Abstract
T cells genetically targeted with a chimeric antigen receptor (CAR) to B-cell malignancies have demonstrated tremendous clinical outcomes. With the proof in principle for CAR T cells as a therapy for B-cell malignancies being established, current and future research is being focused on adapting CAR technology to other cancers, as well as enhancing its efficacy and/or safety. The modular nature of the CAR, extracellular antigen-binding domain fused to a transmembrane domain and intracellular T-cell signaling domains, allows for optimization by replacement of the various components. These modifications are creating a whole new class of therapeutic CARs. In this review, we discuss the recent major advances in CAR design and how these modifications will impact its clinical application.
T-cell-based immunotherapies have come of age as a feasible, safe, and efficacious approach to treating cancer. At the same time, the use of highly personalized, living therapeutics poses multiple challenges. Moreover, immunotherapies involving the genetic modification of T cells, such as those involving expression of chimeric antigen receptors (CAR) to modify T-cell specificity, require an additional level of optimization. In this article, we summarize our current understanding of the key aspects of CAR-T-cell design. This is not meant to be an extensive compilation of the full body of knowledge in the field, but rather an overview of the most relevant finding that have driven the field to its current stage, and those that will likely define its forthcoming directions.
Antigen-specificity for a T cell is encoded by the T-cell receptor (TCR).1 T cells recognize and eradicate infected cells by TCR-mediated detection of microbial antigens, in the form of short amino acids presented by major histocompatibility complex proteins. TCR binding of a specific major histocompatibility complex and peptide combination initiates an intracellular signaling cascade that begins with phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) domains within TCR accessory proteins CD3ζ, CD3γ, CD3δ, and CD3ϵ.2,3 This signaling cascade terminates in T-cell activation and killing of the target cell. T cells can also target cancerous cells by detection of tumor antigens, which can be novel, or normally expressed only in germ cells, or mutated self-antigens (neo-epitopes).4 Investigators have confirmed the power of tumor-reactive T cells by isolating tumor-infiltrating lymphocytes (TILs) from patients with metastatic cancer, expanding them ex vivo, and infusing the TILs back into patients.4,5 Some of these patients have achieved durable complete remission (CRs), which is unheard of with standard cytotoxic chemotherapies.6 However, a major impediment to the adaption of TIL therapy to many cancer patients is the laborious and time-consuming (>2–3 months) process required to achieve a sufficient number of tumor-reactive T cells.7 CARs provided the requisite technological advance and allowed the creation of a large, bulk population of tumor-reactive T cells within as short as 1 week and mediated positive clinical outcomes in many patients with acute or chronic leukemia.8,9
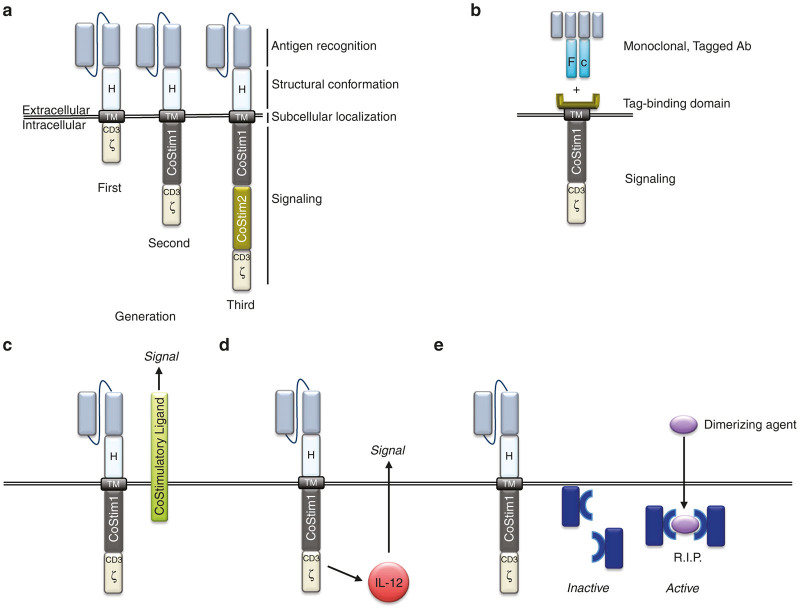
The CAR is a hybrid antigen receptor, part antibody and part TCR, and is composed of an extracellular antigen-binding domain and intracellular signaling domain(s) (Figure 1a).10 Genetic retargeting of a T cell with a CAR endows a new antigen-specificity through the single-chain variable fragment (scFv), which is derived from a tumor-specific antibody.1 The scFv allows the T cell to bind a tumor antigen and T-cell activation is initiated through the intracellular domains, which are derived from CD3ζ ITAM domains.1,3 To complete the genetic construct for the CAR, a hinge and a transmembrane domain (TM), commonly from CD8α or immunoglobulin, bridges the extracellular scFv and intracellular CD3ζ ITAM domains. Early in vitro studies demonstrated that T cells gene-targeted with CARs that have an intracellular signaling domain composed of only CD3ζ, supported T-cell activation and target killing, however, these first-generation CAR T cells had very limited persistence and antitumor efficacy in vivo.11 Since one of the main advantages to the CAR technology is its modular nature it allows continual refinement and modification to optimize T-cell function, which is how first-generation CARs were replaced with second-generation CARs.
Figure 1.
(a) Basic chimeric antigen receptor (CAR) design: antigen recognition domain derived from single chain antibody; hinge domain (H) or spacer; transmembrane domain (TM) providing anchorage to plasma membrane; signaling domains responsible of T-cell activation. First-generation CARs contain a CD3ζ-derived signaling module. Second-generation CARs contain also a costimulatory domain. Third-generation CARs contain two costimulatory domains. (b) Universal CARs: In this design, the intracellular signaling domain is fused to a protein domain that binds a tag (fluorescein isothiocyanate or biotin) on a monoclonal Ab. Therefore, antigen specificity is not linked to the CAR itself, but rather provided by the monoclonal antibodies used in conjunction with CAR-T cells. Antibodies bind to tag binding domain to form the immune receptor. Thus, a given cellular product can be targeted to multiple antigens by using different monoclonal antibodies. (c) Trans signaling: costimulatory ligands can be expressed in combination with CARs to stimulate other cells present in the immune synapsis. (d) Cytokine genes can be coexpressed under the transcriptional control of inducible promoters. Production of IL-12, for instance, under the control of NFAT-responsive promoters has been shown to impact the tumor microenvironment facilitating the generation of an antitumor response. (e) Other accessory molecules: suicide genes. Safety switches can be included in CAR design to initiate the elimination of CAR-T cells in the event of life threatening toxicity. Inactive, monomeric caspase9 subunits can be expressed in viable CAR-T cells, which in the presence of a systemically administered dimerizing agent, will form a lethal dimer causing the rapid clearance of CAR-T cells.
The specificity of a TCR is for only a short peptide (8–12 amino acids) from a foreign (or nonself) antigen; so, there is potential for cross-reactivity to similar sequences of amino acids.12 TCR ligation of host antigen could lead to T-cell activation, autoimmunity, and even death. To minimize this potential, T cells require at least two signals to fully activate.13 The first signal is through the TCR, but the second signal, or costimulation, is mediated through ligation of CD28 by CD80 or CD86, which are normally expressed on antigen-presenting cells (APC).12,13 Therefore, when a T cell encounters a cross-reactive peptide expressed on a normal (non-APC) cell, it is unable to provide costimulation and T-cell activation is unsuccessful. However, when APCs are activated, as during inflammation or infection, they upregulate CD80 and CD86 and can induce both signals 1 and 2, thereby supporting full T-cell activation, target killing, and long-term persistence.12,13 CAR investigators therefore incorporated the two-signal model of T-cell activation by modifying CARs to include a CD28 costimulatory domain in tandem with CD3ζ ITAM domains (Figure 1a).14–16 These second-generation CARs support in vitro T-cell activation and killing, but more importantly also support efficacious in vivo tumor killing and T-cell persistence. It has also been demonstrated that costimulatory domains other than CD28, such as CD27, 4-1BB, and OX40, provide similar in vivo enhancements to CAR T-cell function and persistence.17–19
Second-generation CAR T cells have been confirmed to mediate potent antileukemia responses in phase 1 clinical trials. CR rates up to 90% have been obtained when patients with relapsed and/or refractory B-cell acute lymphoblastic leukemia (B-ALL) were infused with second-generation CD19-targeted T cells that included a CAR with a 4-1BB or CD28 costimulatory domain.20–22 While great success has been noted with targeting CD19, there are significant safety and efficacy concerns with adapting this technology to other cancers. Anticipating these concerns, researchers have again looked to the modular nature of the CAR to further refine and optimize this novel antigen-receptor. For the remainder of this review, we will discuss how modifications to the scFv, hinge/spacer, and intracellular domains may render this engineered cell therapy safer and/or more efficacious.
The Single Chain Variable Fragment
The scFv retargets a bulk population of autologous T cells with a new tumor-reactivity. However, recent studies have demonstrated that the scFv can impact CAR function beyond just tumor-specificity. In fact, investigators have demonstrated aspects of scFv design can modulate the safety and/or efficacy of CAR T cells. For example, the nonhuman origin of the antibodies used to create the scFv has resulted in anti-CAR immune responses, which may limit the persistence of the adoptively transferred CAR T cells.23 Furthermore, this anti-CAR immune response may have more profound implications considering that investigators postulate it resulted in fatal anaphylaxis in patients infused with multiple doses of CAR T cells.24 Therefore, while current lead CARs targeting B-cell malignancies include scFv with mouse origins, CARs targeting other cancers in the developmental pipeline will likely be “humanized” to minimize these immune responses.
The affinity of scFv for its target is another CAR feature being modified to optimize gene-targeted T-cell function. CD19 has been an optimal target to establish the proof-of-principle of adoptive CAR T-cell therapy. It has also demonstrated that on-target/off-tumor toxicity is a concern since patients have been induced into long-term periods of B-cell aplasia.21,25,26 No major complications have been described secondary to B-cell aplasia, presumably due to intervention with gamma globulin and/or antibiotics. However, expression of the target on critical tissues, such as ERBB2 on respiratory epithelium, has resulted in death in at least one patient.27 As CARs are developed against solid tumor malignancies the tumor targets will likely be shared on normal epithelial tissue, thereby increasing the possibility of dangerous complications. However, investigators have recently demonstrated that differential levels of antigen expression and scFv affinity can be used to differentiate between tumor targets and normal tissue. Investigators have demonstrated that low-affinity scFv’s support CAR T-cell-mediated killing of tumor cells that express high level of antigen, but does not support killing of normal cells that express low or normal levels of the same antigen.28,29 This discrimination between levels of antigen expression has been demonstrated both in vitro and in vivo and suggests that scFv affinity can be optimized to increase safety when a target is expressed on normal, healthy tissue.28,29
A breakthrough in CAR design occurred when it was determined that inclusion of CD3ζ (signal 1) in tandem with CD28 costimulation (signal 2) on a single CAR genetic construct could replicate normal TCR activation and costimulation.14–16 However, research has demonstrated that requiring combinatorial ligation of separate, distinct tumor antigens by bispecific CARs can increase safety, while also supporting efficacious cancer killing.30–32 The underlying technology to this CAR advance relies on disassociating the activation and costimulatory domains onto separate complementary CARs. Therefore, CAR1 has only the CD3ζ activation domain, while CAR 2 has only costimulatory domains. Ligation of CAR1 by its antigenic target or ligation of CAR2 by its antigenic target is insufficient by itself to mediate full T-cell activation since they deliver only 1 signal. However, when both CAR1 and CAR2 bind their respective antigens, CD3ζ (signal 1) and costimulation (signal 2) occur in tandem and support T-cell activation and long-term in vivo function. In addition to this novel CAR design, other groups have created similar dual CAR systems and demonstrated the potential for enhancing safety and/or efficacy of tumor targeting.30–32 For example, a second CAR that is specific for a normal tissue antigen can be conjugated to a suppression domain to prevent killing of normal tissue.33 Investigators propose to use this inhibitory bispecific CAR design to prevent normal tissue destruction.33,34 The demonstration that combinatorial antigen ligation and/or signal domain dissociation can be used to regulate T-cell activation and function suggest a robust platform for future CAR design manipulations to further enhance safety and/or function.
While the scFv can be optimized to increase safety, others have replaced the scFv to create a ligand-based CAR or universal CAR. The ligand-based CAR replaces the scFv with a ligand for a tumor marker, for example, a CAR that expresses a ligand for the IL13 receptor (IL13R) allows redirection of T cells to IL13R expressed on glioblastoma.35 While ligands sometimes may have multiple binding partners, this IL13-zetakine is mutated to be highly-specific for the IL13R for glioblastoma. Universal CAR systems have also been developed to broaden the clinical applicability of CAR T-cell therapy (Figure 1b). These universal CARs retarget patients’ T cells and mediate an antitumor response, regardless of cancer diagnosis. This technology relies on replacing the scFv with a binding domain that is specific for a tagged protein or molecule, such as biotin or fluorescein isothiocyanate.36,37 The extracellular portion of the universal CAR is juxtaposed to a transmembrane domain, which is followed by intracellular sequences comprised of T-cell costimulatory and activation domains (Figure 1b). Therefore, after a tumor-specific antibody, labeled with fluorescein isothiocyanate or biotin, binds its target on a tumor cell it can then be bound by the universal CAR, which will support ligation, activation of the T cell, and killing of the tumor cell.36,37 Research has validated the function of both universal CAR T-cell systems in animal models.36,37 While the universal CAR T-cell technology has not been evaluated in patients as of yet, it has the major advantage that it can be applied to the many tumor-specific antibodies that have already been approved for clinical use or are currently in development.
Hinge and Spacer Domains
The hinge, spacer, and transmembrane domains connect the scFv to the activation domains and anchor the CAR in the T-cell membrane.1 Recent studies have demonstrated that these domains can have significant impact on CAR T-cell function and can be optimized to enhance antigen binding and T-cell signaling. This was revealed by demonstrating spacers are required to bind membrane-proximal epitopes and support efficacious target killing.38–40 In contrast, spacers reduce function when the epitope is expressed near the amino terminal portion of the cell surface protein.40 However, spacer length is not the only important consideration. Investigators have also determined that inclusion of Fc domains can support binding of antibodies that activate immune cells.41–44 Due to the abundance of antibodies present in vivo, these CARs can mediate such strong and persistent T-cell activation that it leads to activation-induced T-cell death or activate other immune cells that limit CAR T-cell persistence. Removal of these Fc binding sites enhances in vivo CAR T-cell function and persistence.41–44 These reports suggest that CAR hinge and spacer length and sequence should be optimized based on epitope position. It is important to note that most of this research is based on using an Immunoglobulin-derived hinge and spacer and they have not been evaluated for CD8-derived spacers and hinges.
Intracellular Domains
All the CARs currently tested in the clinic contain either CD28, 4-1BB (CD137) or a combination thereof. However, other alternatives have been explored preclinically. A clear clinical comparison of first- versus second-generation CARs was performed by Savoldo and collaborators. In this work, they administered, to the same patient, T cells expressing a first-generation CAR, along with T cells expressing a second-generation CAR (containing a CD28 costimulation domain). Both CARs had the exact same antigen domain, and their persistence postadministration was analyzed by polymerase chain reaction, showing that the CD28-containg CAR-T cells outperformed the first-generation counterparts. This study provided the ultimate confirmation that CD28 costimulation enhances survival of CAR-T cells in patients.45
While CD28 costimulation is responsible for clonal expansion of activated T cells and secretion of IL-2 in early phases of activation, another costimulatory receptor, 4-1BB (CD137), is associated with long-term survival of T cells. Costimulatory domains derived from 4-1BB have been used in second-46 and third47-generation CARs. Incorporation of this 4-1BB costimulation has been shown to prevent exhaustion due to tonic CD3-signaling present in CD28 CARs.48 Beyond CD28 and 4-1BB, a CD4-associated costimulatory receptor, OX40 (CD134) has been tested preclinically as part of CAR signaling. A third-generation CAR containing CD28 and OX40 costimulation induced superior survival of CCR7(-) T cells than a CD28-only counterpart.49 In addition, a CD28-OX40 CAR induced less secretion of IL-10 than a CD28-based second-generation CAR, without altering the secretion of IFN-γ.50
ICOS, a costimulatory receptor involved in TH17 polarization was also integrated in the intracellular domain of a CAR. Its presence was associated with enhanced in vivo persistence of TH17-polarized CAR-T cells, compared to CD28 or 4-1BB costimulation.51 In turn, CD27 costimulation was shown to provide better survival than CD28.18 In elegant work by Duong and colleagues, a combinatorial approach was used for random generation and selection of intracellular signaling domains. They found that, within a library of CAR constructs containing variable numbers for costimulatory domains, the CAR displaying the greatest antitumor effect was a third-generation design containing DAP10 and CD27 in addition to CD3ζ.52
More recently, a CAR containing a DAP12-derived signaling domain was developed for adoptive transfer of NK cells, showing a more robust performance than CD3ζ-based CARs.53 In T cells, a “KIR-CAR” consisting of a scFv fused to the transmembrane domain of KIR2DS2, along with DAP12 as a separate molecule, displayed more robust antitumor effect in vivo than a second generation, CD3ζ-based CAR. This novel design displayed also superior surface expression than its CD3ζ-counterpart, probably related to different membrane dynamics, which may impact the function of CAR-T cells.54
A thorough understanding of the molecular pathways triggered by CARs, in presence and absence of antigen ligation, will be required to rationally design the most efficient receptors. This will be achieved by the implementation of systems biology approaches to preclinical experimentation, and by integration of the molecular, biological and pharmacodynamics data obtained from the analysis of gene-modified T cells administered in the clinic.55
Joint Expression of CAR and Accessory Genes
CARS have also been combined with accessory proteins to improve the function of T cells, and/or to use lymphocytes as a carrier to deliver a payload that will alter the tumor microenvironment. T cells modified to express a tumor-specific CAR in tandem with a chemokine receptor that binds chemokines present in the tumor microenvironment enhances tumor targeting, while also modulating CAR T-cell homing and trafficking.56 This dual-protein system allows dual targeting as well as functional modification. In another direction with accessory proteins, Zhao et al. demonstrated that a second-generation CAR containing a CD28 costimulatory domain with CD3 ζ, expressed together with 4-1BBLigand in trans (CD28z/41BBL), induced a more potent antitumor effect than a third-generation CAR containing both CD28 and 4-1BB in cis (Figure 1c). In addition, T cells expressing a CAR with the CD28z/41BBL domains, expressed less markers of exhaustion and persisted longer in vivo in a mouse model of B-cell leukemia.57 On the other hand, Condomines et al. reported that the second-generation 28Z design was more efficacious than a first-generation CAR complemented in trans by CD80 costimulation. This observation was associated with a decreased sensitivity to CTLA4-mediated inhibition in T cells expressing the second-generation CAR,58 and suggests that different costimulatory ligands may lead to different outcomes. Finally, CAR-T cells coexpressing CD40L also outperformed second-generation CAR-T cells. This particular design, applied to the treatment of B-cell malignancies, enhanced CAR-T cells by multiple mechanisms including the stimulation of IL-12 secretion by dendritic cells and the induction of costimulatory molecules by CD40-expressing target cells.59
Cytokines have also been explored as combination partners that may enhance the activity and/or persistence of CAR-T cells, resulting in enhanced anti-tumor effects. Hoyos et al. tested the combination of a second-generation CAR targeting CD19, along with interleukin-15 (IL-15) and an inducible suicide gene (iCasp9). The CAR/cytokine/suicide gene trifecta enhanced proliferation, reduced PD-1 expression and provided superior anticancer killing in a humanized mouse model of B-cell lymphoma.60 In another study, Markley and Sadelain showed that anti-CD19 CAR-T cells that constitutively expressed IL-2, IL-7, IL-15, or IL-21 displayed greater antilymphoma activity in vivo than CAR-T cells with no cytokine; IL-7 and IL-21 being the most efficacious.61
Interleukin-12 (IL-12) is a pleiotropic cytokine that may induce potent antitumor responses by stimulating the immune system at multiple levels. Systemic administration of IL-12, however, causes severe toxicity, precluding its clinical application.62 To circumvent this limitation, systemically administered CAR-T cells can be used as “Trojan horses” to deliver IL-12 to the desired tissues (Figure 1d). Using this approach, Pegram and collaborators63 showed that expression of IL-12 by CAR-T cells eliminated the need for lymphodepleting preconditioning, and rendered CAR-T cells resistant to inhibition by regulatory T cells, in a syngeneic mouse model of CD19+ malignancies. Furthermore, their results showed that the mechanism of action of IL-12 was dependent on the presence of both CD8+ and CD4+ T-cell subsets, and of functional IFN-γ secretion.63 IL-12 secretion can be further restricted to sites of T-cell activation by coupling its expression to signaling pathways activated by engagement of CAR. For instance, Zhang and collaborators generated γ-retroviral vectors where the expression of IL-12 by transduced T cells is controlled by a nuclear factor of activated T cells (NFAT)-responsive promoter.64 Using this type of transcriptional control, Chmielewski et al. showed that IL-12 secretion by anti-carcinoembryonic antigen CAR-T cells resulted in elimination of carcinoembryonic antigen-expressing tumor cells, but also of carcinoembryonic antigen-negative cells. This indirect effect was mediated by the activation of a potent macrophage-driven, TNF-α-dependent, cytotoxic effect.65 Chinnasamy et al. similarly demonstrated that T cells targeted to the tumor vasculature, which also expressed NFAT-driven IL-12, modified the tumor microenvironment by eliminating myeloid-derived suppressor cells.66
To this date, the expression of IL-12 by CAR-T cells has been extensively explored in preclinical models, allowing for a better understanding of the biology behind IL-12-driven immunotherapy. However, the clinical efficacy of CAR-T cells armed with IL-12 remains to be demonstrated. A clinical trial of adoptive transfer of TIL expressing NFAT-driven IL-12, in melanoma patients, showed the induction of objective responses in patients treated with T-cell infusions that contained a remarkably lower number of cells than conventionally used in TIL trials. In addition, treatments were administered in absence of preparative chemotherapy and, most importantly, a patient who had failed TIL treatment experienced an objective response upon treatment with TIL+NFAT-IL-12 (ref. 67). These findings suggest that IL-12 expression by adoptively transferred T cells may result in clinical benefit, but the ultimate answer will be provided by randomized clinical trials testing combination treatments versus CAR-T cells alone.
Accessory molecules can also be included in CAR-T cells to serve as safety switches, which allow for the elimination of CAR-T cells from circulation, in the event of T-cell-mediated toxicity. This approach was originally tested in the setting of hematopoietic stem cell transplants, in order to mitigate the symptoms of graft-versus-host disease. Early designs relied on the expression of a protein that metabolized an inactive prodrug, into a cytotoxic metabolite, only in gene-modified cells (Figure 1e). A different approach involves the coexpression of a membrane-bound protein (usually, a truncated receptor lacking the intracellular signaling domain) that can be efficiently targeted by an antibody causing the depletion of cells that express such protein. For instance, Wang et al. 68 described the use of a truncated form of the human epidermal growth factor receptor (huEGFRt) in adoptively transferred cells, which can later be ablated by systemic treatment with a clinical grade antibody against epidermal growth factor receptor. A thorough review of the different suicide genes developed to date has recently been published,69 and therefore we will focus on a more recent technology that is currently being evaluated in a clinical setting: the inducible caspase-9 (iCasp9). This system involves the expression of a synthetic construct consisting of an incomplete proapoptotic caspase-9 (lacking its caspase recruitment domain) fused to a mutated peptide derived from the FKBP12 protein. Interaction of the mutated FKBP12 domain with a small molecule induces dimerization of the fusion protein, resulting in activation of caspase-9-induced apoptosis (Figure 1e).70 Clinical testing proved the efficacy and safety of the iCasp9 system in the context of allogeneic stem-cell transplant, where the suicide gene was expressed in donor T cells adoptively transferred to the patients. Those patients who developed graft-versus-host disease after transfer were treated with an intravenous administration of a clinical grade CID (AP1903), and subsequently experience a rapid and nearly complete elimination of circulating donor T cells.71 Synthetic control switches such as the iCasp9 system will likely be used extensively in clinical trials of adoptive immunotherapy, either for the removal of toxic CAR-T cells, or for a more sophisticated fine tuning of CAR-T-cell activity, as recently described by Wu and collaborators.72 In this elegant work, the authors generated a dissociated CAR that expressed the antigen binding and transmembrane domains in one protein, and the intracellular signaling domains in a separate protein. Both subunits contain a heterodimerization module that, in presence of a small molecule, assembled the CAR into a fully functional unit. Thus, CAR-T-cell activation depends on two input signals: (i) antigen binding, and (ii) presence of dimerizing agent. This design allows for real-time and dose-dependent control of CAR-T-cell activity.
Future Directions
Roughly two decades after its inception, the use of synthetic immune receptors expressed in T cells via genetic manipulation has proven efficacious in treating oncologic patients. Despite reaching this important milestone, the field is still in its infancy and we anticipate that the next few years will see a large increase in the number of scientific papers reporting clinical successes with CAR-T cells. In retrospect, preclinical testing appears as a key component of the clinical success achieved in recent years, providing valuable information such as the need for integrated costimulation for optimal performance of CAR-T cells. Preclinical testing will likely again play a key role in the advancement of this field, now complementing the information collected from clinical samples of patients treated with CAR-T cells. Efforts will be focused on the translation of the clinical success of CD19-targeted therapies to other antigens and, most importantly, to solid tumors. This challenge will require a multidisciplinary approach involving clinicians, clinical laboratories, basic and translational researchers. Capitalizing on the legacy of the genomic era, the functional and structural design of CARs is likely to evolve from mere T-cell activation switches, in response to antigen binding, to more sophisticated pieces of biological engineering. Gene modified T cells will likely become therapeutic agents with complex sensing and effector functions, integrating information from the tumor cells and the tumor microenvironment for increased potency and specificity. The combination of CAR-T cells with other treatment modalities such as oncolytic virotherapy remains largely unexplored, and preclinical modeling will aid in the identification of the best combination strategies to achieve synergism among multiple modalities. At the cellular level, a detailed analysis of the molecular events that govern CAR-T-cell function and persistence will provide the building blocks for the development of the next generation of T-cell therapies.
Marco L Davila receives research funding from the H. Lee Moffitt Cancer Center and Research Institute, American Society of Hematology, and Damon Runyon Cancer Research Foundation. He receives compensation from Celyad and Geneius Biotechnology as a consultant. He receives compensation from Precision Biosciences and Adaptive Biotechnologies as an Advisory Board Member. Daniel Abate-Daga receives research funding from the H. Lee Moffitt Cancer Center and Research Institute, Moffitt’s Lung Cancer Center of Excellence, and a Career Enhancement Award from NIH SPORE grant P50 CA168536.
References
- Sadelain, M, Rivière, I and Brentjens, R (2003). Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 3: 35–45. [DOI] [PubMed] [Google Scholar]
- Abraham, RT and Weiss, A (2004). Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol 4: 301–308. [DOI] [PubMed] [Google Scholar]
- Irving, BA and Weiss, A (1991). The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64: 891–901. [DOI] [PubMed] [Google Scholar]
- Restifo, NP, Dudley, ME and Rosenberg, SA (2012). Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanović, S, Draper, LM, Langhan, MM, Campbell, TE, Kwong, ML, Wunderlich, JR et al. (2015). Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 33: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, ME, Yang, JC, Sherry, R, Hughes, MS, Royal, R, Kammula, U et al. (2008). Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26: 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper, JA, Thomasian, AA, Smith, DM, Gorgas, GC, Wunderlich, JR, Smith, FO et al. (2009). Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J Immunol Methods 345: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyman, D, Stefanski, J, Przybylowski, M, Bartido, S, Borquez-Ojeda, O, Taylor, C et al. (2009). Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 32: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, JN, Feldman, SA, Zhao, Y, Xu, H, Black, MA, Morgan, RA et al. (2009). Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 32: 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, ML, Sauter, C and Brentjens, R (2015). CD19-targeted T cells for hematologic malignancies: clinical experience to date. Cancer J 21: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker, T and Karjalainen, K (1995). Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med 181: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossjohn, J, Gras, S, Miles, JJ, Turner, SJ, Godfrey, DI and McCluskey, J (2015). T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol 33: 169–200. [DOI] [PubMed] [Google Scholar]
- Sharpe, AH and Freeman, GJ (2002). The B7-CD28 superfamily. Nat Rev Immunol 2: 116–126. [DOI] [PubMed] [Google Scholar]
- Maher, J, Brentjens, RJ, Gunset, G, Rivière, I and Sadelain, M (2002). Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 20: 70–75. [DOI] [PubMed] [Google Scholar]
- Hombach, A, Wieczarkowiecz, A, Marquardt, T, Heuser, C, Usai, L, Pohl, C et al. (2001). Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol 167: 6123–6131. [DOI] [PubMed] [Google Scholar]
- Finney, HM, Lawson, AD, Bebbington, CR and Weir, AN (1998). Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol 161: 2791–2797. [PubMed] [Google Scholar]
- Imai, C, Mihara, K, Andreansky, M, Nicholson, IC, Pui, CH, Geiger, TL et al. (2004). Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18: 676–684. [DOI] [PubMed] [Google Scholar]
- Song, DG, Ye, Q, Poussin, M, Harms, GM, Figini, M and Powell, DJ Jr (2012). CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119: 696–706. [DOI] [PubMed] [Google Scholar]
- Hombach, AA and Abken, H (2013). Of chimeric antigen receptors and antibodies: OX40 and 41BB costimulation sharpen up T cell-based immunotherapy of cancer. Immunotherapy 5: 677–681. [DOI] [PubMed] [Google Scholar]
- Davila, ML, Riviere, I, Wang, X, Bartido, S, Park, J, Curran, K et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude, SL, Frey, N, Shaw, PA, Aplenc, R, Barrett, DM, Bunin, NJ et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, DW, Kochenderfer, JN, Stetler-Stevenson, M, Cui, YK, Delbrook, C, Feldman, SA et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, MC, Popplewell, L, Cooper, LJ, DiGiusto, D, Kalos, M, Ostberg, JR et al. (2010). Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 16: 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus, MV, Haas, AR, Beatty, GL, Albelda, SM, Levine, BL, Liu, X et al. (2013). T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 1: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos, M, Levine, BL, Porter, DL, Katz, S, Grupp, SA, Bagg, A et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3: 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, JN, Dudley, ME, Feldman, SA, Wilson, WH, Spaner, DE, Maric, I et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Yang, JC, Kitano, M, Dudley, ME, Laurencot, CM and Rosenberg, SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X, Jiang, S, Fang, C, Yang, S, Olalere, D, Pequignot, EC et al. (2015). Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res 75: 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso, HG, Hurton, LV, Najjar, A, Rushworth, D, Ang, S, Olivares, S et al. (2015). Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res 75: 3505–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss, CC, Condomines, M, Cartellieri, M, Bachmann, M and Sadelain, M (2013). Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 31: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada, Z, Hegde, M, Byrd, T, Shaffer, DR, Ghazi, A, Brawley, VS et al. (2013). TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids 2: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis, E, Poussin, M, Klattenhoff, AW, Song, D, Sandaltzopoulos, R, June, CH et al. (2013). Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 1: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov, VD, Themeli, M and Sadelain, M (2013). PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 5: 215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov, VD, Sadelain, M and Kloss, CC (2014). Novel approaches to enhance the specificity and safety of engineered T cells. Cancer J 20: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, CE, Badie, B, Barish, ME, Weng, L, Ostberg, JR, Chang, WC et al. (2015). Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res 21: 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska, K, Lanitis, E, Poussin, M, Lynn, RC, Gavin, BP, Kelderman, S et al. (2012). A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res 72: 1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada, K, Geng, D, Sakoda, Y, Bansal, N, Srivastava, R, Li, Z et al. (2012). Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 18: 6436–6445. [DOI] [PubMed] [Google Scholar]
- James, SE, Greenberg, PD, Jensen, MC, Lin, Y, Wang, J, Till, BG et al. (2008). Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol 180: 7028–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haso, W, Lee, DW, Shah, NN, Stetler-Stevenson, M, Yuan, CM, Pastan, IH et al. (2013). Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest, RD, Hawkins, RE, Kirillova, N, Cheadle, EJ, Arnold, J, O’Neill, A et al. (2005). The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 28: 203–211. [DOI] [PubMed] [Google Scholar]
- Hudecek, M, Sommermeyer, D, Kosasih, PL, Silva-Benedict, A, Liu, L, Rader, C et al. (2015). The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 3: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach, A, Hombach, AA and Abken, H (2010). Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther 17: 1206–1213. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda, M, Mardiros, A, Urak, R, Wang, X, Hoffman, LJ, Bernanke, A et al. (2015). Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther 23: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almåsbak, H, Walseng, E, Kristian, A, Myhre, MR, Suso, EM, Munthe, LA et al. (2015). Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model. Gene Ther 22: 391–403. [DOI] [PubMed] [Google Scholar]
- Savoldo, B, Ramos, CA, Liu, E, Mims, MP, Keating, MJ, Carrum, G et al. (2011). CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 121: 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, DL, Hwang, WT, Frey, NV, Lacey, SF, Shaw, PA, Loren, AW et al. (2015). Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7: 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammana, S, Huang, X, Wong, M, Milone, MC, Ma, L, Levine, BL et al. (2010). 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther 21: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, AH, Haso, WM, Shern, JF, Wanhainen, KM, Murgai, M, Ingaramo, M et al. (2015). 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach, AA, Chmielewski, M, Rappl, G and Abken, H (2013). Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther 24: 259–269. [DOI] [PubMed] [Google Scholar]
- Hombach, AA, Heiders, J, Foppe, M, Chmielewski, M and Abken, H (2012). OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 1: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedan, S, Chen, X, Madar, A, Carpenito, C, McGettigan, SE, Frigault, MJ et al. (2014). ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong, CP, Westwood, JA, Yong, CS, Murphy, A, Devaud, C, John, LB et al. (2013). Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS One 8: e63037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer, K, Cartellieri, M, Michen, S, Wiedemuth, R, Müller, N, Lindemann, D et al. (2015). DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol 194: 3201–3212. [DOI] [PubMed] [Google Scholar]
- Wang, E, Wang, LC, Tsai, CY, Bhoj, V, Gershenson, Z, Moon, E et al. (2015). Generation of potent T-cell immunotherapy for cancer using DAP12-based, multichain, chimeric immunoreceptors. Cancer Immunol Res 3: 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate-Daga, D, Hanada, K, Davis, JL, Yang, JC, Rosenberg, SA and Morgan, RA (2013). Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood 122: 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi, A, De Angelis, B, Rooney, CM, Zhang, L, Mahendravada, A, Foster, AE et al. (2009). T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113: 6392–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z, Condomines, M, van der Stegen, SJ, Perna, F, Kloss, CC, Gunset, G et al. (2015). Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condomines, M, Arnason, J, Benjamin, R, Gunset, G, Plotkin, J and Sadelain, M (2015). Tumor-targeted human T cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition. PLoS One 10: e0130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran, KJ, Seinstra, BA, Nikhamin, Y, Yeh, R, Usachenko, Y, van Leeuwen, DG et al. (2015). Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther 23: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos, V, Savoldo, B, Quintarelli, C, Mahendravada, A, Zhang, M, Vera, J et al. (2010). Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24: 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley, JC and Sadelain, M (2010). IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood 115: 3508–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Car, BD, Eng, VM, Lipman, JM and Anderson, TD (1999). The toxicology of interleukin-12: a review. Toxicol Pathol 27: 58–63. [DOI] [PubMed] [Google Scholar]
- Pegram, HJ, Lee, JC, Hayman, EG, Imperato, GH, Tedder, TF, Sadelain, M et al. (2012). Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119: 4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L, Feldman, SA, Zheng, Z, Chinnasamy, N, Xu, H, Nahvi, AV et al. (2012). Evaluation of γ-retroviral vectors that mediate the inducible expression of IL-12 for clinical application. J Immunother 35: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski, M, Kopecky, C, Hombach, AA and Abken, H (2011). IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 71: 5697–5706. [DOI] [PubMed] [Google Scholar]
- Chinnasamy, D, Yu, Z, Kerkar, SP, Zhang, L, Morgan, RA, Restifo, NP et al. (2012). Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 18: 1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L, Morgan, RA, Beane, JD, Zheng, Z, Dudley, ME, Kassim, SH et al. (2015). Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 21: 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X, Chang, WC, Wong, CW, Colcher, D, Sherman, M, Ostberg, JR et al. (2011). A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118: 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, BS, Lamb, LS, Goldman, F and Di Stasi, A (2014). Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol 5: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, L, Freeman, KW, Khan, T, Pham, E and Spencer, DM (1999). Improved artificial death switches based on caspases and FADD. Hum Gene Ther 10: 2273–2285. [DOI] [PubMed] [Google Scholar]
- Di Stasi, A, Tey, SK, Dotti, G, Fujita, Y, Kennedy-Nasser, A, Martinez, C et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, CY, Roybal, KT, Puchner, EM, Onuffer, J and Lim, WA (2015). Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350: aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]