Abstract
STUDY QUESTION
What effect does diet-induced obesity have on endometrial stromal cell (ESC) decidualization?
SUMMARY ANSWER
Diet-induced obesity impairs ESC decidualization.
WHAT IS KNOWN ALREADY
Decidualization is important for successful implantation and subsequent health of the pregnancy. Compared with normal-weight women, obese women have lower pregnancy rates (both spontaneous and by assisted reproductive technology), higher rates of early pregnancy loss and poorer oocyte quality.
STUDY DESIGN, SIZE, DURATION
Beginning at 6 weeks of age, female C57Bl/6J mice were fed either a high-fat/high-sugar diet (HF/HS; 58% Fat Energy/Sucrose) or a diet of standard mouse chow (CON; 13% Fat) for 12 weeks. At this point, metabolic parameters were measured. Some of the mice (n = 9 HF/HS and 9 CON) were mated with reproductively competent males, and implantation sites were assessed. Other mice (n = 11 HF/HS and 10 CON) were mated with vasectomized males, and artificial decidualization was induced. For in vitro human studies of primary ESCs, endometrial tissue was obtained via biopsy from normo-ovulatory patients without history of infertility (obese = BMI > 30 kg/m2, n = 11 and lean = BMI < 25 kg/m2, n = 7) and from patients consented for hysterectomies for a benign indication (n = 4). In vitro studies were also performed with immortalized human ESCs. ESCs were decidualized in culture for nine 9 days in the presence or absence of palmitic acid (PA), and the degree of decidualization was assessed by measuring expression of decidualization markers.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The sizes of implantation sites and fetuses were analyzed in mice mated with reproductively competent males. In mice mated with vasectomized males, decidualization was induced, and uterine tissues were analyzed via hematoxylin and eosin staining, quantitative RT–PCR (RT–qPCR), and western blots. Human ESCs were cultured in vitro and induced to decidualize by treatment with cAMP and medroxyprogesterone. The level of expression of decidualization markers was assessed by RT–qPCR (mRNA) and western blotting (protein). ATP content of ESCs was measured, and levels of autophagy were assessed by western blotting of the autophagy regulators acetyl coa carboxylase (ACC) and ULK1 (Ser 317). Autophagic flux was measured by western blot of the marker LC3b-II.
MAIN RESULTS AND THE ROLE OF CHANCE
Mice exposed to an HF/HS diet became obese and metabolically impaired. HF/HS-exposed mice mated to reproductively competent males had smaller implantation sites in early pregnancy (P <0.001) and larger fetuses at term (P <0.05) than CON-exposed mice. In the artificial decidualization experiments, mice exposed to the HF/HS diet developed 50% smaller deciduomas than mice exposed to CON diet (P< 0.001). Human ESCs cultured in the presence of PA had markedly decreased mRNA expression of the decidualization markers, decidual prolactin (PRL) (P< 0.0001) and insulin-like growth factor binding protein 1 (IGFBP1) (P< 0.0001). Expression of PRL and IGFBP1 by mRNA were also significantly lower in early follicular phase ESCs of obese women than in those of normal-weight women (P< 0.05). Protein expression of phosphorylated ACC and phosphorylated ULK1, both activated forms, were lower in deciduomas of HF/HS mice than in those of control mice (P < 0.01). In immortalized human ESCs, LC3b-II levels were higher in decidualized cells than in controls, indicating increased autophagy. PA treatment abrogated this increase.
LIMITATIONS, REASONS FOR CAUTION
Many aspects of obesity and metabolic impairment could contribute to the decidualization defects observed in the HF/HS-exposed mice. Although our findings suggest that both autophagy and decidualization are impaired by exposure to PA, the underlying mechanisms should be elucidated. Finally, our human patient sample size was small.
WIDER IMPLICATIONS OF THE FINDINGS
Although many factors contribute to poor reproductive outcome and early pregnancy loss in obese women, our study suggests the importance of decidualization defects. Such defects may contribute to compromised endometrial receptivity and poor implantation. If defects in autophagy contribute to impaired decidualization, therapeutics could be developed to improve this process and thus improve implantation and pregnancy outcomes in obese women.
STUDY FUNDING/COMPETING INTEREST(S)
Grants include NIH 5T32HD040135-12 (J.S.R.), R01 HD065435 (K.H.M.), NIH T32 HD049305 (J.L.S.) and ACOG Research Grant (M.B.S.). The authors report no conflicts of interest.
Keywords: obesity, decidualization, implantation, reproduction, autophagy
Introduction
According to the Centers for Disease Control and Prevention, in 2009–2010, 35.7% of US adults, and more than 30% of reproductive-age women, were obese (BMI ≥ 30 kg/m2) (Ogden, 2012). Moreover, the World Health Organization reported that, in 2008, 35% of reproductive-age women worldwide were overweight (BMI ≥ 25 kg/m2) and an estimated 297 million women over the age of 20 years were obese (http://www.who.int/gho/en/). Numerous negative effects of obesity on overall health have been well established, such as increased risk of cardiovascular disease, Type 2 diabetes, stroke and all-cause mortality (Flegal et al., 2013). Additionally, recent research is revealing the negative impacts of obesity on reproduction. For example, obesity has been linked to infertility (Talmor and Dunphy, 2015), poor oocyte quality (Luzzo et al., 2012), increased early pregnancy loss (Bellver and Pellicer, 2004, Boots et al., 2014), decreased success of achieving pregnancy even with assisted reproductive technology (ART) (Bellver et al., 2010; Moragianni et al., 2012), decreased ability to carry a healthy pregnancy to term and poor neonatal outcomes (Galliano and Bellver, 2013; Gaudet et al., 2012).
The pathologies underlying decreased pregnancy rates and increased early pregnancy loss in obese women are unclear. Notably, obese recipients of oocytes from normal-weight donors have better pregnancy rates than obese recipients of autologous oocytes, suggesting that poor oocyte quality is partly to blame (Bellver et al., 2013; Jungheim et al., 2013). However, in a paper examining 9587 first cycles of assisted reproduction in which donor oocytes were used, Bellver et al. (2013) showed that implantation, pregnancy and live birth rates were significantly reduced as BMI of the recipients increased. Moreover, this group has shown that the endometrium of obese patients has decreased expression of specific genes important in early pregnancy (Bellver et al., 2011). Additionally, obese women have higher rates of recurrent early pregnancy loss than normal-weight women, despite having euploid embryos (Boots et al., 2014). Together, these findings point to an important role of the endometrial environment in obesity-related reproductive impairments.
One key aspect of early pregnancy is decidualization, a hormonally mediated process by which endometrial stromal cells (ESCs) proliferate and differentiate into decidual cells (Large and DeMayo, 2012). In mice, this process is triggered by the attachment and implantation of the blastocyst, whereas in humans, this differentiation occurs in the luteal phase of each menstrual cycle, independent of embryo implantation (Large and DeMayo, 2012). The decidua has numerous roles, including maternal–fetal exchange of gas and nutrients, immunomodulation, invasion of the trophoblast and placentation. Successful decidualization is essential to establish and support a healthy pregnancy; mice lacking key genes that are important in decidualization have reduced fertility (Benson et al., 1996) and pregnancy complications such as preterm birth (Hirota et al., 2010). Here, to explore the cause of abnormal implantation and early pregnancy loss seen in obesity, we investigated the effects of obesity and exposure to excess fatty acids on decidualization in both mice and human endometrial cells.
Materials and Methods
Animal care and feeding
All mouse studies were approved by the Animal Studies Committee at Washington University School of Medicine, St Louis, MO, USA, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Six-week-old female C57Bl/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed five per cage and given access to water and fed either a high fat/high sucrose (HF/HS; Test Diet 58R3, 58% fat energy) (total n = 20) or control chow (Con; Lab Diet 5053, 13% Fat) (n = 19) for 12 weeks. Both diets were from Lab Diet/Test Diet, St Louis, MO, USA.
Assessment of body composition and metabolic parameters
After the 12-week feeding period, mice were weighed, and body composition analysis was performed using the EchoMRI-100TM machine (Echo Medical Systems, Houston, TX, USA). For metabolic assessments, mice were fasted for 14 h and injected i.p. with 10% glucose at a dose of 1 mg/g of body weight. A Contour TS (Bayer, Parsippany, NJ, USA) glucometer was used to measure glucose at 0, 30, 60, and 120 min by sampling from the tail tip. After mice were fasted for 4 h, serum triglycerides and cholesterol were measured by using the infinity triglyceride and cholesterol reagent kits (Fisher Diagnostics), serum fatty acids were measured with the NEFA-HR (2) assay kit (Wako Diagnostics, Mountain View, CA, USA), and serum insulin was measured with the rat/mouse insulin enzyme-linked immunosorbent assay kit (EMD Millipore, Billerica, MA, USA). All kits were used as per manufacturer’s instructions.
Assessment of decidualization after natural mating
Twenty-five HF/HS- and 25 CON-fed female mice were mated to CON-fed males. The day on which a vaginal plug was detected was defined as day post-coitus (dpc) 0.5. On dpc 5.5, a subset (n = 6 HF/HS and 6 CON) of mice was anesthetized, and 1% Evan's blue dye (Sigma, St Louis, MO, USA) in phosphate-buffered saline (PBS) was injected retro-orbitally and the mice were sacrificed that day and the number of implantation sites was noted. On dpc 8.5, another subset (n = 14 HF/HS and 14 CON) of mice was anesthetized, and 1% Evan's blue dye (Sigma) in PBS was injected retro-orbitally (Yardeni et al., 2011). Mice were then sacrificed, uteri were harvested and each implantation site was measured with calipers and weighed. Another subset of mice (n = 5 HF/HS and 5 CON) was sacrificed on dpc 18.5. Uteri were harvested, and each fetus and placenta was measured with calipers and weighed. In a separate cohort of mice, six HF/HS- and six CON-fed female mice were mated to CON-fed males.
Artificially induced deciduoma
HF/HS- or CON-fed female mice were made pseudo-pregnant by mating with vasectomized males as described previously (Deb et al., 2006). Decidualization was induced on the fourth day of pseudo-pregnancy by placing a silk thread in the right uterine horn. The left uterine horn was used as an internal control. After 72 h, uteri were removed, and each uterine horn was weighed separately.
Immunoblotting of mouse samples
Total cell and tissue lysates were prepared in RIPA buffer [25 mM Tris–HCl, 150 mM NaCl, 1.0% NP-40, 1.0% deoxycholic acid, 0.1% sodium dodecyl sulphate (SDS), 2 mM EDTA] containing 1 mM phenylmethylsulphonyl fluoride and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Proteins were resolved by SDS–polyacrylamide gel electrophoresis and standard immunoblotting procedures were followed. Blots were probed in 5% bovine serum albumin (BSA) in Tris-buffered saline Tween-20 (TBST) for 16 h at 4°C with the following primary antibodies (all purchased from Cell Signaling Technology, Danvers, MA, USA): anti-p-Unc-51-like kinase 1 (ULK1) (1:500), anti-ULK1 (1:500), anti-p-Acetyl-CoA carboxylase (pACC; 1:1500), anti-ACC (1:2000) and anti-GAPDH (1:5000). Blots were then probed with horse-radish peroxidase-conjugated anti-rabbit immunoglobulin G (Jackson ImmunoResearch, West Grove, PA, USA; 1:5000). Quantity One software (Bio-Rad, Hercules, CA, USA) was used for signal quantification.
Isolation of human ESCs and culture conditions
The Human Research Protection Office of Washington University approved protocols, and written informed consent was obtained prior to performing endometrial biopsy. Primary endometrial tissue was obtained from human uteri at the time of hysterectomy being conducted for benign indications (n = 16) and used for in vitro studies with palmitic acid (PA) treatment described below. For all these primary cell cultures, the method of culturing ensures the persistence of endometrial epithelial cells in prolonged culture to be negligible. All patients were between the ages of 25–40 years old without a history of infertility and not on hormonal treatment. Additionally, endometrial pipelle biopsies were performed from normal weight (BMI < 25 kg/m2, n = 7) and obese (BMI > 30 kg/m2, n = 11) normo-ovulatory women in the early follicular phase of the cycle. Inclusion criteria included: regular cycles and proven fertility (birth of one child after spontaneously conceived pregnancy). Exclusion criteria included any endocrine disorders (including diabetes), hormonal treatments for 3 months prior to study and known endometriosis. After biopsy, uterine tissue was transported to the laboratory in normal saline.
Human primary ESCs were isolated as previously described (Frolova et al., 2011; Tsai et al., 2013) with a few modifications. Tissue was minced, digested in Dulbecco's modified Eagle's medium (DMEM):F12 containing collagenase (2 mg/ml; Invitrogen, Carlsbad, CA, USA) and deoxyribonuclease (15 U/ml; Roche, Basel, Switzerland) for 30 min (shaking for 1 min every 10 min) at 37°C, and then passed through a 40 µm sieve. Approximately 2 × 105 cells per well were plated in 6-well cell culture plates. Cells were cultured for 12–14 days (to recover from effects of endogenous hormones) in DMEM:F12 media supplemented with 10% heat-inactivated charcoal dextran-treated fetal bovine serum and 50 µg/ml penicillin/streptomycin. Human telomerase-immortalized ESCs (hESC-Ts), either the generous gift of Dr Krikun from Yale University (Krikun et al., 2006) or purchased from ATCC (Manassas, VA, USA), were cultured in the same medium containing puromycin dihydrochloride (Sigma) to maintain telomerase expression. After reaching 70–80% confluency, cells were decidualized by treatment with 1 µM medroxyprogesterone-17-acetate (MPA; Sigma) and 0.5 mM N6,2′-O-dibutyryladenosine cAMP sodium salt (db-cAMP; Sigma) for 3–9 days, depending on the experiment. In some experiments, 100 µM PA, sonicated in 0.6 mM BSA, was added to the wells on Days 0, 3 and 6, and cells were harvested on Day 9. Control wells received ethanol/BSA. On the day of harvest, trypan blue staining was performed to ensure cell survival rates were similar in all treatment groups.
RNA isolation and real-time RT–PCR
Total RNA was isolated by using the RNeasy mini kit, including on-column DNase digestion (Qiagen, Valencia, CA, USA). The Quantitect Reverse Transcription kit (Qiagen, Hilden, Germany) was used with 1 µg of total RNA in 20 µl reactions. Subsequent real-time PCR (Table I) was performed on the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR green fluorescence and 300 nM gene-specific primers, as described previously (Frolova et al., 2011; Tsai et al., 2013). Values were normalized to 18s rRNA and ribosomal protein 36B4 transcripts in murine and human cells, respectively (Schroder et al., 2009).
Table I.
Primer sequence for decidualization markers.
| Gene symbol | Primer sequence |
|---|---|
| PRL | Forward 5′-AAGCTGTAGAGATTGAGGAGCAAA-3′ Reverse 5′-TCAGGATGAACCTGGCTGACTA-3′ |
| IGFBP1 | Forward 5′-CGAAGACTCTCCATGTCACCA-3′ Reverse 5′-TGTCTCCTGTGCCTTGGCTAAAC-3′ |
| 36B4 | Forward 5′-GACACCCTCCAGGAAGCGA-3′ Reverse 5′-GTGTTCGACAATGGCAGCAT-3′ |
| Prp | Forward 5′-TCCTGGCCAATA ATGCTGCCATTG-3′ Reverse 5′-AGCAGCCATTCTCTCCTGTTTGAC-3′ |
| 18s | Forward 5′-TTCCTTACCTGGTTGATCCTGCCA-3′ Reverse 5′-AGCCATTCGCAGTTTCACTGTACC-3′ |
PRL, prolactin; IGFBP1, insulin-like growth factor binding protein 1; Prb, prolactin-related protein.
Autophagic flux assay
On Day 9 of decidualization, cells were treated for 3 h with 100 nM Bafilomycin A1 (BafA1) or dimethylsulphoxide vehicle. Cells were lysed in RIPA buffer and assayed by immunoblotting with an anti-LC3b-II antibody (Novus, St Louis, MO, USA). Autophagic flux was quantified by comparing LC3b-II band densities, normalized to GAPDH loading control, in BafA1-treated cells.
ATP assay
The assay was performed in 20 µl of cell extract. Blank, ATP standards and cell extracts were added to 20 µl of ATP reagent (60 mM Tris–HCl pH 8.1, 0.04% BSA, 4 mM MgCl2, 2 mM dithiothreitol, 600 µM d-glucose, 250 µM NADP+, 4 µg/ml Hexokinase without (NH4)2SO4 and 1 µg/ml G6PDH) and incubated at room temperature for 20 min. The reaction was terminated by adding 10 µl of 0.3 N NaOH and incubating at 60°C for 20 min. A 1 ml mixture of 6 N NaOH, 10 mM Imidazole base and 0.01% H2O2 was then added, the sample was incubated at 60°C for 20 min, and then fluorescence at 340 nm was measured by using a fluorometer. The fluorescence of NADP+ is indicative of ATP levels.
Statistical analysis
Data are expressed as mean ± SEM. Differences between control and experimental values were compared using appropriate parametric and non-parametric tests for continuous variables, including Student's t-test for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. For multiple comparisons between more than two groups, analysis of variance with the Bonferroni correction was used. Significance was defined as P < 0.05. The statistical analysis software used was GraphPad Prism6 (LaJolla, CA, USA).
Results
Diet-induced obesity impairs metabolism in mice
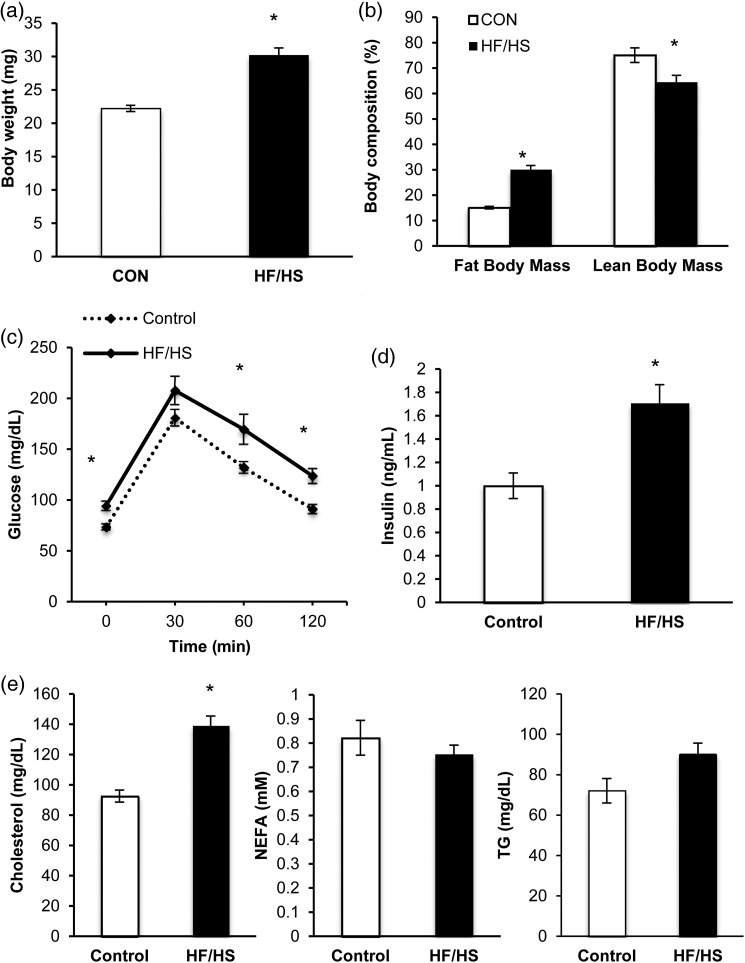
We fed female mice either a control (CON; 13% kcal fat) or a high-fat, high-sucrose (HF/HS; 59% kcal fat, 26% kcal sucrose) diet for 12 weeks starting at 6 weeks of age. At the end of this period, the HF/HS-exposed mice were significantly heavier and had higher percentage fat body mass than the CON-exposed mice (Fig. 1a and b). Furthermore, the HF/HS-exposed mice had impaired glucose tolerance and elevated fasting serum insulin levels (Fig. 1c and d). Finally, although we observed no difference in serum levels of non-esterified fatty acids, serum levels of cholesterol and triglycerides were elevated in the HF/HS mice (Fig. 1e); however, the difference in triglycerides between groups was not significant.
Figure 1.
High-fat/high-sucrose feeding leads to obesity and altered glucose metabolism in mice. (a) Body weight, (b) fat and lean body mass, (c) glucose tolerance test, (d) serum insulin levels and (e) serum lipids [cholesterol, non-esterified fatty acids (NEFA) and triglycerides (TG)] measured after 12 weeks of control (CON) or high-fat/high-sucrose (HF/HS) diet. Values are expressed as mean ± SE. *P < 0.01, as analyzed by Student’s t-tests. n = 27 control fed mice and n = 25 HF/HS-fed mice.
Effects of diet-induced obesity on sizes of implantation sites and term fetuses in mice
As a first test of the effects of diet-induced obesity on the uterus and offspring, we mated CON- and HF/HS-exposed female mice to CON-exposed males and visualized the implantation sites in early pregnancy (dpc 5.5). We found that implantation sites in HF/HS-exposed mice were significantly greater in number than CON-exposed females, although this difference was relatively small (Fig. 2a). These findings are consistent with a slightly increased 1-cell zygote number in prior studies (Igosheva et al., 2010). Next, we mated CON- and HF/HS-exposed female mice to CON-exposed males and visualized the implantation sites at a slightly later time in early pregnancy (dpc 8.5). We found that the number of implantation sites on dpc 8.5 was significantly lower in the HF/HS-exposed mice when compared with the CON mice (Fig. 2b). In addition, we found that implantation sites in HF/HS-exposed mice were of more variable diameter and significantly smaller than those in CON-exposed mice (Fig. 2d and e). The difference between dpc 5.5 and 8.5 suggests an early loss, consistent with the clinical pattern in obesity (Boots et al., 2014). Finally, we investigated fetal number and size at term, on dpc 18.5 (Fig. 2c). Although there was no difference in litter size, fetuses of HF/HS mice weighed significantly more than the fetuses of control mice (Fig. 2f and g). Additionally, placentas were significantly heavier. Previous work from our lab showed that fetuses resulting from PA-exposed blastocysts were smaller at dpc 14.5 than controls (Jungheim et al., 2011) and fetuses on dpc 14.5 of mice fed a high-fat diet were growth restricted (Jungheim et al., 2010). Together, these results suggest that implantation and early fetal growth are impaired in obese mice but that fetuses can catch up, and overcompensate to become macrosomic, later in development.
Figure 2.
Diet-induced obesity in mice leads to altered implantation, size and shape of sites in early pregnancy and fetal macrosomia at term. (a) Implantation site number at dpc 5.5 (n = 6 mice in each group); (b) at dpc 8.5 (n = 14 mice I each group); and (c) at dpc 18.5 (n = 5 in each group). (d) Representative images of implantation sites at dpc 8.5 from CON- and HF/HS-exposed mice. (e) Diameter and weight at dpc 8.5 (n = 14 mice in each group). (f) Representative images of term (dpc 18.5) CON and HF/HS fetuses (n = 5 each group). (g) Fetal and placental weights on dpc 18.5 (n = 5 mice in each group). Values are expressed as mean ± SE. *P < 0.05, ***P < 0.0001 as analyzed by the Mann–Whitney U-test.
Diet-induced obesity impairs decidualization in mice
The above results led us to wonder whether decidualization was impaired in the HF/HS-exposed mice. To test this possibility, we used a previously established method of inducing decidualization (Deb et al., 2006; Herington and Bany, 2007) in pseudo-pregnant HF/HS- and CON-exposed mice. Whereas CON-exposed mice formed robust deciduomas, the stimulated uterine horns from HF/HS-exposed mice formed much smaller deciduomas (Fig. 3a). We weighed the deciduomas and found that those of HF/HS-exposed mice were 50% lighter than those of CON-exposed mice (Fig. 3b). The weights of the unstimulated horns did not differ between the groups. Morphological examination of stained sections (hematoxylin and eosin) revealed a marked increase in the number of decidual cells and size of the stromal compartment of the stimulated horns from CON-exposed mice but not in those from HF/HS-exposed mice (Fig. 3c). Consistent with this observation, mRNA expression level of prolactin-related protein (Prp), a well-established marker of decidualization in mice (Rasmussen et al., 1996), was significantly lower in deciduomas from HF/HS-exposed mice than in those from CON-exposed mice (Fig. 3d). Together, these data indicate that decidualization is impaired in mice that are obese from consuming an HF/HS diet.
Figure 3.
HF/HS diet impairs artificial ESC decidualization in mice. (a) Representative images of deciduomas formed in pseudo-pregnant CON- and HF/HS-exposed mice. (b) Weights and (c) representative cross-sections of deciduomas. (d) mRNA expression level of Prp in deciduomas (n = 10 Con, n = 11 HF/HS). Values are expressed as mean ± SE. *P < 0.05, as analyzed by the Mann–Whitney U-test.
Progesterone produced by the ovary is required to induce decidualization, so we wondered whether impaired decidualization was due to reduced progesterone production in the HF/HS-exposed mice. However, the serum levels of progesterone at the time of decidualization were identical between the HF/HS- and CON-exposed mice (mean progesterone = 14 ng/ml in each group—data not shown). This finding implicates the endometrium, not the ovary, in the decidualization defect in HF/HS-exposed mice.
Autophagy is up-regulated during murine decidualization
Decidualization is a highly energetic process that requires the ESCs to first proliferate extensively and then differentiate into secretory epithelial-like cells. Thus, we hypothesized that the cellular recycling pathway, autophagy, is important to ensure that the cells are able to meet the energetic demands of decidualization. To investigate this possibility, we examined protein levels of key regulators of autophagy. When cellular energy status is low, AMP kinase (AMPK) is activated (Weikel et al., 2015), leading to the inhibitory phosphorylation of acetyl-CoA carboxylase (ACC). Additionally, activated AMPK phosphorylates and thereby activates the serine/threonine kinase ULK1, which drives autophagy. Thus, levels of p-ACC and p-ULK1 are indicators of autophagy level in cells. Consistent with our hypothesis, we found that expression levels of ACC, p-ACC and p-ULK1 were higher in stimulated uterine horns than in unstimulated horns (Fig. 4a), suggesting that autophagy is induced during decidualization. However, levels of these markers increased significantly less in the HF/HS-exposed mice than in the CON-exposed mice, suggesting that autophagy was up-regulated more in decidualizing cells in CON-exposed mice than HF/HS-exposed mice (Fig. 4b).
Figure 4.
Autophagy is up-regulated during murine decidualization. (a) Representative western blots show relative protein levels of acetyl-CoA carboxylase (ACC), phosphorylated-ACC, phosphorylated-Unc-like kinase 1 (ULK1), ULK1 and loading control GAPDH in unstimulated (US) and stimulated (S) uterine horns from CON- and HF/HS-exposed mice. (b) Quantification of band intensities (n = 6–8 mice per group). Values are expressed as mean ± SE. *P < 0.01 between the Con and HF/HS groups, $P < 0.05 between the US and S groups. The Mann–Whitney U-test was used to analyze the data.
PA impairs human ESC decidualization in vitro
We next wanted to determine whether similar effects occur in human ESCs. Thus, we first assessed the effects of PA on decidualization of immortalized human ESCs. We chose PA because it is a major component of the mouse HF/HS diet. Additionally, PA is the most abundant unsaturated fatty acid in the current Western diet and contributes to hyperlipidemia and its subsequent negative long-term health effects (Kien et al., 2014). A dose–response experiment revealed that exposure to a PA concentration of 100 μM resulted in the greatest effect on suppressing decidualization, as measure by PRL mRNA expression without causing cell death (Supplementary Fig. S1). After 9 days of decidualization, cells treated with PA expressed significantly lower mRNA levels of insulin-like growth factor binding protein 1 (IGFBP1) and PRL (Fig. 5a), two well-established markers of decidualization (Zhu et al., 2014) than control cells. We used trypan blue staining to confirm that there was no difference cell number between the PA-treated and control cells at Day 9.
Figure 5.
PA impairs human ESC decidualization in vitro. Expression levels of IGFBP1 and decidual prolactin (PRL) mRNA in (a) immortalized and (b) primary human ESCs in the presence or absence of PA. (c) Expression of IGFBP1 and PRL mRNA in decidualized endometrial cells from obese (BMI > 30 kg/m2) and lean (BMI < 25 kg/m2) women (n = 11 obese, n = 7 lean). Values are expressed as mean fold change over undecidualized ± SE. *P < 0.001 as analyzed by the Mann–Whitney U-test.
Similar to our findings in immortalized cells, IGFBP1 and PRL mRNA expression was reduced in primary human ESCs decidualized for 3 days in the presence of PA (Fig. 5b). Finally, we stratified the primary ESCs by BMI of the patients and compared mRNA expression of IGFBP1 and PRL in cells decidualized in the absence of PA. We found that these markers were expressed at lower levels in the cells obtained from obese women (BMI > 30 kg/m2) than in those from normal-weight women (BMI < 25 kg/m2) (Fig. 5c). From these data, we conclude that exposure to high levels of fatty acids and obesity can also cause impairment in decidualization of human ESCs.
Autophagy is up-regulated during human ESC decidualization
To assess the role of autophagy in human ESC decidualization, we performed an autophagic flux assay (Fig. 6a). This assay measures levels of lipidated LC3b, termed LC3b-II. LC3b-II localizes to autophagosomes (Barth et al., 2010) and is one of the best-characterized markers of autophagy. However, because autophagy is a dynamic process in which the end-point is fusion of the autophagosome with a lysosome and protein degradation, it is not always sufficient to measure steady-state levels of LC3b-II. Instead, bafilomycin A1 (BafA1) can be used to block autophagy to lysosomal degradation; accumulation of LC3b-II in these conditions indicates an increase in autophagic flux. Consistent with our findings in the mouse uterus showing that expression of autophagy regulators increases in decidualized endometrium (Fig. 4), we found that levels of LC3b-II tended to increase with decidualization in human ESCs (P= 0.06) in the presence of BafA1 (Fig. 6b) but this was not statistically significant. In contrast, we observed significantly lower levels of LC3b-II in PA-treated undecidualized and decidualized ESCs (Fig. 6b). Taken together, these data suggest that autophagy increases during decidualization of human endometrial cells and is impaired by exposure to excess fatty acid.
Figure 6.
Autophagy is up-regulated during human ESC decidualization. (a) Western blot of LC3b-II and GAPDH (loading control) in control (Con) and decidualized (Dec) human ESCs in the presence or absence of PA and in the presence or absence of Bafilomycin A1 (BafA1). (b) Quantification of LC3b-II level relative to GAPDH in BafA1-treated cells (n = 3–7 treatment wells per condition). Values are expressed as mean ± SE. *P<0.001, as analyzed by analysis of variance with the Bonferroni correction for multiple comparisons. This experiment was performed in triplicate.
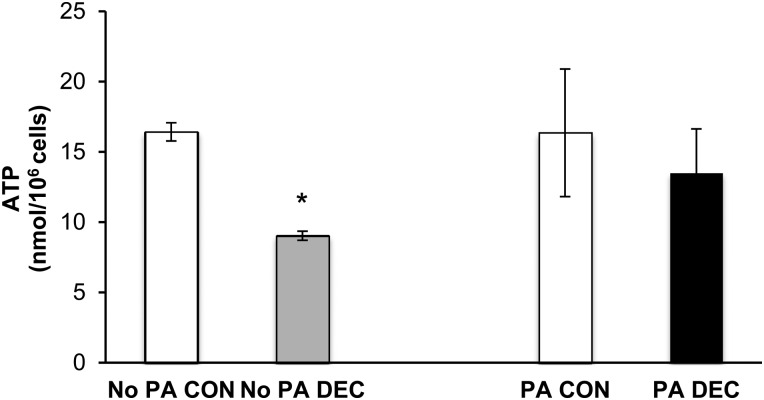
ATP levels increase during human ESC decidualization
Finally, given that autophagy is a cellular mechanism to recycle nutrients under conditions of energy depletion (Rabinowitz and White, 2010), we wondered whether ATP levels in human ESCs were affected by decidualization and PA treatment. We found that ATP levels decreased significantly, by ∼50%, upon decidualization of untreated cells but did not change upon decidualization of PA-treated cells (Fig. 7).
Figure 7.
ATP levels decrease during human ESC decidualization. ATP concentration in control (Con) and decidualized (Dec) human ESCs in the presence or absence of PA (n = 3 treatment wells per condition). Values are expressed as mean ± SE. *P< 0.001 as analyzed by Student’s t-tests.
Discussion
Here, we have presented the first evidence, in both mice and humans, that obesity and exposure to a high-fat diet impair ESC decidualization. We showed by two different methods, natural mating and induced deciduoma formation, that decidualization is impaired in a mouse model of high-fat-diet-induced obesity and metabolic syndrome. Likewise, we found that human ESCs exposed to PA or isolated from obese women had reduced ability to decidualize. Our working model is that in the presence of excess free fatty acids, the ESCs do not experience a drop in ATP and thus do not activate autophagy as efficiently. As a result, they are unable to properly decidualize. In support of this idea, we found that autophagic flux increased during decidualization of human cells and was decreased in the presence of PA. Likewise, we observed increased activity of the autophagy regulators AMPK and ULK1 in deciduomas from CON- but not HF/HS-exposed mice. Additionally, a similar phenomenon of decreased autophagy as a result of exposure to excess free fatty acids has been demonstrated in other cell types such as human aortic endothelial cells and hepatocytes (Las et al., 2011; Mei et al., 2011; Weikel et al., 2015). Finally, recent literature has provided evidence that autophagy is also a key factor in implantation (Armant, 2011; Lee et al., 2011).
Lipotoxicity has been shown to be damaging to many cell types such as myocytes, hepatocytes and β cells (Malhotra and Kaufman, 2007), in addition to oocytes (Wu et al., 2010). Therefore, similar detrimental effects of free fatty acid accumulation and lipotoxicity in the endometrium may contribute to pregnancy complications in our mouse model and in obese women. This thought is also supported by evidence that obese women have recurrent early pregnancy loss, despite euploid embryos (Boots et al., 2014), pointing to the endometrial environment as a contributor to pregnancy loss. In this study, we exposed human endometrial cells to PA because this fatty acid is common in the Western diet. Additionally, intake of high levels of PA has been linked to increased cardiovascular disease risk (WHO, 2003) and other negative health effects. As we observed with ESCs, in vitro studies have demonstrated negative effects of free fatty acids, including PA, on other cell types such as hepatocytes (Ricchi et al., 2009) and fetal calvarial cells (Chen et al., 2012). Thus, in addition to the defect we observed in autophagy, lipotoxicity may contribute to the impaired decidualization we report here in mice and human samples.
The mechanism by which diet-induced obesity may impair ESC decidualization is likely multifactorial. Recent literature has pointed to autophagy as being a key factor in implantation (Armant, 2011, Lee et al., 2011). Autophagy is a cellular degradation process by which the cell recycles its contents to generate energy in high demand states and is regulated by cellular energy and nutrient-sensing enzymes, such as AMPK and the mammalian target of rapamycin (mTOR), respectively. Autophagy has not yet been shown to be critical for decidualization, however, given decidualization is a high-energy process of cellular proliferation and differentiation, it seems likely that autophagy would be activated during decidualization. Consistent with this idea, our data show that ATP levels in immortalized human ESCs drop during decidualization. Increased autophagic flux was also observed in decidualized human ESCs confirming a role for autophagy in decidualization. As the main energy-sensing enzyme in the cell, AMPK is activated in response to an increased ratio of AMP to ATP. This activation has been shown to induce autophagy through both the inhibition of mTOR and the activation of ULK1, a key initiator of the autophagic process (Egan et al., 2011; Kim et al., 2011). Our data show increased AMPK and ULK1 activity in deciduomas from control mice, further supporting the concept that autophagy is induced during decidualization. This phenomenon has been demonstrated in other cell types such as human aortic endothelial cells and hepatocytes (Las et al., 2011; Mei et al., 2011; Weikel et al., 2015). Our data in human ESCs exposed to PA as well as HF/HS-fed mice support this concept. Furthermore, autophagy and decidualization were impaired in PA-exposed human ESCs and HF/HS-fed mice, suggesting that impaired autophagy may play an underlying role in decreased decidualization and obesity-associated subfertility.
Recently, one study examined the eutopic and ectopic endometrium from patients with endometriosis and reported that secretory phase ESC from these patients demonstrated reduced evidence of autophagy (Mei et al., 2015). In a series of experiments, they concluded that estradiol suppressed autophagy and progesterone reversed these effects in the ESC from patients with endometriosis. It was not determined if this hormone effect was via the estrogen or progesterone receptor, or if this occurred by other non-genomic mechanisms. Whether this abnormal autophagy in human ESC is the cause or consequence of endometriosis was not addressed.
Obesity after menopause is directly related to endometrial cancer (Moley and Colditz, 2016), and it is possible that mechanisms implicated in the development of uterine cancer may provide alternative pathways to consider to explain poor decidualization in obese women. For instance, a recent study compared gene expression profiles in visceral fat versus subcutaneous fat versus endometrium from patients with or without uterine cancer (Modesitt et al., 2012). They found a distinct up-regulation of uterine lipogenesis enzymes suggesting a metabolism favoring fatty acid synthesis from carbohydrate precursor. This is unlikely in our study since we found a decrease in ACC1; however, future studies examining endometrial signaling pathways in obesity-related endometrial cancer may be helpful.
Decidualization is essential not only for implantation but also for placentation, fetal health and maintenance of pregnancy (Galliano and Bellver, 2013). Thus, it may seem surprising that the fetuses of HF/HS mice on dpc 18.5 were macrosomic compared with controls, especially given our previous report that fetuses resulting from PA-exposed blastocysts were smaller than controls at dpc 14.5 (Jungheim et al., 2011) and fetuses on dpc 14.5 of obese mice were growth restricted (Jungheim et al., 2010). We interpret this to mean that catch-up growth occurred in the HF/HS-fed offspring over the last few days of gestation when the majority of fetal growth occurs. This idea is consistent with our previous report that 13-week-old offspring from dams fed an HF/HS diet dams exhibit catch-up growth (Jungheim et al., 2010). At this point, it remains unclear whether or not impaired decidualization in HF/HS-fed mice is the underlying mechanism driving increased fetal growth. However, given the role of decidualization in maintaining a healthy gestation and placentation, an altered uterine environment early in pregnancy could set the stage for altered fetal growth later on. Clinically, this phenotype is consistent with increased incidence of fetal macrosomia in obese and diabetic women (Gaudet et al., 2014) and may suggest a role for impaired decidualization in altered fetal growth during human pregnancy.
Strengths of our study include incorporation of both mouse and human experiments to investigate our hypothesis, use of in vivo and in vitro experiments as well as use of clinical samples from obese and normal-weight women. Additionally, we incorporated two methods of examining mouse decidualization. Weaknesses of our study include a small sample size in the human biopsy samples and inability to demonstrate a definitive cause-and-effect relationship of autophagy defects and impaired decidualization. However, our data are the first to show that diet-induced obesity impairs ESC decidualization both in vivo in an obese mouse model and in vitro in an immortalized and primary human ESC culture model. When this key process of early pregnancy is impaired, it can have both negative short-term effects on implantation and long-term fetal effects. If our hypothesis that autophagy is a key player in decidualization is borne out by further studies, the clinical implications could be important. For example, autophagic activators may be a potential therapeutic regimen for obese women with recurrent pregnancy loss or infertility, both common and increasing problems seen among the growing obese patient population.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Acknowledgements
We thank Andrea M. Drury for her help with mouse experiments and Deborah Frank, PhD, for aiding in the editing of this manuscript.
Authors' roles
J.S.R. conceived the study, performed experimental work and wrote the manuscript. J.L.S. aided in study design, experimental work and edited the manuscript. A.L.M., M.B.S., Z.A., C.S., and M.M.-Y.C. aided with experimental work. K.H.M. conceived, supervised and mentored all studies and edited the manuscript.
Funding
This work was supported by the following grants: National Institutes of Health 5T32HD040135-12 (J.S.R.), R01 HD065435 (K.H.M.), National Institutes of Health T32 HD049305 (J.L.S.), American College of Obstetrics and Gynecology Research Grant (M.B.S.).
Conflict of interest
None declared.
References
- Armant DR. Autophagys expanding role in development: implantation is next. Endocrinology 2011;152:1739–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol 2010;221:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellver J, Pellicer A. Impact of obesity on spontaneous abortion. Am J Obstet Gynecol 2004;190:293–294. [DOI] [PubMed] [Google Scholar]
- Bellver J, Ayllon Y, Ferrando M, Melo M, Goyri E, Pellicer A, Remohi J, Meseguer M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril 2010;93:447–454. [DOI] [PubMed] [Google Scholar]
- Bellver J, Martinez-Conejero JA, Labarta E, Alama P, Melo MA, Remohi J, Pellicer A, Horcajadas JA. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril 2011;95:2335–2341, 2341.e1–8. [DOI] [PubMed] [Google Scholar]
- Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril 2013;100:1050–1058. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996;122:2687–2696. [DOI] [PubMed] [Google Scholar]
- Boots CE, Bernardi LA, Stephenson MD. Frequency of euploid miscarriage is increased in obese women with recurrent early pregnancy loss. Fertil Steril 2014;102:455–459. [DOI] [PubMed] [Google Scholar]
- Chen JR, Zhang J, Lazarenko OP, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J 2012;26:1131–1141. [DOI] [PubMed] [Google Scholar]
- Deb K, Reese J, Paria BC. Placenta and Trophoblast: Methods and Protocols. Totowa, NJ: Humana Press Inc., 2006. [Google Scholar]
- Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011;7:643–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova AI, O'Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol 2011;25:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano D, Bellver J. Female obesity: short- and long-term consequences on the offspring. Gynecol Endocrinol 2013;29:626–631. [DOI] [PubMed] [Google Scholar]
- Gaudet L, Tu X, Fell D, El-Chaar D, Wu Wen S, Walker M. The effect of maternal Class III obesity on neonatal outcomes: a retrospective matched cohort study. J Matern Fetal Neonatal Med 2012;25:2281–2286. [DOI] [PubMed] [Google Scholar]
- Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. BioMed Res Int 2014;2014:640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bany BM. The conceptus increases secreted phosphoprotein 1 gene expression in the mouse uterus during the progression of decidualization mainly due to its effects on uterine natural killer cells. Reproduction 2007;133:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 2010;120:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PloS One 2010;5:e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 2010;151:4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Louden ED, Chi MM, Frolova AI, Riley JK, Moley KH. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biol Reprod 2011;85:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod 2013;28:2720–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr 2014;99:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011;13:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Lockwood C. The immortalization of human endometrial cells. Methods Mol Med 2006;121:79–83. [DOI] [PubMed] [Google Scholar]
- Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol 2012;358:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in beta-cells. J Biol Chem 2011;286:42534–42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Oh HA, Song H, Jun JH, Roh CR, Xie H, Dey SK, Lim HJ. Autophagy regulates embryonic survival during delayed implantation. Endocrinology 2011;152:2067–2075. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 2012;7:e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 2007;9:2277–2293. [DOI] [PubMed] [Google Scholar]
- Mei S, Ni HM, Manley S, Bockus A, Kassel KM, Luyendyk JP, Copple BL, Ding WX. Differential roles of unsaturated and saturated fatty acids on autophagy and apoptosis in hepatocytes. J Pharmacol Exp Ther 2011;339:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod 2015;30:1677–1689. [DOI] [PubMed] [Google Scholar]
- Modesitt SC, Hsu JY, Chowbina SR, Lawrence RT, Hoehn KL. Not all fat is equal: differential gene expression and potential therapeutic targets in subcutaneous adipose, visceral adipose, and endometrium of obese women with and without endometrial cancer. Int J Gynecol Cancer 2012;22:732–741. [DOI] [PubMed] [Google Scholar]
- Moley KH, Colditz GA. Effects of obesity on hormonally driven cancer in women. Sci Transl Med 2016;8:323ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril 2012;98:102–108. [DOI] [PubMed] [Google Scholar]
- Ogden CL. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 2012;82:1–8. [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science 2010;330:1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen CA, Hashizume K, Orwig KE, Xu L, Soares MJ. Decidual prolactin-related protein: heterologous expression and characterization. Endocrinology 1996;137:5558–5566. [DOI] [PubMed] [Google Scholar]
- Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol 2009;24:830–840. [DOI] [PubMed] [Google Scholar]
- Schroder AL, Pelch KE, Nagel SC. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine 2009;35:211–219. [DOI] [PubMed] [Google Scholar]
- Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol 2015;29:498–506. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Schulte M, O'Neill K, Chi MM, Frolova AI, Moley KH. Glucosamine inhibits decidualization of human endometrial stromal cells and decreases litter sizes in mice. Biol Reprod 2013;89:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Glucose and palmitate uncouple AMPK from autophagy in human aortic endothelial cells. Am J Physiol Cell Physiol 2015;308:C249–C263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization.Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series, No. 916 World Health Organization, 2003; p. 88. [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010;151:5438–5445. [DOI] [PubMed] [Google Scholar]
- Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Animal 2011;40:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene 2014;551:1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.