Abstract
Deep brain stimulation (DBS), applying high-frequency electrical stimulation to deep brain structures, has now provided an effective therapeutic option for treatment of various neurological and psychiatric disorders. DBS targeting the internal segment of the globus pallidus, subthalamic nucleus, and thalamus is used to treat symptoms of movement disorders, such as Parkinson’s disease, dystonia, and tremor. However, the mechanism underlying the beneficial effects of DBS remains poorly understood and is still under debate: Does DBS inhibit or excite local neuronal elements? In this short review, we would like to introduce our recent work on the physiological mechanism of DBS and propose an alternative explanation: DBS dissociates input and output signals, resulting in the disruption of abnormal information flow through the stimulation site.
Keywords: deep brain stimulation, stereotactic surgery, globus pallidus, subthalamic nucleus, Parkinson’s disease, dystonia
Conventional stereotactic surgery, such as pallidotomy and thalamotomy that make small lesions in the globus pallidus and thalamus, went through a renaissance as treatment for Parkinson’s disease in early 1990s (Laitinen and others 1992). Around the same time, deep brain stimulation (DBS) that applies high-frequency electrical stimulation through chronically implanted electrodes into a specific target in the subcortical structures was put into practical use (Benabid and others 1991, 1994). DBS was soon found to be an effective and safe alternative to lesion therapy, because it was reversible and adjustable. DBS has now been widely accepted as an effective surgical treatment for movement disorders. DBS targeting the ventral intermediate nucleus of the thalamus dramatically reduces essential and resting tremor (Benabid and others 1991, 1996). DBS targeting the subthalamic nucleus (STN) and the internal segment of the globus pallidus (GPi) has been largely used for treatment of advanced Parkinson’s disease and dyskinesia, a major side effect of l-DOPA treatment (Deep-Brain Stimulation for Parkinson’s Disease Study Group 2001; Kringelbach and others 2007; Limousin and others 1995; Vitek 2008; Wichmann and Delong 2006). GPi-DBS has marked effects on the improvement of dystonic symptoms (Ostrem and Starr 2008). DBS is also applied for treatment of pain, epilepsy, and neuropsychiatric disorders, such as obsessive compulsive disorders, Tourett’s syndrome and depression (Wichmann and Delong 2006).
However, despite clinical benefits of DBS, the exact mechanism underlying its effectiveness has remained to be clarified and there are still several controversies about its action mechanism: Does DBS inhibit or excite local neuronal elements? (Deniau and others 2010; Kringelbach and others 2007; Perlmutter and Mink 2006; Vitek 2008; Wichmann and Delong 2006). Since DBS brings about similar beneficial effects to those of lesion therapy, it was initially believed to inhibit local neuronal elements (“inhibition hypothesis”). Actually, STN-DBS and GPi-DBS inhibited firings of neighboring neurons. On the other hand, it is not surprising that DBS excites local neuronal elements just as single stimulation does (“excitation hypothesis”). STN-DBS and GPi-DBS excited their efferents and provided effects on the GPi and thalamus, respectively.
In this short review, first, we will summarize current concepts regarding the pathophysiology of Parkinson’s disease and other movement disorders, because DBS is considered to normalize, or at least change, the pathophysiological states of movement disorders. Second, we will critically review “inhibition hypothesis” and “excitation hypothesis” as the mechanism of DBS. Finally, we would like to introduce our recent work on the physiological mechanism of DBS and propose an alternative explanation: DBS dissociates input and output signals, resulting in the disruption of abnormal information flow through the stimulation site (“disruption hypothesis”) (Chiken and Nambu 2013).
Pathophysiology of Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disorder characterized by the progressive loss of nigrostriatal dopaminergic neurons originating from the substantia nigra pars compacta. The loss of dopaminergic neurons induces severe motor and non-motor dysfunctions, such as akinesia, tremor, rigidity, postural instability, cognitive impairments and depression. Three models have been proposed to explain the pathophysiology of Parkinson’s disease.
Firing Rate Model
Dopamine provides tonic excitatory inputs to striatal direct pathway neurons projecting to the GPi and tonic inhibitory inputs to striatal indirect pathway neurons projecting to the external segment of the globus pallidus (GPe), and dopamine depletion reduces these tonic excitatory and inhibitory inputs (Albin and others 1989; DeLong 1990; Gerfen and others 1990; Mallet and others 2006). Both of these changes are thought to increase mean firing rates of GPi and substantia nigra pars reticulata (SNr) neurons by reduced inhibitory inputs through the striato-GPi/SNr direct pathway and increased excitatory inputs through the striato-GPe-STN-GPi/SNr indirect pathway. Such increased mean firing rates in the output nuclei of the basal ganglia seem to induce decreased activity in thalamic and cortical neurons, resulting in akinesia (“firing rate model”). These firing rate changes of the basal ganglia, that is, increased mean firing rates in the GPi and STN and decreased mean firing rates in the GPe, were confirmed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced parkinsonian monkeys (Bergman and others 1994; Boraud and others 1996, 1998; Filion and Tremblay 1991; Heimer and others 2002; Miller and DeLong 1987; Soares and others 2004; Wichmann and others 2002). Moreover, lesioning of the STN or GPi, whose activity was increased, had beneficial effects on Parkinson’s disease (Baron and others 2000, 2002; Bergman and others 1990), supporting this “firing rate model.” This model seems to be applicable to hyperkinetic disorders, such as dystonia and hemiballism that exhibit involuntary movements, as well. It was reported that firing rates in the GPe and GPi were decreased in human patients of dystonia (Starr and others 2005; Tang and others 2007; Vitek and others 1999; Zhuang and others 2004) and an animal model of dystonia (Chiken and others 2008). The development of the involuntary movements can be explained as the result of reductions in inhibitory inputs to the thalamus from the GPi.
Firing Pattern Model
Dopamine depletion enhances connections between the GPe and STN, and promotes oscillatory activity in the basal ganglia. Oscillatory and/or synchronized firings of the basal ganglia disable individual neurons to process and relay motor-related information, resulting in the failure of appropriate movements (“firing pattern model”) (Bergman and others 1998). Abnormal firing patterns, such as bursts and oscillations, were recorded in the GPe, GPi and STN of parkinsonian monkeys (Bergman and others 1994; Heimer and others 2002, 2006; Raz and others 2000; Tachibana and others 2011; Wichmann and Soares 2006) and parkinsonian patients (Levy and others 2000). Oscillatory local filed potentials (LFPs), especially those in the beta frequency band, were also observed in parkinsonian patients using DBS electrodes (Brown 2003; Brown and others 2001; Brown and Williams 2005; Gatev and others 2006; Hammond and others 2007).
Dynamic Activity Model
In the normal state, signals through the cortico-STN-GPi/SNr hyperdirect, cortico-striato-GPi/SNr direct, and cortico-striato-GPe-STN-GPi/SNr indirect pathways cause dynamic activity changes in the GPi (see Fig. 3C) and release only a selected motor program at a selected timing with a clear boundary between the selected and other unnecessary competing motor programs (Nambu 2008; Nambu and others 2015). In Parkinson’s disease, dopamine depletion reduces movement-related GPi inhibition through the direct pathway and facilitates movement-related GPi excitation through the hyperdirect and indirect pathways (Boraud and others 2000; Degos and others 2005; Kita and Kita 2011; Leblois and others 2006). These changes shorten and narrow movement-related GPi inhibition, which leads to the reduction of disinhibition in the thalamus and cortex, resulting in akinesia (“dynamic activity model”). In hyperkinetic disorders, movement-related inhibition in the GPi through the direct pathway is enhanced, and GPi excitation through the hyperdirect and indirect pathways is reduced. These dynamic changes induce excessive, uncontrolled disinhibition in the thalamus and cortex, leading to involuntary movements (Chiken and others 2008; Nambu and others 2011; Nishibayashi and others 2011).
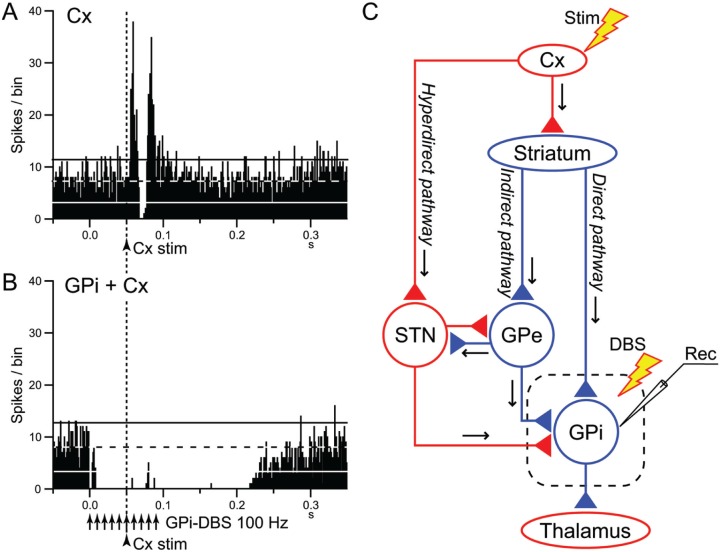
Figure 3.
Deep brain stimulation (DBS) of the internal segment of the globus pallidus (GPi) disrupts information flow through the GPi. (A, B) Effects of local GPi-DBS on cortically evoked responses of a GPi neuron in a normal monkey. PSTHs in response to a single-pulse stimulation of the primary motor cortex (Cx) (arrowhead with dotted line) without (A) and with GPi-DBS (arrows) (B) are shown. In (B), cortical stimulation was applied 50 ms after the initiation of GPi-DBS. The cortically evoked responses were entirely inhibited during GPi-DBS. (C) Schematic diagram showing the cortico-basal ganglia pathways and stimulating (Stim and DBS) and recording (Rec) sites. Cortically evoked early excitation, inhibition and late excitation in (A) are mediated by the hyperdirect, direct, and indirect pathways, respectively. Cx, cerebral cortex; GPe, external segment of the globus pallidus; STN, subthalamic nucleus. Red and blue triangles represent glutamatergic excitatory and GABAergic inhibitory terminals, respectively. Modified from Chiken and Nambu (2013).
“Inhibition Hypothesis”: DBS Inhibits Local Neuronal Elements
Both DBS and lesion therapy have similar beneficial effects on the alleviation of symptoms. STN-DBS (Benazzouz and others 1993; Benabid and others 1994; Limousin and others 1995) showed similar effects on parkinsonian motor signs to STN-lesion (Aziz and others 1991; Bergman and others 1990; Levy and others 2001) or STN-blockade (Luo and others 2002). Thus, DBS was initially believed to inhibit local neuronal elements (“inhibition hypothesis”). Actually, the most common effects of STN-DBS and GPi-DBS are the reduction of the firing rates of neighboring neurons. Suppression of neuronal activity was recorded around the stimulating sites of STN-DBS in parkinsonian patients (Filali and others 2004; Welter and others 2004), parkinsonian monkeys (Meissner and others 2005; Moran and others 2011) and parkinsonian rats (Shi and others 2006; Tai and others 2003). However, a limited number of STN neurons showed complete cessation of firings, and other STN neurons exhibited residual neuronal activity during STN-DBS (Meissner and others 2005; Tai and others 2003; Welter and others 2004). Inhibitory effects of GPi-DBS on firings of neighboring neurons were also reported in parkinsonian patients (Dostrovsky and others 2000; Lafreniere-Roula and others 2010; Wu and others 2001), parkinsonian monkeys (Boraud and others 1996) and normal monkeys (Fig. 1A) (Chiken and Nambu 2013). GPi-DBS induced complete inhibition of local neuronal firings more commonly than STN-DBS.
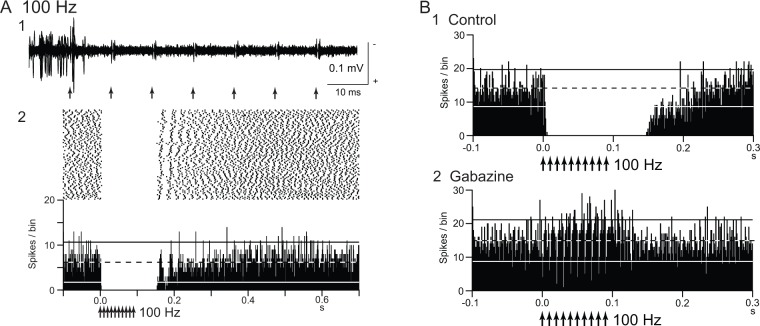
Figure 1.
Deep brain stimulation (DBS) inhibits local neuronal firings. (A) Responses of an internal pallidal (GPi) neuron to local repetitive high-frequency stimulation (GPi-DBS; 30 µA, 100 Hz, 10 pulses; arrows) in a normal monkey. Raw traces of spike discharges after removing the stimulus artifacts (1) and raster and peristimulus time histograms (PSTHs; 100 trials; binwidth, 1 ms) (2) are shown. Spontaneous discharge of the GPi neuron was completely inhibited by GPi-DBS. (B) Effect of local injection of gabazine (GABAA receptor antagonist) in the vicinity of the recorded GPi neuron. The inhibition induced by GPi-DBS (1) was abolished after gabazine injection (2). Modified from Chiken and Nambu (2013).
The “inhibition hypothesis” fits well with the “firing rate model” and “firing pattern model” of movement disorders. DBS reduces abnormally increased firings or abnormal firing patterns in the STN and GPi and ameliorates parkinsonian motor symptoms. However, it seems to be difficult to explain why GPi-DBS can treat dystonic symptoms, in which the GPi shows low activity.
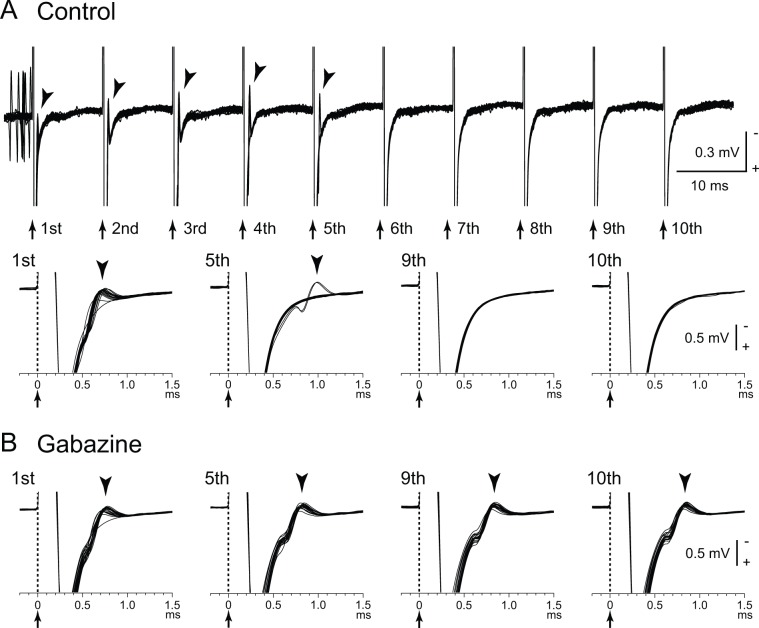
Several possible mechanisms can account for the inhibitory responses during DBS: (1) depolarization block, (2) inactivation of voltage-gated currents (Beurrier and others 2001; Do and Bean 2003; Shin and others 2007), and (3) activation of inhibitory afferents (Boraud and others 1996; Chiken and Nambu 2013; Deniau and others 2010; Dostrovsky and others 2000; Dostrovsky and Lozano 2002; Johnson and McIntyre 2008; Liu and others 2008; Meissner and others 2005). Our recent study (Chiken and Nambu 2013) confirmed that inhibitory responses induced by GPi-DBS were mediated by GABA receptors (Fig. 1B). The GPi receives inhibitory GABAergic inputs from the striatum and GPe (Shink and Smith 1995; Smith and others 1994), and these inhibitory GABAergic afferents are considered to be activated by GPi-DBS. The GPi also receives excitatory glutamatergic inputs from the STN, and these afferents should also be activated. However, GABAergic terminals are predominant (Shink and Smith 1995), and thus, GABAergic inhibition probably overwhelms glutamatergic excitation. GPi-stimulation induced directly-evoked spikes, which are characterized by a short and constant latency (Fig. 2A). GPi-DBS also suppressed such directly evoked spikes by strong GABAergic inhibition (Fig. 2B). In contrast to the GPi, GPe-DBS induced complex responses composed of excitation and inhibition in neighboring GPe neurons (Chiken and Nambu 2013). Since GABAergic terminals on GPe neurons are less dense than those on GPi neurons (Shink and Smith, 1995), glutamatergic excitation can be observed in the GPe. Similarly, STN-DBS generated both excitatory and inhibitory postsynaptic potentials in STN neurons through activation of both glutamatergic and GABAergic afferents (Lee and others 2004). Thus, DBS activates afferent axons in the stimulated nucleus, and the net effects vary depending on the composition of the inhibitory and excitatory axon terminals.
Figure 2.
Directly evoked spikes of internal pallidal (GPi) neurons are inhibited during GPi–deep brain stimulation (DBS). (A) Raw traces showing directly evoked spikes (arrowheads) of a GPi neuron by GPi-DBS (40 µA, 100 Hz, 10 pulses; arrows with dotted lines) in a normal monkey. Traces with long (top) and short (bottom) time scales are shown. GPi-DBS failed to evoke spikes (from 6th to 10th stimuli). (B) Effects of local gabazine injection on the inhibition of directly evoked GPi responses. Gabazine injection decreased the failure rate, and each stimulus successfully evoked spikes (5th, 9th, and 10th stimuli). Modified from Chiken and Nambu (2013).
“Excitation Hypothesis”: DBS Excites Local Neuronal Elements
It is not surprising that DBS excites local neuronal elements just as single stimulation does (“excitation hypothesis”). Directly evoked spikes were induced by GPi-DBS in GPi neurons (Johnson and McIntyre 2008; McCairn and Turner 2009). GPi-DBS reduced firings in thalamic neurons of parkinsonian monkeys (Anderson and others 2003) and dystonia patients (Pralong and others 2003; Montgomery 2006) through the inhibitory GPi-thalamic projections. STN-DBS increased firings in GPi neurons of parkinsonian monkeys (Hashimoto and others 2003) and GPi/GPe neurons (Reese and others 2011) and SNr neurons (Galati and others 2006) of parkinsonian patients through the excitatory STN-GPi/SNr/GPe projections. A modeling study has suggested that subthreshold DBS suppresses intrinsic firings in the cell bodies, while suprathreshold DBS induces spikes at the stimulus frequency in the axons without corresponding firings in the cell bodies (McIntyre and others 2004). Thus, although stimulation may fail to activate the cell bodies due to strong GABAergic inhibition, it can still excite the efferent axons and provide spikes to the target nucleus at the stimulus frequency. Other studies showed that GPi-DBS induced multiphasic responses consisting of excitation and inhibition in the GPi of parkinsonian monkeys (Bar-Gad and others 2004; Erez and others 2009; McCairn and Turner 2009) and dystonia hamsters (Leblois and others 2010). It was recently reported that GPe-DBS changed firing patterns in STN, GPi and thalamic neurons of parkinsonian monkeys and improved motor signs, suggesting the GPe as a potential target for DBS (Vitek and others 2012).
Deep brain stimulation also excites afferent axons antidromically. STN-DBS activated GPi neurons antidromically in parkinsonian monkeys (Moran and others 2011), probably by current spread to the lenticular fasciculus, a part of GPi-thalamic fibers (Miocinovic and others 2006). GPi-DBS activated thalamic neurons antidromically in dystonia patients, probably by activation of thalamic axons passing in the vicinity of GPi-DBS electrodes (Montgomery 2006). STN-DBS with low intensity induced GABAergic inhibition in the SNr through antidromic activation of GPe neurons projecting to both the STN and SNr, while STN-DBS with higher intensity induced glutamatergic excitation in the SNr through the STN-SNr projections (Deniau and others 2010; Maurice and others 2003). STN-DBS activated neurons in the motor cortex antidromically (Degos and others 2013; Q. Li and others 2012, S. Li and others 2007). Recent development of optogenetic study showed that selective stimulation of cortico-STN afferent axons without activation of STN efferent axons ameliorated the symptoms of parkinsonian mice (Gradinaru and others 2009).
The “excitation hypothesis” fits well with the “firing pattern model” of movement disorders, but not with the “firing rate model”. Excitation and/or excitation-inhibition reach the target nucleus along efferent pathways, or antidromic activation reaches the original region along afferent pathways. These activity changes may alter the firing rates and patterns, and normalize or suppress abnormal firings of target nucleus (Anderson and others 2003; Degos and others 2013; Deniau and others 2010; Hammond and others 2007; Hashimoto and others 2003; Johnson and McIntyre 2008; Q. Li and others 2012; S. Li and others 2007; Vitek 2008). However, the precise mechanism how DBS normalizes firing patterns remains to be elucidated.
“Disruption Hypothesis”: DBS Disrupts Abnormal Information Flow
We recently examined the effects of GPi-DBS on the responses of GPi neurons evoked by motor cortical stimulation in normal monkeys (Fig. 3) (Chiken and Nambu 2013). Cortical stimulation induces a triphasic response composed of early excitation, inhibition and late excitation in the GPi (Fig. 3A), which are mediated by the hyperdirect, direct, and indirect pathways, respectively (Nambu and others 2000, 2002) (Fig. 3C). GPi-DBS completely inhibited both cortically evoked responses and spontaneous discharges by strong GABAergic inhibition (Fig. 3B), suggesting that it blocks information flow through the GPi (“disruption hypothesis”) (Fig. 4). STN-DBS may similarly block transmission of signals through the STN: Maurice and others (2003) examined the effects of STN-DBS on cortically evoked responses in SNr neurons of normal rats. Cortically evoked early and late excitation was abolished or largely reduced during STN-DBS, whereas cortically evoked inhibition was preserved, suggesting that information flow through the hyperdirect and indirect pathways was blocked by STN-DBS without interrupting the direct pathway.
Figure 4.
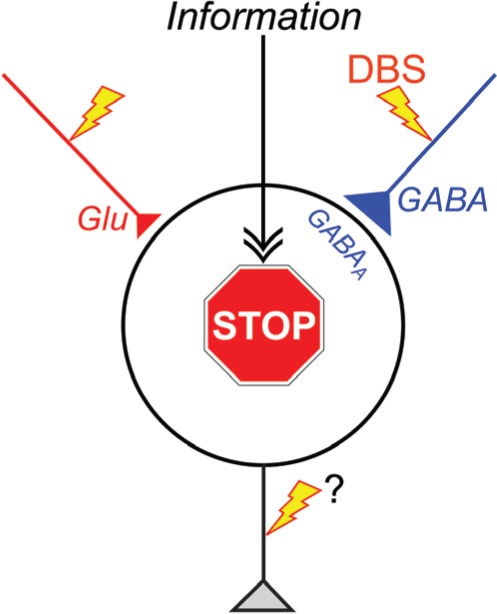
“Disruption hypothesis” explaining the mechanism underlying the effectiveness of deep brain stimulation (DBS). DBS activates axon terminals in the stimulated nucleus, induces extensive release of neurotransmitters, such as GABA and glutamate (Glu), and dissociates inputs and outputs in the stimulated nucleus. Thus, DBS results in disruption of the abnormal information flow through the cortico-basal ganglia loop in the pathological conditions. GABAA, GABAA receptors.
The “disruption hypothesis” fits well with the “firing rate model,” “firing pattern model,” and “dynamic activity model.” Since abnormally increased firings, abnormal firing patterns, or abnormal dynamic activity changes in the basal ganglia are transmitted to the thalamus and motor cortex, and finally induce motor symptoms, disrupting such abnormal information flow through the GPi and STN can suppress the expression of motor symptoms. The GPi is an output nucleus of the basal ganglia, and thus GPi-DBS disrupts all information outflow from the basal ganglia. On the other hand, the GPe-STN reciprocal connections produce abnormal firing patterns in Parkinson’s disease (“firing pattern model”), and interruption of information flow through the STN reduces them. In addition, the hyperactivity along the hyperdirect and indirect pathways are suggested in Parkinson’s disease (“firing rate model” and “dynamic activity model”), and interruption of information flow through the STN blocks such hyperactivity. The “disruption hypothesis” can explain the long-standing paradox as well: DBS produces similar therapeutic effects to lesion therapy because both DBS and lesions interrupt abnormal information flow. Another paradox is that GPi-DBS has therapeutic effects to both Parkinson’s disease and dystonia. Parkinsonian symptoms are induced by increased firing rates (“firing rate model”), abnormal firing patterns (“firing pattern model”) or reduced movement-related inhibition (“dynamic activity model”) in the GPi. In the case of dystonia, signals through the hyperdirect, direct, and indirect pathways may induce a sequence of bursts and pauses in the GPi, and subsequent inhibition and rebound bursts in the thalamus and cortex, leading to the manifestation of involuntary movements (“dynamic activity model”). GPi-DBS blocks abnormal information flow responsible for motor symptoms in both diseases. Other research groups have also proposed similar ideas of functional disconnection of the stimulated elements (Anderson and others 2006; Deniau and others 2010; Moran and others 2011).
Concluding Remarks
Deep brain stimulation has a variety of effects on neurons in the stimulated nucleus of the cortico-basal ganglia loop through orthodromic activation of efferent axons, antidromic and orthodrimic activation of afferent axons. The total effects may vary depending on the composition of neuronal elements in the stimulated nucleus. Here, we have suggested a common key mechanism of DBS: DBS dissociates input and output signals in the stimulated nucleus and disrupts abnormal information flow through the cortico-basal ganglia loop in the pathological conditions (“disruption hypothesis”) (Fig. 4). Understanding the exact mechanism of DBS will lead us to better therapeutic options, toward improvements and upgrading of DBS.
Acknowledgments
The authors thank Shigeki Sato, Kanako Awamura, and Hitomi Isogai for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research authorship, and/or publication of this article: This work was supported by CREST and Strategic Japanese-German Cooperative Programme from Japan Science and Technology Agency, and a Grant-in-Aid for Scientific Research (A) (26250009) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
References
- Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–75. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Postupna N, Ruffo M. 2003. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89:1150–60. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Hu B, Iremonger K, Kiss ZH. 2006. Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest. J Neurosci 26:841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz TZ, Peggs D, Sambrook MA, Crossman AR. 1991. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord 6:288–92. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. 2004. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci 24:7410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MS, Vitek JL, Bakay RA, Green J, McDonald WM, Cole SA, and others. 2000. Treatment of advanced Parkinson’s disease by unilateral posterior GPi pallidotomy: 4-year results of a pilot study. Mov Disord 15:230–7. [DOI] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. 2002. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci 22:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, and others. 1996. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 84:203–14. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, and others. 1991. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337:403–6. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, and others. 1994. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg 62:76–84. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. 1993. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci 5:382–9. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, and others. 1998. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci 21:32–8. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. 1990. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249:1436–8. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. 1994. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72:507–20. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. 2001. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85:1351–6. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. 1996. High frequency stimulation of the internal globus pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215:17–20. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross CE. 2000. Ratio of inhibited-to-activated pallidal neurons decreases dramatically during passive limb movement in the MPTP-treated monkey. J Neurophysiol 83:1760–3. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. 1998. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res 787:157–60. [DOI] [PubMed] [Google Scholar]
- Brown P. 2003. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18:357–63. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. 2001. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 21:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Williams D. 2005. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 116:2510–9. [DOI] [PubMed] [Google Scholar]
- Chiken S, Nambu A. 2013. High-frequency pallidal stimulation disrupts information flow through the pallidum by GABAergic inhibition. J Neurosci 33:2268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiken S, Shashidharan P, Nambu A. 2008. Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J Neurosci 28:13967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep-Brain Stimulation for Parkinson’s Disease Study Group. 2001. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med 345:956–63. [DOI] [PubMed] [Google Scholar]
- Degos B, Deniau JM, Chavez M, Maurice N. 2013. Subthalamic nucleus high-frequency stimulation restores altered electrophysiological properties of cortical neurons in parkinsonian rat. PloS One 8:e83608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos B, Deniau JM, Thierry AM, Glowinski J, Pezard L, Maurice N. 2005. Neuroleptic-induced catalepsy: electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J Neurosci 25:7687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–5. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Degos B, Bosch C, Maurice N. 2010. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci 32:1080–91. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. 2003. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39:109–20. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. 2000. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84:570–4. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Lozano AM. 2002. Mechanisms of deep brain stimulation. Mov Disord 17(suppl 3):S63–8. [DOI] [PubMed] [Google Scholar]
- Erez Y, Czitron H, McCairn K, Belelovsky K, Bar-Gad I. 2009. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 29:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. 2004. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156:274–81. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. 1991. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547:142–51. [PubMed] [Google Scholar]
- Galati S, Mazzone P, Fedele E, Pisani A, Peppe A, Pierantozzi M, and others. 2006. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson’s disease. Eur J Neurosci 23:2923–8. [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. 2006. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 21:1566–77. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, and others. 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–32. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. 2009. Optical deconstruction of parkinsonian neural circuitry. Science 324:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. 2007. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30:357–64. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. 2003. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23:1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H. 2002. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J Neurosci 22:7850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. 2006. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of Parkinsonism. J Neurosci 26:8101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. 2008. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 100:2549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kita T. 2011. Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J Neurosci 31:10311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. 2007. Translational principles of deep brain stimulation. Nat Rev Neurosci 8:623–35. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, Kim E, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. 2010. High-frequency microstimulation in human globus pallidus and substantia nigra. Exp Brain Res 205:251–61. [DOI] [PubMed] [Google Scholar]
- Laitinen LV, Bergenheim AT, Hariz MI. 1992. Ventroposterolateral pallidotomy can abolish all parkinsonian symptoms. Stereotact Funct Neurosurg 58:14–21. [DOI] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bezard E, Bioulac B, Gross CE, Boraud T. 2006. Temporal and spatial alterations in GPi neuronal encoding might contribute to slow down movement in Parkinsonian monkeys. Eur J Neurosci 24:1201–8. [DOI] [PubMed] [Google Scholar]
- Leblois A, Reese R, Labarre D, Hamann M, Richter A, Boraud T, and others. 2010. Deep brain stimulation changes basal ganglia output nuclei firing pattern in the dystonic hamster. Neurobiol Dis 38:288–98. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chang SY, Roberts DW, Kim U. 2004. Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg 101:511–7. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. 2000. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20:7766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, Saint-Cyr J, and others. 2001. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain 124:2105–18. [DOI] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan Danny CW, Qian Z-M, Yung Ken KL, Ko H, and others. 2012. Therapeutic deep brain stimulation in parkinsonian rats directly influences motor cortex. Neuron 76:1030–41. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. 2007. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98:3525–37. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, and others. 1995. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345:91–5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Postupna N, Falkenberg J, Anderson ME. 2008. High frequency deep brain stimulation: what are the therapeutic mechanisms? Neurosci Biobehav Rev 32:343–51. [DOI] [PubMed] [Google Scholar]
- Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, and others. 2002. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science 298:425–9. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. 2006. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 26:3875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Thierry AM, Glowinski J, Deniau JM. 2003. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci 23:9929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. 2009. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol 101:1941–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Walter BL, Vitek JL. (2004). How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol 21:40–50. [DOI] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, and others. 2005. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128:2372–82. [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. (1987) Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MB, Jayaraman A. editors. The basal ganglia II: structure and functions—current concepts. New York: Plenum; p 415–27. [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, and others. 2006. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 96:1569–80. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr. 2006. Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 117:2691–702. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Belelovsky K, Bar-Gad I. 2011. Dynamic stereotypic responses of basal ganglia neurons to subthalamic nucleus high-frequency stimulation in the parkinsonian primate. Front Syst Neurosci 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. 2008. Seven problems on the basal ganglia. Curr Opin Neurobiol 18:595–604. [DOI] [PubMed] [Google Scholar]
- Nambu A, Chiken S, Shashidharan P, Nishibayashi H, Ogura M, Kakishita K, and others. 2011. Reduced pallidal output causes dystonia. Front Syst Neurosci 5:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tachibana T, Chiken S. 2015. Cause of parkinsonian symptoms: firing rate, firing pattern or dynamic activity changes? Basal Ganglia 5:1–6. [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, and others. 2000. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol 84:289–300. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. 2002. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43:111–7. [DOI] [PubMed] [Google Scholar]
- Nishibayashi H, Ogura M, Kakishita K, Tanaka S, Tachibana Y, Nambu A, and others. 2011. Cortically evoked responses of human pallidal neurons recorded during stereotactic neurosurgery. Mov Disord 26:469–76. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Starr PA. 2008. Treatment of dystonia with deep brain stimulation. Neurotherapeutics 5:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. 2006. Deep brain stimulation. Annu Rev Neurosci 29:229–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E, Debatisse D, Maeder M, Vingerhoets F, Ghika J, Villemure JG. 2003. Effect of deep brain stimulation of GPI on neuronal activity of the thalamic nucleus ventralis oralis in a dystonic patient. Neurophysiol Clin 33:169–73. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. 2000. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci 20:8559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R, Leblois A, Steigerwald F, Potter-Nerger M, Herzog J, Mehdorn HM, and others. 2011. Subthalamic deep brain stimulation increases pallidal firing rate and regularity. Exp Neurol 229:517–21. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. 2006. Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test. Synapse 59:445–57. [DOI] [PubMed] [Google Scholar]
- Shin DS, Samoilova M, Cotic M, Zhang L, Brotchie JM, Carlen PL. 2007. High frequency stimulation or elevated K+ depresses neuronal activity in the rat entopeduncular nucleus. Neuroscience 149:68–86. [DOI] [PubMed] [Google Scholar]
- Shink E, Smith Y. 1995. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol 358:119–41. [DOI] [PubMed] [Google Scholar]
- Smith Y, Wichmann T, DeLong MR. 1994. Synaptic innervation of neurones in the internal pallidal segment by the subthalamic nucleus and the external pallidum in monkeys. J Comp Neurol 343:297–318. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. 2004. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci 24:6417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr., Ostrem JL, Simmons D, and others. 2005. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol 93:3165–76. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Iwamuro H, Kita H, Takada M, Nambu A. 2011. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci 34:1470–84. [DOI] [PubMed] [Google Scholar]
- Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. 2003. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J 17:1820–30. [DOI] [PubMed] [Google Scholar]
- Tang JK, Moro E, Mahant N, Hutchison WD, Lang AE, Lozano AM, and others. 2007. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. J Neurophysiol 98:720–9. [DOI] [PubMed] [Google Scholar]
- Vitek JL. 2008. Deep brain stimulation: how does it work? Cleve Clin J Med 75(suppl 2):S59–65. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, and others. 1999. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol 46:22–35. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. 2012. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp Neurol 233:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Houeto JL, Bonnet AM, Bejjani PB, Mesnage V, Dormont D, and others. 2004. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol 61:89–96. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. 2006. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52:197–204. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, Soares J. 2002. Slow oscillatory discharge in the primate basal ganglia. J Neurophysiol 87:1145–8. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. 2006. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol 95:2120–33. [DOI] [PubMed] [Google Scholar]
- Wu YR, Levy R, Ashby P, Tasker RR, Dostrovsky JO. 2001. Does stimulation of the GPi control dyskinesia by activating inhibitory axons? Mov Disord 16:208–16. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Li Y, Hallett M. 2004. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol 115:2542–57. [DOI] [PubMed] [Google Scholar]