing novel objects and block patterns, but showed no evidence for face recognition. These findings suggest that young children with ASD have specific impairments in face recognition, and that they may have advantage over TD controls when processing complex nonsocial stimuli.
Keywords: Autism, ASD, Face processing, Visual recognition, Eye-tracking
Introduction
Autism spectrum disorder (ASD) is characterized by impairments in social interaction, communication difficulties, and restricted and repetitive behaviors (APA 1994). In younger children with ASD, these communication and social deficits are often manifested in the lack of social overtures including limited initiation of joint attention and eye contact (Chawarska et al. 2009, 2007; Landa et al. 2007; Wetherby et al. 2004). The importance of face-to-face interactions in mediating social exchange, combined with difficulty maintaining eye contact and limited sensitivity to facial cues such as gaze direction and emotional expression in ASD, has led to great interest in face processing in individuals with ASD.
In experimental contexts, children and adults with ASD often attend to faces; however they do not seem to extract the same crucial information from the face as typically developing individuals (Chawarska and Shic 2009). They have difficulties in processing social cues such as emotional expression (Golan et al. 2006; Hobson et al. 1988; Kätsyri et al. 2008) and eye gaze (Dalton et al. 2005; Joseph and Tanaka 2003; Senju et al. 2003), as well as abstracting invariant features necessary for face recognition (Annaz et al. 2009; Boucher and Lewis 1992; Chawarska and Shic 2009; Klin et al. 1999). Imaging and electrophysiological studies suggest that deficits in face processing in ASD are also reflected in atypical brain responses to these highly socially relevant stimuli. These atypicalities have been suggested to be a result of abnormal development of the amygdala-fusiform system, the neural network involved in face processing (Dalton et al. 2005; Pelphrey et al. 2005, 2002; Schultz et al. 2000). ERP studies have found slower face-processing components in adolescents and adults with ASD (McPartland et al. 2004; O’Connor et al. 2005) and have shown that the brain electrical responses in individuals with ASD, in contrast to typically developing individuals, does not differ during presentations of familiar versus unfamiliar faces (Dawson et al. 2002).
The majority of studies on face recognition in ASD populations have examined older children and adults. However, elementary face recognition skills develop within the first months of life. Attention to the face and face processing is likely to be one of the earliest expressions of social engagement. Newborns exhibit a visual preference for faces over complex patterns (Goren et al. 1975; Maurer and Barrera 1981; Morton and Johnson 1991) and are able to rapidly form representations of faces for recognition (Bushnell 2001; Pascalis et al. 1995; Walton and Bower 1993). Additionally, young infants in electrophysiological studies exhibit differential event-related brain potentials to familiar versus unfamiliar faces (de Haan and Nelson 1997). Thus, findings in work with older children with ASD may be tapping into the developmental endpoints of an otherwise protracted developmental process.
In contrast to a visual processing deficit for social information, several groups have reported enhanced perceptual processing of nonsocial information in individuals with ASD (see Mottron et al. 2006 for a review). For example, individuals with autism are better than normal controls in extracting embedded figures during a block design task (Shah and Frith 1993). Individuals with ASD are faster than typical children when engaged in visual search tasks (Plaisted et al. 1998) and respond atypically to the interference of local features in a number identification task (Rinehart et al. 2000). In addition, children with ASD are not as susceptible to visual illusions as their typically developing peers. (Happé 1996; though see Ropar and Mitchell 1999). More recently, it has been suggested that children with ASD utilize a more detail-oriented style of processing visual information (Muller and Nussbeck 2008; Sasson et al. 2008). Although very young children have not routinely been included in experimental paradigms investigating these phenomena, behavioral studies reveal that children with ASD begin to show abnormal visual exploration of objects by their first birthday (Ozonoff et al. 2008) and typically show relative strength on tasks involving visual discrimination and categorization skills (Chawarska et al. 2009).
The primary goal of this study is to replicate and extend our previous findings on face recognition (Chawarska and Shic 2009) in toddlers with ASD. Although face recognition impairments have been found in children with ASD as young as 2 years of age (Chawarska and Shic 2009; Chawarska and Volkmar 2007), it is unclear if these impairments are restricted to faces or represent a generalized deficit in fast and implicit processing of novel stimuli, regardless of its social content. To address this question, in this study we examine face, object, and complex block pattern recognition in 3-year-old children with ASD and compare their performance to age-matched typically developing (TD) controls.
Recognition of faces, objects, and block patterns was tested using the fixed-trial Visual Paired Comparison (VPC) paradigm (Fantz 1964; Pascalis and de Haan 2003). The VPC paradigm typically consists of a Familiarization and Recognition phase. In the Familiarization phase, a stimulus is presented until the subject has observed the stimulus for a predetermined amount of time. The succeeding Recognition phase consists of the familiarized stimulus presented simultaneously with a novel stimulus from the same class. In the VPC paradigm, recognition of a previously familiarized stimulus can be inferred from a significantly longer looking time at the novel stimulus (novelty preference) or the familiar stimulus (familiarity preference) (Kaplan et al. 1990; Pascalis and de Haan 2003).
We hypothesize that children with ASD will not show a global deficit in recognition, but rather the impairment will be specific to social stimuli. Additionally, we predict that both typically developing children and those with ASD will process the simple nonsocial stimuli in a similar way resulting in successful recognition of objects. Finally, we hypothesize the TD group will find the complex nonsocial stimuli too difficult to encode in the given amount of time while the ASD group will exhibit superior performance.
Methods
Participants
The cohort consisted of 21 children diagnosed with ASD and 21 typically developing children. Participants with ASD were recruited through a university-based research center specializing in developmental disorders. Typically developing children were recruited through flyers and email lists distributed throughout the community, and any child between the age of 36–48 months who did not present with developmental delays in the intake interview or developmental assessment was included in the study as a control participant. Best estimate clinical diagnosis of ASD was based on direct assessment consisting of the Mullen Scales of Early Learning (Mullen 1995), Autism Diagnostic Observation Schedule-Generic (ADOS-G) (Lord et al. 2000), Vineland Adaptive Behavior Scales-II (Sparrow et al. 1984), and Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al. 2003) given between 36–48 months of age. Typical development was confirmed in our TD sample with average scores on nonverbal scales in the Mullen. Verbal scales were not administered with typically developing controls.
The ASD group was made up of 90% males and 10% females, while the TD group was 52% males and 48% females (see Table 1). The gender distribution as well as cognitive level was significantly different between diagnostic groups. Within the TD children, however, nonverbal developmental quotient did not differ between gender groups.
Table 1.
Participant characterization
| Characterization variable | ASD (n = 21) | TD (n = 21) |
|---|---|---|
| Chronological age (months) | 39 (10) | 36 (7) |
| Male:female | 19:2* | 11:10* |
| Verbal DQa | 63 (36) | – |
| Nonverbal DQa | 79 (28)* | 106 (14)* |
| Social affect (SA)b | 13 (5) | – |
| Restricted repetitive behaviors (RRB)b | 6 (1) | – |
| Communication standard scorec | 67 (9) | – |
| Socialization standard scorec | 79 (18) | – |
p<.05
Mullen Scales of Early Learning (Mullen 1995)
Autism Diagnostic Observation Schedule-G (Lord et al. 2000)
Vineland Adaptive Behavior Scales (Sparrow et al. 1984)
Stimuli
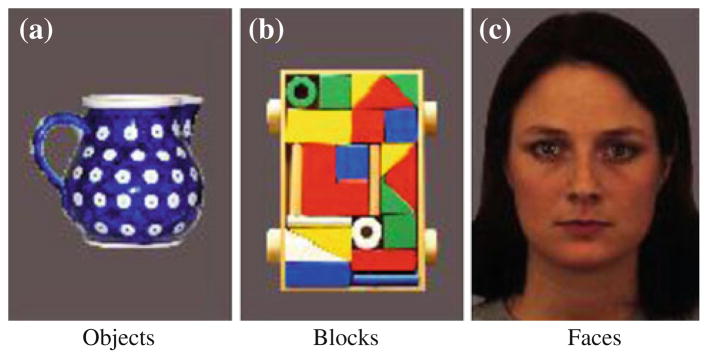
All stimuli were color images of affectively neutral female faces (Lundqvist et al. 1998), common objects, and colorful geometric block patterns (see Fig. 1). Objects consisted of teapots, vases, and bowls with complex patterns and colors. The block stimuli consisted of a rectangular frame filled with geometric shapes of varying colors that made distinct patterns. All stimuli were standardized using Adobe Photoshop to equate illumination, color, and size. Each image was displayed on a light grey background measuring approximately 12.6 × 17.6 visual degrees.
Fig. 1.
Images employed in object (a), block (b), and face (c) conditions
Apparatus
Stimuli were displayed on a 20″ widescreen LCD monitor (1280 × 800 pixels) using Neurobehavioral Systems Presentation software (Neurobehavioral Systems 2005). Children were placed into a car seat located 75 cm away from the monitor with their eye-level at the center of the monitor. Visual scanning patterns were recorded using a SensoMotoric Instruments iView X RED 60 Hz eye tracking system (Sensomotoric Instruments 2005).
Procedure
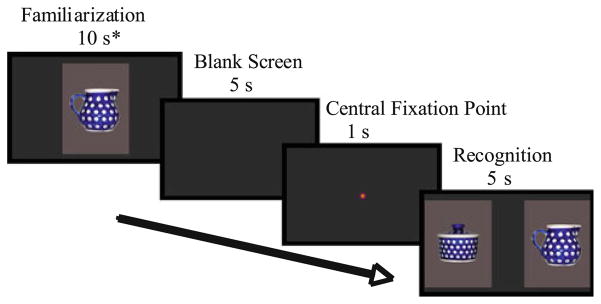
Each child was exposed to one of two orderings of stimuli: (a) Objects, Blocks, Faces or (b) Blocks, Faces, Objects. Preliminary analysis revealed no significant effect of order, so the data were collapsed across order for final analysis. Each eyetracking session began with a movie presentation to get the child settled, followed by a five-point calibration procedure which was repeated until successful. Only one child (typically developing) was unable to complete this initial calibration step, preventing him from continuing the experiment. Each of the three conditions consisted of six trials; each trial included a Familiarization and Recognition phase (see Fig 2). During the Familiarization phase, an image was presented on the screen until the child looked at the screen for 10 s. The looking time was determined by trained research assistants who monitored the child through a live feed of the entire head, independent of the eyetracking camera. The research assistants pressed a computer key any time the child was judged to be looking at the screen. A child was judged to be looking at the screen when their eyes and head were oriented toward the screen and a reflection of the screen appeared on his or her cornea. Cumulative looking time was calculated by a computer program that would advance the experiment to the next phase in the trial once the child had reached the 10 s criterion. The Familiarization phase was followed by a blank grey screen for 5 s and a re-centering stimulus for 1 s, completing a 6 s intertrial interval (ITI). The re-centering stimulus was a bright, animated circle that appeared in the center of the screen accompanied by a loud sound. This typically attracted the child’s attention, but children were not required to look at this image in order for the experiment to continue. The Recognition phase then began and consisted of a side-by-side presentation of the novel and familiar stimuli for 5 s. Location of the novel stimulus was counterbalanced across the trials. The re-centering stimulus was presented before each image in the Familiarization and Recognition phases. No instructions were given to the children other than general statements to redirect the child’s attention such as “Look at the screen” and “What do you see?”
Fig. 2.
Example of visual paired comparison paradigm. * Duration of familiarization was dependent on the child’s looking time; the 10 s shown here indicates 10 s of accumulated looking
Results
Valid Trials
Trials were excluded on the basis of insufficient or poor quality of the recording or eyetracking data loss due to excessive subject movement or inattention. Children contributing less than two valid trials per condition were excluded from analysis. Out of six possible trials, the ASD group had an average of 4.6 (SD = 1.1), 4.3 (SD = 1.0), and 4.9 (SD = 1.2) valid trials in the Face, Object, and Block Pattern conditions, respectively, as compared to 4.6 (SD = 1.2), 3.9 (SD = 1.1), and 4.2 (SD = .8), in the TD group. There were no significant between-group differences regarding the number of valid trials, nor the proportion of children who were dropped due to lack of attention.
Attention During Trials
The amount of time spent looking at the screen in the Familiarization and Recognition phases in each condition are displayed in Table 2. Total time spent looking at the Familiarization phase is less than the 10 s criterion due to movement and blinking that causes minor gaps in the eyetracking data. Univariate ANOVAs revealed no significant differences in looking time between the diagnostic groups during the Familiarization or Recognition phase in each condition. Additionally there were no significant differences in duration of looking or novelty preference between the two different orders of stimuli shown to each diagnostic group. Data regarding visual attention toward the re-centering stimulus was not analyzed as we used the recalibration and error measurement system of Shic (2008), however trials with insufficient eyetracking data were excluded from analysis.
Table 2.
Average (SD) total duration of looking at stimuli in familiarization and recognition phases
| Stimulus | Looking time ASD | TD |
|---|---|---|
| Familiarization phase | ||
| Faces | 7.8 s (1.7) | 8.1 s (1.5) |
| Objects | 8.0 s (1.5) | 8.9 s (1.7) |
| Blocks | 8.1 s (1.7) | 7.7 s (1.9) |
| Recognition phase | ||
| Faces | 3.9 s (.8) | 4.2 s (.5) |
| Objects | 3.8 s (.7) | 3.9 s (.8) |
| Blocks | 3.2 s (.8) | 3.0 s (.8) |
Recognition
In each condition, novelty preference ratio was calculated as a ratio of looking time at the novel stimulus to total looking time at both the novel and familiar stimulus. Novelty preference ratios were averaged over trials so that a single novelty preference was calculated for each child. There were no significant differences in novelty ratios for male and female participants within the TD or ASD group. When novelty preference ratios for each group were compared to each other within stimulus sets, only the performance during the social stimuli was significantly different between groups (Faces: p<.0001, Objects: p = .890, Blocks: p = .748). However, when one sample t-tests against chance level (.50) were conducted for each condition, the standard analysis for determining novelty preference in this type of paradigm (Pascalis et al. 1998; Rose et al. 1982), typically developing children displayed a novelty preference significantly above chance for faces and objects, while children with ASD displayed a novelty preference significantly above chance for blocks and objects (see Table 3).
Table 3.
Novelty preference ratio in face, object, and block pattern conditions
| n | Mean (SD) | t-test | p-value | |
|---|---|---|---|---|
| ASD | ||||
| Faces | 18 | .50 (.06) | −.24 | .810 |
| Objects | 12 | .57 (.11) | 2.3 | .043* |
| Blocks | 15 | .54 (.06) | 2.2 | .047* |
| TD | ||||
| Faces | 17 | .60 (.07) | 5.8 | .001* |
| Objects | 14 | .57 (.10) | 2.5 | .027* |
| Blocks | 15 | .53 (.09) | 1.1 | .284 |
Significant at p<.05
Discussion
This study replicates and extends our previous findings (Chawarska and Shic 2009) and elucidates the relative strengths and weaknesses of children with ASD in the recognition of social and nonsocial stimuli. The purpose of this study was to ascertain if recognition deficits in young children with ASD are specific to faces or if there is a generalized processing impairment that extends to nonsocial stimuli as well. We incorporated both social and nonsocial stimuli into the framework of a Visual Paired Comparison paradigm, splitting the nonsocial stimuli into categories of simple and complex.
Consistent with previous literature (e.g. Chawarska and Shic 2009; Klin et al. 1999) and supporting our original hypothesis, children with ASD, unlike their typical peers, had difficulties encoding and recognizing faces. They were however adept at encoding and recognizing objects and complex block patterns, revealing a specific social impairment and a specific advantage for complex nonsocial stimuli. This visual processing advantage has been noted in a variety of studies (e.g. Happé and Frith 2006; Plaisted et al. 1998; Rinehart et al. 2000; Sasson et al. 2008) in which individuals with ASD have a more detail-oriented style of processing allowing them to display superior performance in certain tasks, such as the block design task. To our knowledge, this visual processing advantage has not yet been documented in children as young as 3 years of age. It is also important to note that within the block pattern stimulus set the two diagnostic groups did not differ from each other in their recognition abilities. It seems that the large amount of variance observed in the novelty preference of the typical group could be leading to the lack of significance against chance. It is perhaps the case that the ability to encode these difficult block figures is just emerging for 3-year-old typically developing children and thus some children in our sample were able to recognize the blocks, while others were not. Further study is needed to disambiguate this developmental issue.
Our study illustrates that in the absence of competing stimuli, 3-year-old children with ASD are not less motivated to examine social stimuli as they required the same amount of time to reach the familiarization criterion for every condition, including faces, as their typical peers. Additionally, since the study was designed to equate the total looking time towards the familiarization images across all subjects, the failure to recognize faces cannot be explained in terms of decreased overall attention towards faces. This suggests impairment in children with ASD that extends beyond simply attention toward social stimuli, and lies more specifically in the processing of social stimuli. A question thus presents itself: what are the origins of the less effective face processing strategies as seen in 2 (Chawarska and Shic 2009) and 3-year-olds? Impairments in face recognition might be driven by limited attention to faces early in development leading to the development of immature or atypical face processing strategies reflected in ineffective processing of these complex and highly socially relevant stimuli. Given that this deficit appears so early in life, early intervention targeting the processing of social information could be pivotal for later development of social motivation and attention.
Limitations of this study include the lack of a non-autistic developmentally delayed control group, especially due to the significant differences in nonverbal skills between the TD and ASD group. However the double dissociation between face and block recognition suggests a non-cognitive origin for the observed pattern of results. Language abilities were not formally assessed for the typically developing group and therefore we cannot draw conclusions about how language might affect recognition for the typical group. However the stimuli included in the experiment were chosen to be unfamiliar to 3-year-old children so that language would play a minimal role in the encoding and recognition process. The role of language in the encoding and recognition of stimuli in ASD and TD children should be further examined.
Future studies should focus on studying in greater depth the process that is directly responsible for impaired face recognition including factors associated with scanning strategies, attention regulation, learning, and memory.
Acknowledgments
This study was supported by the National Alliance for Autism Research foundation, NIMH Studies to Advance Autism Research and Treatment grant U54 MH66494, Autism Centers of Excellence grant P50 MH081756 project 2 (PI: KC), and NIMH grant T32 MH18268 (FS). We would like to thank Celine Saulnier for her contribution to the characterization of the participants in this study and Jessica Reed, Brittany Butler, Rebecca Doggett, Paula Ogston, and Joslin Latz for their assistance in high-quality data collection. We would especially like to express our gratitude to the children and families who participated and continue to participate in our research.
Footnotes
A preliminary version of this study was presented at the 9th Annual International Meeting for Autism Research (IMFAR) in Philadelphia, PA, USA, May, 2010.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Annaz D, Karmiloff-Smith A, Johnson MH, Thomas MS. A cross-syndrome study of the development of holistic face recognition in children with autism, Down syndrome, and Williams syndrome. Journal of Experimental Child Psychology. 2009;102(4):456–486. doi: 10.1016/j.jecp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry. 1992;33(5):843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Bushnell I. Mother’s face recognition in newborn infants: Learning and memory. Infant and Child Development. 2001;10(1–2):67–74. doi: 10.1002/icd.248. [DOI] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: Short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50(10):1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39(12):1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F. Impairments in monkey and human face recognition in 2-year-old toddlers with Autism Spectrum Disorder and Developmental Delay. Developmental Science. 2007;10(2):266–279. doi: 10.1111/j.1467-7687.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- Fantz RL. Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science. 1964;146(3644):668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Golan O, Baron-Cohen S, Hill JJ, Golan Y. The “Reading the Mind in Films” task: Complex emotion recognition in adults with and without autism spectrum conditions. Social Neuroscience. 2006;1(2):111. doi: 10.1080/17470910600980986. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56(4):544–549. [PubMed] [Google Scholar]
- Happé FG. Studying weak central coherence at low levels: Children with autism do not succumb to visual illusions. A research note. Journal of Child Psychology and Psychiatry. 1996;37(7):873–877. doi: 10.1111/j.1469-7610.1996.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988;79(4):441. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2003;44(4):529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Werner J, Rudy J. Habituation, sensitization, and infant visual attention. Advances in Infancy Research. 1990;6:61–109. [Google Scholar]
- Kätsyri J, Saalasti S, Tiippana K, von Wendt L, Sams M. Impaired recognition of facial emotions from low-spatial frequencies in Asperger syndrome. Neuropsychologia. 2008;46(7):1888–1897. doi: 10.1016/j.neuropsychologia.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29(6):499–508. doi: 10.1023/A:1022299920240. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Psychology section. Karolinska Institutet; 1998. The Karolinska Directed Emotional Faces—KDEF, CD ROM from Department of Clinical Neuroscience. [Google Scholar]
- Maurer D, Barrera M. Infants’ perception of natural and distorted arrangements of a schematic face. Child Development. 1981;52(1):196–202. [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review. 1991;98(2):164–181. doi: 10.1037/0033-295X.98.2.164. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc; 1995. AGS Edition ed. [Google Scholar]
- Muller C, Nussbeck S. Do children with autism spectrum disorders prefer to match pictures based on their physical details or their meaning? Journal of Mental Health Research in Intellectual Disabilities. 2008;1(3):140–155. doi: 10.1080/19315860801988244. [DOI] [Google Scholar]
- Neurobehavioral Systems. Presentation. 2005. [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain and Cognition. 2005;59(1):82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism: The International Journal of Research and Practice. 2008;12(5):457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Haan M. Recognition memory and novelty preference: what model? In: Hayne H, Fagen J, editors. Progress in infancy research. Vol. 3. Lawrence Erlbaum; Mahwah: 2003. pp. 95–119. [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. Journal of Experimental Psychology Learning, Memory, and Cognition. 1998;24:249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates: A replication and an extension. Infant Behavior and Development. 1995;18(1):79–85. doi: 10.1016/0163-6383(95)90009-8. [DOI] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32(4):249–261. doi: 10.1023/A:1016374617369. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: A research note. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(05):777–783. doi: 10.1017/S0021963098002613. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Atypical interference of local detail on global processing in high-functioning autism and asperger’s disorder. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(06):769–778. doi: 10.1111/1469-7610.00664. [DOI] [PubMed] [Google Scholar]
- Ropar D, Mitchell P. Are individuals with autism and Asperger’s syndrome susceptible to visual illusions? Journal of Child Psychology and Psychiatry. 1999;40(8):1283–1293. doi: 10.1111/1469-7610.00544. [DOI] [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar P, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18(5):704–713. doi: 10.1037/0012-1649.18.5.704. [DOI] [Google Scholar]
- Rutter M, Le Couter A, Lord C. ADI-R: Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1(1):31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Senju A, Yaguchi K, Tojo Y, Hasegawa T. Eye contact does not facilitate detection in children with autism. Cognition. 2003;89(1):B43–B51. doi: 10.1016/S0010-0277(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Sensomotoric Instruments. iView X (TM) RED. 2005. [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34(8):1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Shic F. PhD thesis. Yale University; 2008. Computational methods for eye-tracking analysis: Applications to autism. [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales: Survey from manual. Circle Pines, MN: American Guidance Service, Inc; 1984. [Google Scholar]
- Walton GE, Bower TGR. Newborns form “Prototypes” in less than 1 minute. Psychological Science. 1993;4(3):203–205. [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2004;34(5):473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]