Abstract
Objectives:
The composition of the medical costs incurred by people treated for basal cell and squamous cell carcinomas (hereafter keratinocyte cancers) is not adequately understood. We sought to compare the medical costs of individuals with or without keratinocyte cancers.
Methods:
We used national health insurance data to analyze the direct medical costs of 2000 cases and 2000 controls nested within the QSkin prospective cohort study (n = 43,794) conducted in Australia. We reconstructed the medical history of patients using medical and pharmaceutical item codes and then compared the health service costs of individuals treated for keratinocyte cancers with those not treated for keratinocyte cancers.
Results:
Individuals treated for keratinocyte cancers consumed on average AUD$1320 per annum more in medical services than those without keratinocyte cancers. Only 23.2% of costs were attributed to the explicit treatment of keratinocyte cancers. The principal drivers of the residual costs were medical attendances, surgical procedures on the skin, and histopathology services. We found significant positive associations between history of treatment for keratinocyte cancers with treatments for other health conditions, including melanoma, cardiovascular disease, lipidemia, osteoporosis, rheumatoid arthritis, colorectal cancer, prostate cancer, and tuberculosis.
Conclusion:
Individuals treated for keratinocyte cancers have substantially higher medical costs overall than individuals without keratinocyte cancers. The direct costs of skin cancer excision account for only one-fifth of this difference.
Keywords: Basal cell carcinoma, squamous cell carcinoma, keratinocyte cancer, costs of keratinocyte cancer comorbidities
Introduction
Keratinocyte cancer (KC) is the collective term for basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) of the skin.1 Due to its high incidence, the aggregate costs of treating KC remains a concern in North America,2–5 Europe,6–9 Australia,10 New Zealand,11 and Brazil.12 KC is the most costly cancer to treat in Australia10 and the fifth most costly to treat in the United States.13 The increasing incidence of KC in North America,13,14 Europe,15 and Australia10 motivates the need for a thorough understanding of the medical costs of treating people with KC.
While several broad estimates of the cost of KC treatment have been published,3,5–10,12,16 few studies have used individual-level data to explore direct personal costs in detail. Due to a high incidence and low mortality rate, KC data are seldom collected by national cancer registries.17 In the very few jurisdictions where data collection is mandated (e.g. Demark), incomplete case registration18 can result in bias if registered patients are systematically different from the unregistered patients.19 Thus, obtaining accurate KC data is challenging, and there are limited data that adequately describe the costs of KC treatments.
Patients with KC can be affected by comorbid disease. The epidemiological literature remains inconclusive about incidence of correlated comorbidities, and the economic literature has not accurately determined the costs of treatment. KC is reported to be positively correlated with melanoma,20,21 other cancers,16,20–22 and several chronic diseases,18,23 and due its association with vitamin D,24–26 it is negatively correlated with second solid primary cancers27 and prostate cancer.25
To explore the burden of KC in detail, we used national health insurance data obtained through record-linkage to a very large prospective cohort study that was established specifically to study cancers of the skin. Here, we report an analysis in which we measured and categorized medical treatments utilized by a cohort of patients treated for KC.
Materials and methods
In 2011, the QSkin Sun and Health Study was initiated in Queensland, Australia, to investigate the role of environmental and genetic factors in the etiology of skin cancer.28 An invitation and survey was sent to 193,344 persons selected at random from the Queensland Electoral Roll (voter registration is compulsory by law in Queensland, Australia, for all persons over the age of 18 years). Potential participants were aged 40–69 years and frequency matched by age and sex to the Queensland population, of whom 43,794 agreed to participate in the study.28,29 The respondents were asked to report demographic and socioeconomic characteristics as well as clinical data including skin phenotype, medical history, and exposure to sunlight.28 In total 39,033 participants gave their consent for linkage to individual health service utilization data from 30 June 2011 to 30 June 2012 retained by the national health insurance scheme, Medicare Australia.
Medicare Australia is composed of two major funding instruments, the Medicare Benefits Scheme (MBS) and the Pharmaceutical Benefits Scheme (PBS). The MBS subsidizes fee-for-service medical care provided by Australia’s medical practitioners as well as diagnostic, therapeutic, and imaging services. The MBS lists thousands of subsidized clinical services; each identified by a unique item code and assigned a scheduled subsidy. The PBS is a parallel funding instrument, which provides Australian residents affordable access to a wide range of medications listed on the PBS. The survey responses were linked to 12 months of health service utilization data, denoted by MBS and PBS item numbers. Ethical approval for the study was received from the QIMR Berghofer Medical Research Institute Human Research Ethics Committee and the Department of Health and the Queensland University of Technology.
The treatment of KC will typically involve local excision or biopsy of the suspect skin lesion. Depending on the clinical staging at the time of presentation, other KC treatment costs can include attendance fees for general practitioners (GPs) and specialist practitioners, surgeries, anesthetic, skin grafting, inpatient care, and after care. In our data, a KC was identified if an individual had utilized any 1 of 41 MBS item codes, which explicitly described the excision of a BCC, SCC, or ablation of malignant neoplasm of skin (see Table 2). A sub-sample of 2000 randomly selected individuals with KC was matched 1:1 on gender and 5-year age categories to a group of controls.
Table 2.
List of 41 medical services on the MBS for the treatment of KC: total number of procedures in KC treatment group (n = 2000) and mean cost per 100 patients.
| MBS item description | Number of procedures | Cost per 100 KC patients |
|---|---|---|
| Removal of malignant neoplasm of skin by serial curettage or carbon dioxide laser excision–ablation: <10 lesions (30196) | 799 | AUD$3711 |
| Removal of malignant neoplasm of skin by serial curettage or carbon dioxide laser excision–ablation: >10 lesions (30197) | 15 | AUD$467.56 |
| Removal of malignant neoplasm of skin by cryotherapy: <10 lesions (30202) | 230 | AUD$425.43 |
| Removal of malignant neoplasm of skin by cryotherapy: >10 lesions (30203) | 28 | AUD$202.58 |
| Removal of malignant neoplasm of skin and cartilage by cryotherapy: >10 lesions (30205) | 0 | AUD$0.00 |
| Mircographically controlled serial excision of skin tumor with histological examination: <6 lesions (31000) | 19 | AUD$800.26 |
| Mircographically controlled serial excision of skin tumor with histological examination: 7–12 lesions (31001) | 8 | AUD$464 |
| Mircographically controlled serial excision of skin tumor with histological examination: >13 lesions (31002) | 1 | AUD$55 |
| Removal from nose, eyelid, lip, ear, digit, or genitalia by surgical excision | ||
| Removal of BCC or SCC with malignancy confirmed: <10 mm diameter (31255) | 318 | AUD$2992 |
| Removal of residual BCC or SCC by original GP, specimen sent to histology: original tumor < 10 mm diameter (31256) | 14 | AUD$116 |
| Removal of residual BCC or SCC by nonoriginal GP, specimen sent to histology: original tumor < 10 mm diameter (31257) | 4 | AUD$49 |
| Removal of recurrent BCC or SCC, malignancy confirmed by histology: original tumor < 10 mm diameter (31258) | 2 | AUD$14 |
| Removal of BCC or SCC with malignancy confirmed: >10 mm diameter (31260) | 84 | AUD$972 |
| Removal of residual BCC or SCC by original GP, specimen sent to histology: original tumor > 10 mm diameter (31261) | 5 | AUD$82 |
| Removal of residual BCC or SCC by nonoriginal GP, specimen sent to histology: original tumor > 10 mm diameter (31262) | 1 | AUD$21 |
| Removal of recurrent BCC or SCC, malignancy confirmed by histology: original tumor > 10 mm diameter (31263) | 0 | AUD$0 |
| Removal from face, neck (anterior to the sternomastoid muscles), or lower leg (mid-calf to ankle) by surgical excision | ||
| Removal of BCC or SCC, malignancy confirmed by histology: <10 mm diameter (31265) | 595 | AUD$4765 |
| Removal of residual BCC or SCC, by original GP, specimen sent to histology: original tumor < 10 mm diameter (31266) | 14 | AUD$115 |
| Removal of residual BCC or SCC, by nonoriginal GP, specimen sent to histology: original tumor < 10 mm diameter (31267) | 3 | AUD$21 |
| Removal of recurrent BCC or SCC, malignancy confirmed by histology: original tumor > 10 mm diameter (31268) | 2 | AUD$15 |
| Removal of BCC or SCC with malignancy confirmed: 10–20 mm diameter (31270) | 229 | AUD$2549 |
| Removal of residual BCC or SCC by original GP, specimen sent to histology: original tumor 10–20 mm diameter (31271) | 3 | AUD$33 |
| Removal of residual BCC or SCC by nonoriginal GP, specimen sent to histology: original tumor 10–20 mm diameter (31272) | 0 | AUD$0 |
| Removal of recurrent, BCC or SCC, malignancy confirmed: original tumor 10–20 mm diameter (31273) | 2 | AUD$21 |
| Removal of BCC or SCC, malignancy confirmed: >20 mm diameter (31275) | 54 | AUD$653 |
| Removal of residual BCC or SCC, by original GP, specimen sent to histology: original tumor > 20 mm diameter (31276) | 1 | AUD$6 |
| Removal of residual BCC or SCC, by nonoriginal GP: original tumor > 20 mm diameter (31277) | 1 | AUD$5 |
| Removal of recurrent BCC or SCC, malignancy confirmed: original tumor > 20 mm diameter (31278) | 0 | AUD$0 |
| Removal from other body areas by surgical excision | ||
| Removal of BCC or SCC, malignancy confirmed by histology: <10 mm diameter (31280) | 1049 | AUD$7258 |
| Removal of residual BCC or SCC, by original GP, specimen sent to histology: original tumor < 10 mm diameter (31281) | 15 | AUD$100 |
| Removal of residual BCC or SCC, by nonoriginal GP, specimen sent to histology: original tumor < 10 mm diameter (31282) | 1 | AUD$8 |
| Removal of recurrent BCC or SCC, malignancy confirmed by histology: original tumor > 10 mm diameter (31283) | 0 | AUD$0 |
| Removal of BCC or SCC with malignancy confirmed: 10–20 mm diameter (31285) | 435 | AUD$3891 |
| Removal of residual BCC or SCC by original GP, specimen sent to histology: original tumor 10–20 mm diameter (31286) | 4 | AUD$31 |
| Removal of residual BCC or SCC by nonoriginal GP, specimen sent to histology: original tumor 10–20 mm diameter (31287) | 1 | AUD$9 |
| Removal of recurrent, BCC or SCC, malignancy confirmed: original tumor 10–20 mm diameter (31288) | 0 | AUD$0 |
| Removal of BCC or SCC, malignancy confirmed: >20 mm diameter (31290) | 58 | AUD$547 |
| Removal of residual BCC or SCC, by original GP, specimen sent to histology: original tumor > 20 mm diameter (31291) | 0 | AUD$0 |
| Removal of residual BCC or SCC, by nonoriginal GP: original tumor > 20 mm diameter (31292) | 3 | AUD$52 |
| Removal of recurrent BCC or SCC, malignancy confirmed: original tumor > 20 mm diameter (31293) | 2 | AUD$18 |
| Removal of recurrent BCC or SCC, by nonoriginal GP, malignancy confirmed: tumor size unspecified (31295) | 8 | AUD$120 |
| Total | 4008 | AUD$30,589 |
MBS: Medicare Benefits Scheme; KC: keratinocyte cancer; BCC: basal cell carcinoma; SCC: squamous cell carcinoma; GP: general practitioner.
The costs of medical and pharmaceutical services, as identified by MBS and PBS item codes, were summed for each individual. This estimate included the value of the MBS subsidy plus the individual co-payment. Where Medicare Australia fully reimburses the physician for their service, the co-payment will be zero. Costs are presented in 2012 AUD$. Health service utilization, by cohort, is compared in league tables for frequency and cost.
The MBS and PBS item codes were also used to develop a clinical profile—albeit limited—for both cohorts. While in principle, there is no limit to the number of diseases, which one could consider, limited resources constrained the number of comorbidities, which were identified in the data. A literature review identified 17 comorbidities, which were reported to be correlated (positively or negatively) with KC. The selected comorbidities included three autoimmune diseases30 (asthma, rheumatoid arthritis,31 and multiple sclerosis32), two mental illnesses (depression33 and anxiety34), cardiovascular disease35 and two associated risk factors (hypertension and hyperlipidemia), diabetes,36 four cancers (melanoma,37 breast,38 prostate,38 and colorectal38), osteoporosis,39,40 Parkinson’s disease,41 tuberculosis,42 and bronchitis.40 The 17 selected comorbidities provide a good proxy for “health” insofar as they include 7 of the 10 most frequently managed chronic problems by Australia’s GPs.43
The Merck manual44 was reviewed to compile a comprehensive list of diagnostic tests, medical and surgical procedures, and pharmaceuticals used to manage each comorbidity listed. Each therapeutic intervention was then matched to the corresponding item codes identified in a search of the MBS45 and PBS46 websites. A total of 1500 MBS and PBS item codes were used to identify the 17 comorbidities that are documented in the supplementary material provided by Rowell et al.47 While not every treatment citied in the Merck manual could uniquely identify a diagnosis (e.g. analgesia), many treatments were able to identify a probable diagnosis (e.g. antihypertensive medication suggests hypertension). Unadjusted and adjusted odds ratios (controlled for age, gender, education, employment, health insurance status, and country of birth) were derived for each diagnosis using logistic regression. While treatments can be prescribed for “off-label” indications (e.g. antihypertensive medications can be prescribed for anxiety), the estimated odds ratio will provide a defensible approximation of the true odds ratio if the probability of “off-label” prescription is equal for cases and controls.
Medical treatments were tabulated for cases and controls. Frequency and costs differences were estimated for each medical service. The results are presented in league tables, which report statistically significant (p < 0.1) results. Student’s t-tests were used to report differences in the mean cost of medical care for cases and controls. Wilcoxon rank-sum tests were used to test differences in median costs. Chi-square tests were used to assess differences between those with and without KC for categorical data.
Results
Table 1 reports the demographic characteristics of sample of KC cases and controls matched by age group and sex drawn from the QSkin study. Despite matching on 5-year age groups, the cases with KC were on average 1.5 years older than the controls. Cases were also more likely to be white, born in Australia, less likely to be employed full-time, and not to have private health insurance. The average per person cost for all medical services was AUD$2096 (median = AUD$1230). Individuals treated for KC consumed an average cost of AUD$2756 in medical services (median = AUD$1762), while the controls consumed AUD$1436 (median = AUD$729). The difference, AUD$1320 (p < 0.01), was attributed to the additional cost of all medical treatments utilized by people with a KC.
Table 1.
Demographic characteristics of people treated with or without KC in Queensland, 2010.
| Characteristics | Cases (n = 2000) | Controls (n = 2000) | p-value |
|---|---|---|---|
| Female | 984 (49.2) | 1000 (50) | 0.613 |
| Private health insurance | 553 (27.7) | 619 (31) | 0.022 |
| Born in Australia | 1728 (86.4) | 1562 (78.1) | <0.01 |
| Age group in years | <0.01 | ||
| 40–49 | 420 (21) | 616 (30.8) | |
| 50–59 | 684 (34.2) | 573 (28.7) | |
| 60–69 | 896 (44.8) | 811 (40.6) | |
| Education | 0.037 | ||
| Nil | 184 (9.27) | 152 (7.7) | |
| School certificate | 365 (18.4) | 308 (15.6) | |
| High school | 349 (17.6) | 349 (17.7) | |
| Trade | 161 (8.11) | 178 (9) | |
| Certificate or diploma | 323 (16.3) | 362 (18.3) | |
| University | 442 (22.3) | 481 (24.4) | |
| Missing | 160 8.06 | 144 7.3 | |
| Ethnicity | <0.01 | ||
| White skin | 1926 (97.1) | 1845 (93.5) | |
| Black skin | 0 (0) | 4 (0.2) | |
| Asian | 2 (0.1) | 24 (1.2) | |
| Aboriginal and Torres Strait Islander | 2 (0.1) | 4 (0.2) | |
| Other | 2 (0.1) | 7 (0.4) | |
| Mixed | 33 (1.7) | 69 (3.5) | |
| Missing | 19 (0.97) | 21 (1.1) | |
| Employment | 0.01 | ||
| Full time | 770 (38.8) | 842 (42.7) | |
| Part time | 290 (14.6) | 272 (13.8) | |
| Home | 131 (6.6) | 150 (7.6) | |
| Unemployed | 28 (1.41) | 31 (1.6) | |
| Student | 14 (0.71) | 10 (0.5) | |
| Retired | 606 (30.5) | 520 (26.3) | |
| Other | 47 (2.4) | 69 (3.5) | |
| Missing | 98 4.9 | 80 4.1 | |
Case and controls matched on 5-year age categories and gender.
p-values calculated on the basis of χ2 statistics.
Table 2 lists the frequencies and costs for 41 medical services, which explicitly indicated the diagnosis and/or treatment of a KC. In our sample, 4008 KC treatments were provided at a total cost of AUD$611,778. The average cost per treatment was AUD$153. The average cost per treated individual was AUD$306, of which the public subsidy was AUD$241, and the co-payment was AUD$65. The mean number of treatments for KC was 2.0 per year. Of the individuals treated for KC, 777 (38.9%) received at least two treatments and 234 (11.7%) received at least four treatments.
By location, the most costly KC service was removal from “other body areas” followed by “face, neck or lower leg” (see Figure 1). By size of lesion, KCs < 10 mm in diameter accounted for 51% of direct treatment costs (see Figure 2).
Figure 1.
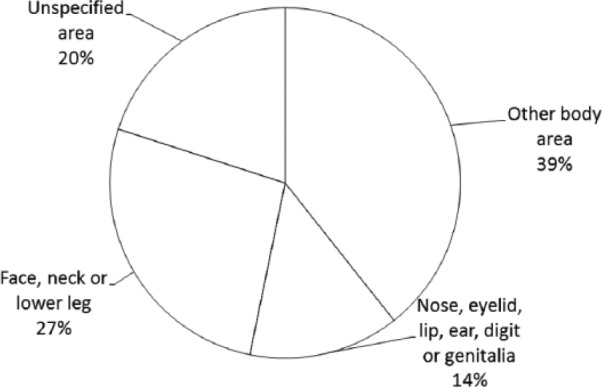
Keratinocyte cancer: proportion of costs by location of lesion.
(1) Removal from other body area (MBS items 31280, 31281, 31282, 31283, 31285, 31286, 31287, 31288, 31290, 31291, 31292, 31293, and 31295); (2) removal from nose eyelid, lip ear, digit, or genitalia (MBS items 31255, 31256, 31257, 31258, 31260, 31261, 31262, and 31263); (3) removal from face, neck, or lower leg (MBS items 31265, 31266, 31267, 31268, 31270, 31271, 31272, 31273, 31275, 31276, 31277, and 31278); and (4) unspecified area (30196, 30197, 30202, 30203, 30205, 31000, 31001, and 31002).
Figure 2.
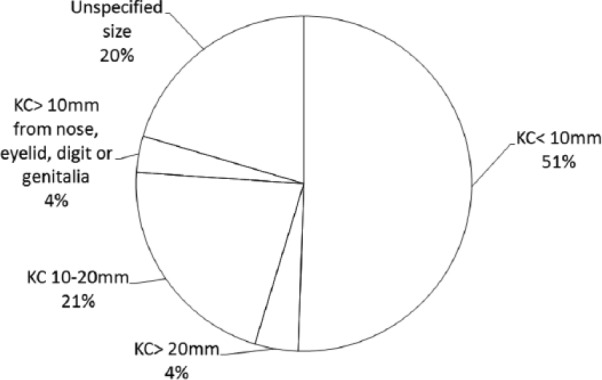
Keratinocyte cancer: proportion of costs by size of lesion treated.
(1) KC < 10 mm (MBS items 31255, 31256, 31257, 31258, 31265, 31266, 31267, 31268, 31280, 31281, 31282, and 31283); (2) KC > 20 mm (MBS items 31275, 31277, 31290, 31276, 31278, 31291, 31292, and 31293); (3) KC 10–20 mm (MBS items 31270, 31285, 31287, 31271, 31272, 31273, 31286, and 31288); (4) KC > 10 mm removed from nose, eyelid, lip, ear, digit, or genitalia (MBS items 31260, 31261, 31262, and 31263); and (5) unspecified size (MBS items 30196, 30197, 30202, 30203, 30205, 31000, 31001, 31002, and 31295).
Patients treated for KC experienced considerably greater comorbidity compared with patients not treated for KC. The KC cohort utilized an additional 366 medical attendances, 198 histopathology services, and 47 other surgical procedures on the skin (see Table 3). Other skin-related pathology (diagnostic skin biopsy, pre-malignant skin lesions, neoplastic skin lesions, treatment of wound by practice nurse and microscopy, and culture of the skin) and general diagnostic tests (serum chemistry, erythrocyte count, urine examination, thyroid-stimulating hormone (TSH) tests, and quantitation of a drug level) were also significantly greater among individuals treated for KC.
Table 3.
Twenty medical services with the greatest difference in rates between the KC and non-KC patient groups (rates of service per 100 patients).
| MBS description | With KC | Without KC | Difference | Wilcoxon rank-sum |
|---|---|---|---|---|
| Medical attendancesa | 1079 | 713 | 366 | <0.01 |
| Histopathologyb | 223 | 25 | 198 | <0.01 |
| Surgical procedures on the skinc | 55 | 8 | 47 | <0.01 |
| Diagnostic biopsy of skin or mucous membrane (30071) | 104 | 10 | 94 | <0.01 |
| Premalignant skin lesions (30192) | 46 | 9 | 37 | <0.01 |
| General serum chemistry 5 or more tests (66512) | 138 | 101 | 37 | <0.01 |
| Erythrocyte count, hematocrit (65070) | 104 | 73 | 31 | <0.01 |
| Treatment of wound by practice nurse (10996) | 21 | 3 | 18 | <0.01 |
| Pre-anesthesia brief consultation (17610) | 34 | 17 | 17 | <0.01 |
| Neoplastic skin lesions (30195) | 16 | 3 | 13 | <0.01 |
| Psoralen and ultraviolet A or B therapy (14050) | 9 | 0 | 9 | 0.01 |
| Electrocardiogram (11700) | 24 | 15 | 9 | <0.01 |
| Urine examination (69333) | 27 | 20 | 7 | <0.01 |
| Trigeminal nerve injection (18236) | 5 | 0 | 5 | <0.01 |
| Review a GP management plan (732) | 15 | 10 | 5 | <0.01 |
| Thyroid-stimulating hormone test (66716) | 35 | 31 | 4 | 0.04 |
| Quantitation of a drug level (66812) | 4 | 1 | 4 | 0.01 |
| Micro or cult skin (69306) | 6 | 2 | 4 | 0.01 |
| Foot, ankle, leg, knee, or femur (57521) | 16 | 12 | 3 | 0.02 |
| Subsequent optometric consultation (10918) | 19 | 16 | 3 | 0.07 |
MBS: Medicare Benefits Scheme; KC: keratinocyte cancer; GP: general practitioner.
Service definitions by MBS item numbers.
Medical attendances: 3, 23, 36, 44, 193, 197, 199, 2501, 2503, 2504, 2517, 2521, 2525, 2546, 2552, 5000, 5020, 5040, 5060, 86014, 104, 105, 110, and 116.
Histology: 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836, and 72838.
Other surgical procedures on skin: single-stage local flap: 45200, 45203, 45206, 45000, and 45003; free grafting: 45400, 45403, 45439, 45442, 45445, 45448, and 45451; lip, eyelid, or ear, full thickness wedge: 45665; H-flap or double advancement flap: 45207; vermilionectomy: 45668 and 45669; whole thickness reconstruction of eyelid: 45614; tumor, cyst, ulcer, or scar removal: 31200, 31205, 31210, 31215, 31220, 31225, 31230, 31235, 31240, and 52045; lens replacement: 42702.
Table 4 reports that the KC group incurred an additional AUD$22,296 for medical attendances, AUD$17,908 for histopathology, and AUD$13,202 for surgical procedures on the skin, per 100 treated patients. Premalignant skin lesions and neoplastic skin lesions cost an additional AUD$1203 and AUD$634 per 100 treated patients, respectively. Treatment of malignant melanoma costs an extra AUD$371 per 100 treated patients. Diagnostic tests also figured prominently, and computed tomogragphy (CT) scans (56507, 56807, 57341, and 56001) cost an additional AUD$2421. Table 4 includes a number of treatments denoted by superscript d, which could be attributed to the management of KC. If all services were initiated to treat a KC or complication of KC, this would imply an additional annual cost of AUD$21,391 per 100 KC patients or an average of AUD$214–AUD$306 per year as indicated in Table 2 (AUD$30,589 per 100 patients).
Table 4.
Twenty medical services with the greatest difference in costs between the KC and non-KC patient groups (costs per 100 patients).
| Service description (MBS no.) | Average cost per service (in 2012 AUD$) | With KC | Without KC | Difference | Wilcoxon rank-sum p-value |
|---|---|---|---|---|---|
| Medical attendancesa | AUD$79 | AUD$63,407 | AUD$41,111 | AUD$22,296 | <0.01 |
| Histopathologyb | AUD$104 | AUD$20,642 | AUD$2734 | AUD$17,908 | <0.01 |
| Surgical procedures on the skinc,d | AUD$259 | AUD$14,081 | AUD$879 | AUD$13,202 | <0.01 |
| Diagnostic biopsy of skin (30071)d | AUD$42 | AUD$4311 | AUD$401 | AUD$3911 | <0.01 |
| Anesthesia for procedures on the skin (20100)d | AUD$350 | AUD$1678 | AUD$17 | AUD$1660 | <0.01 |
| Pre-anesthesia brief consultation (17610) | AUD$73 | AUD$2522 | AUD$1278 | AUD$1244 | <0.01 |
| Premalignant skin lesions (30192)d | AUD$33 | AUD$1489 | AUD$286 | AUD$1203 | <0.01 |
| CT scan of chest, abdomen, and pelvis (56507) | AUD$474 | AUD$2134 | AUD$1138 | AUD$996 | <0.01 |
| Assistance at operation (51303) | AUD$373 | AUD$1901 | AUD$1062 | AUD$839 | <0.01 |
| Anesthesia for procedures on nose (20100)d | AUD$459 | AUD$896 | AUD$115 | AUD$781 | <0.01 |
| CT scan of upper abdomen and pelvis (56807) | AUD$560 | AUD$1904 | AUD$1176 | AUD$728 | 0.06 |
| Knee arthroscopy (49561) | AUD$1323 | AUD$1323 | AUD$595 | AUD$728 | 0.06 |
| Neoplastic skin lesions (30195)d | AUD$50 | AUD$777 | AUD$143 | AUD$634 | <0.01 |
| Dosimetry (15562) | AUD$1225 | AUD$796 | AUD$184 | AUD$613 | 0.03 |
| General serum chemistry 5 or more tests (66512) | AUD$17 | AUD$2299 | AUD$1688 | AUD$611 | <0.01 |
| Psoralen and ultraviolet A or B therapy (14050) | AUD$44 | AUD$387 | AUD$0 | AUD$387 | 0.01 |
| Malignant melanoma < 10 mm (31325) | AUD$247 | AUD$396 | AUD$25 | AUD$371 | <0.01 |
| CT with surgery (57341) | AUD$447 | AUD$536 | AUD$179 | AUD$358 | 0.01 |
| Review a GP management plan (732) | AUD$70 | AUD$1025 | AUD$676 | AUD$349 | <0.01 |
| CT of brain without contrast (56001) | AUD$206 | AUD$587 | AUD$247 | AUD$340 | <0.01 |
MBS: Medicare Benefits Scheme; KC: keratinocyte cancer; GP: general practitioner; CT: computed tomogragphy.
Service definitions by MBS item numbers.
Medical attendances (see Table 3).
Histopathology (see Table 3).
Other surgical procedures on skin (see Table 3).
Procedures potentially indicated to treat KC or consequence of KC.
Table 5 reports the percentages of cases and controls who received treatment for 17 prespecified comorbidities with unadjusted and two adjusted odds ratios. On crude (unadjusted) analysis, we found patients treated for KC were significantly more likely to also receive treatments for three cancers (melanoma, colorectal, and prostate), cardiovascular disease, hyperlipidemia, osteoporosis, rheumatoid arthritis, and tuberculosis. After controlling for socioeconomic factors correlated with KC (age, gender, education, employment, private health insurance, and born in Australia), significant associations persisted for osteoporosis, rheumatoid arthritis, colorectal cancer, prostate cancer, and melanoma retained a positive correlation with KC. Only hypertension had an adjusted odds ratio of <1.
Table 5.
Seventeen comorbid diseases for individuals treated for KC.
| Comorbidities | Patients with KC n (%) | Patients without KC n (%) | Unadjusted odds ratio (p-value) | Adjusted odds ratio* (p-value) |
|---|---|---|---|---|
| Cardiovascular disease | 778 (38.9) | 707 (35.35) | 1.16 (0.02) | 0.98 (0.81) |
| Lipidemiaa | 657 (32.85) | 407 (20.35) | 1.16 (0.03) | 1.01 (0.91) |
| Hypertensiona | 359 (17.95) | 364 (18.2) | 0.98 (0.84) | 0.76 (<0.01) |
| Depressiona | 311 (15.55) | 302 (15.1) | 1.04 (0.69) | 1.01 (0.91) |
| Osteoporosis | 364 (18.2) | 303 (15.15) | 1.25 (0.01) | 1.16 (0.1) |
| Rheumatoid arthritisa | 306 (15.3) | 239 (11.95) | 1.33 (<0.01) | 1.21 (0.06) |
| Asthmaa | 257 (12.85) | 261 (13.05) | 1.02 (0.85) | 0.92 (0.41) |
| Diabetesa | 202 (10.1) | 179 (8.95) | 1.14 (0.22) | 1.04 (0.75) |
| Colorectal cancer | 188 (9.4) | 146 (7.3) | 1.32 (0.02) | 1.26 (0.05) |
| Prostate cancer | 195 (9.75) | 132 (6.6) | 1.53 (<0.01) | 1.38 (0.01) |
| Breast cancer | 100 (5) | 91 (4.55) | 1.1 (0.51) | 1.08 (0.63) |
| Anxiety | 60 (3) | 67 (3.35) | 0.89 (0.53) | 0.83 (0.33) |
| Melanoma | 50 (2.5) | 8 (0.4) | 6.38 (<0.01) | 6.15 (<0.01) |
| Bronchitisa | 45 (2.25) | 40 (2) | 1.13 (0.58) | 1 (0.99) |
| Tuberculosis | 31 (1.55) | 19 (0.95) | 1.64 (0.09) | 1.58 (0.13) |
| Parkinson’s disease | 14 (0.7) | 8 (0.4) | 1.76 (0.21) | 1.55 (0.34) |
| Multiple sclerosis | 3 (0.15) | 5 (0.25) | 0.6 (0.48) | 0.55 (0.41) |
KC: keratinocyte cancer; GP: general practitioner.
The cases and controls were matched on gender and 5-year age groups.
Seven of the 10 most frequently managed problems by Australia’s GPs. The three nonincluded categories were Checkup all, immunization or vaccination, and prescription.
Odds ratios adjusted for age, gender, education, employment, private health insurance, and born in Australia.
Discussion
The average costs of all medical services utilized by individuals with and without KC were AUD$2756 and AUD$1436, respectively. The mean difference, AUD$1320, was equal to the cost of all additional medical treatments consumed by individuals in the KC cohort over a 12-month period. The average cost of a KC excision per patient was AUD$306. The difference, AUD$1014 (AUD$1320–AUD$306), was consumed on nonspecific medical treatments for KC (e.g. physician fees and histopathology) and correlated disease.
Individuals with KC had a lower socioeconomic status than people without a KC. They were less likely to be in full-time employment (38.8% vs 42.7%), more likely to be retired (30.5% vs 26.3%), and reported lower rates of private health insurance (27.7% vs 31.0%). The comparatively lower socioeconomic status may be a cause of much of the comorbidity we observed in this cohort. In addition to significant costs for medical attendances, other surgical procedures on the skin and histopathology, the league tables also indicate that individual treatments for cardiovascular disease and oncology contribute substantially.
Our analyses have sought to provide an overview of the costs of comorbid diseases that commonly affect patients with KC. While the correlation between melanoma and KC has been well documented,20,21 our analysis indicates that the costs of comorbid disease lie elsewhere. In our sample, only 2.5% of the patients treated for KC were also treated for melanoma (see Table 5), suggesting that the majority of comorbid costs were due to treatments for other conditions. After patients treated for melanoma were removed from the analysis, the average cost of comorbid disease attributable to KC was reduced from AUD$1320 (full sample) to AUD$1296 (AUD$2728–AUD$1432). The removal of osteoarthritis (AUD$1225), rheumatoid arthritis (AUD$1289), colorectal cancer (AUD$1285), and prostate cancer (AUD$1266) from the sample resulted in greater reductions in medical costs attributable to patients with KC.
While our results are broadly consistent with other published research, there are some important differences. A Danish population-based case–control study reported positive correlations between BCC and connective tissue disease, transplants, and lymphoma and between SCC and leukemia, lymphoma, and skin diseases.18 The unadjusted odds ratios indicated that KC was significantly correlated (p < 0.1) with rheumatoid arthritis and three cancers (colorectal, prostate, and melanoma). In addition, we found a positive association with cardiovascular disease, lipidemia, osteoporosis, and tuberculosis. Ong et al.20 analyzed the International Classification of Diseases-10 (ICD-10) codes in 8 million hospitalized patients in the United Kingdom in patients aged 45–69 years treated for KC. They reported statistically significant relative risks for melanoma (9.4), breast cancer (1.25), prostate cancer (1.21), colon cancer (1.30), and rectal cancer (2.59). The corresponding unadjusted odds ratios from our analysis were 6.38 (melanoma), 1.53 (prostate cancer), and 1.32 (colorectal cancer); however, the unadjusted odds ratio for breast cancer (1.1) did not reach statistical significance.
The adjusted odds ratios for cardiovascular disease, lipidemia, and tuberculosis were not statistically significant. The adjusted odds ratios for the three cancers (colorectal, prostate, and melanoma), osteoporosis, and rheumatoid arthritis remained >1. Notably, the adjusted odds ratio for hypertension was <1. This suggests that, perhaps, the association between KC and cardiovascular disease and lipidemia may be due to behavioral characteristics associated with socioeconomic status, for example, poor understanding of risk factors, whereas the association between KC and other cancers may instead be due to underlying biological factors. The adjusted odds ratio for antihypertensive medications (0.76) was unexpected and not easily explained.
Although we considered the possibility that KC might offer some “protective effect” against diseases mediated by ultraviolet (UV) light (e.g. second solid primary cancers27 and prostate cancer25), we found no evidence of a negative correlation between KC and treatments for these diseases. Research has confirmed that Australians increase their exposure to UV radiation due a concern about vitamin D deficiency.48 While the importance of vitamin D for bone health should not be ignored, these results suggest that public concern for vitamin D deficiency should not be allowed to undermine public health campaigns to minimize exposure to UV radiation.
The principal strength of our study was our capacity to access cost data obtained for a large population-based sample. These survey data were linked to medical and pharmaceutical item codes, which enabled the medical costs of individual treated for a KC to be analyzed. First, we were able to report on the cost of 41 medical services explicitly attributable to the management of KC. Second, coding algorithms were used to identify treatments for 17 other diseases, identified by the literature as potentially correlated with KC. While our list was not intended to be all-encompassing, we believe that it does include most of the likely possibilities. The 17 comorbidities that we identified include 8 of the 20 most frequently managed problems by Australia’s GPs.43
Our definition of KC did not include the growing number topical agents now available to treat KC. Because most topical agents used for KC treatment do not incur a Medicare rebate, we could not identify such instances in our cohort. This would only bias our estimates if patients used only topical agents to treat their cancers. In our sample, we could identify only 25 individuals who were prescribed imiquimod (PBS item numbers: 02546B, 04134N, and 04559Y), a topical treatment for KC. A cross tabulation indicated that 21 of these individuals had received other KC treatments, and only 4 were coded as having received no other KC treatment. Hence, the 41 selected MBS codes provide a workable definition of a KC treatment. While administrative data are potentially prone to misclassification errors, particularly for inferring diagnoses, it has been recently shown in these data that Medicare records for skin cancer treatments have very high validity when compared against histopathology reports.49 Therefore, we believe that misclassification of skin cancer history is very unlikely to have biased our estimates of treatment costs to any substantial extent.
The principal limitation of our analysis was an inability to differentiate between generic medical treatments used to manage KC and the treatment of any correlated diseases. Itemized costs directly related to treatment can only account for 23.2% of the additional AUD$1320 of medical services used. Ideally, interrogation of the medical record would enable these AUD$1014 of residual costs to be differentiated. However, with a large dataset, this method may be prohibitively costly, and where patients present with multiple complaints, some allocation overheads (e.g. attendance fees) would be required. Any future analysis, which could differentiate between these two classes of medical costs, would be beneficial, as it would help policy-makers to tailor preventive health-care messages and target the delivery of medical resource to this cohort.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for the study was received from the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (P1309), the Department of Health and the Queensland University of Technology.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Rowell received funding through a postdoctoral fellowship from the National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Sun and Health. Prof. Whiteman is supported by a Research Fellowship from the NHMRC. The QSkin study is supported by a Program Grant from the NHMRC (552429).
Informed consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Albert MR, Weinstock MA. Keratinocyte carcinoma. CA Cancer J Clin 2003; 53: 292–302. [DOI] [PubMed] [Google Scholar]
- 2. Chen JG, Fleischer AB, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg 2001; 27: 1035–1038. [DOI] [PubMed] [Google Scholar]
- 3. Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol 2003; 48: 425–429. [DOI] [PubMed] [Google Scholar]
- 4. Krueger H. The economic burden of skin cancer in Canada: current and projected. Final report, Canadian Partnership against Cancer, Toronto, ON, Canada, 26 February 2010. [Google Scholar]
- 5. Rogers HW, Coldiron BM. Analysis of skin cancer treatment and costs in the United States Medicare population, 1996–2008. Dermatol Surg 2013; 39: 35–42. [DOI] [PubMed] [Google Scholar]
- 6. Nilsson GH, Carlsson L, Dal H, et al. Skin diseases caused by ultraviolet radiation: the cost of illness. Int J Technol Assess Health Care 2003; 19: 724–730. [DOI] [PubMed] [Google Scholar]
- 7. Tinghog G, Carlsson P, Synnerstad I, et al. Societal cost of skin cancer in Sweden in 2005. Acta Derm Venereol 2008; 88: 467–473. [DOI] [PubMed] [Google Scholar]
- 8. Stang A, Stausberg J, Boedeker W, et al. Nationwide hospitalization costs of skin melanoma and non-melanoma skin cancer in Germany. J Eur Acad Dermatol Venereol 2008; 22: 65–72. [DOI] [PubMed] [Google Scholar]
- 9. Bentzen J, Kjellberg J, Thorgaard C, et al. Costs of illness for melanoma and nonmelanoma skin cancer in Denmark. Eur J Cancer Prev 2013; 22: 569–576. [DOI] [PubMed] [Google Scholar]
- 10. Fransen M, Karahalios A, Sharma N, et al. Non-melanoma skin cancer in Australia. Med J Aust 2012; 197: 565–568. [DOI] [PubMed] [Google Scholar]
- 11. O’Dea D. The costs of skin cancer to New Zealand. Wellington, New Zealand: The Cancer Society of New Zealand, 2009. [Google Scholar]
- 12. Souza RJ, Mattedi AP, Correa MP, et al. An estimate of the cost of treating non-melanoma skin cancer in the state of Sao Paulo, Brazil. An Bras Dermatol 2011; 86: 657–662. [DOI] [PubMed] [Google Scholar]
- 13. Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States. Arch Dermatol 2006; 2010(146): 283. [DOI] [PubMed] [Google Scholar]
- 14. Hayes RC, Leonfellner S, Pilgrim W, et al. Incidence of nonmelanoma skin cancer in New Brunswick, Canada, 1992 to 2001. J Cutan Med Surg 2006; 11: 45–52. [DOI] [PubMed] [Google Scholar]
- 15. Levi F, Te V, Randimbison L, et al. Trends in skin cancer incidence in Vaud: an update, 1976–1998. Eur J Cancer Prev 2001; 10: 371–373. [DOI] [PubMed] [Google Scholar]
- 16. Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 2008; 100: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisch M, Melbye M. New primary cancers after squamous cell skin cancer. Am J Epidemiol 1995; 141: 916–922. [DOI] [PubMed] [Google Scholar]
- 18. Jensen AØ, Olesen AB, Dethlefsen C, et al. Chronic diseases requiring hospitalization and risk of non-melanoma skin cancers—a population based study from Denmark. J Invest Dermatol 2007; 128: 926–931. [DOI] [PubMed] [Google Scholar]
- 19. Jensen AØ, Lamberg AL, Olesen AB. Epidemiology of non-melanoma skin cancer. In: Jemec GB, Kemény L, Miech D. (eds) Non-surgical treatment of keratinocyte skin cancer. Heidelberg: Springer, 2010; pp.15–24. [Google Scholar]
- 20. Ong ELH, Goldacre R, Hoang U, et al. Subsequent primary malignancies in patients with nonmelanoma skin cancer in England: a National Record-Linkage Study. Cancer Epidemiol Biomarkers Prev 2014; 23: 490–498. [DOI] [PubMed] [Google Scholar]
- 21. Cantwell M, Murray L, Catney D, et al. Second primary cancers in patients with skin cancer: a population-based study in Northern Ireland. Br J Cancer 2009; 100: 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wassberg C, Thörn M, Yuen J, et al. Second primary cancers in patients with squamous cell carcinoma of the skin: a population-based study in Sweden. Int J Cancer 1999; 80: 511–515. [DOI] [PubMed] [Google Scholar]
- 23. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012; 143: 390.e1–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 2007; 32: 210–216. [DOI] [PubMed] [Google Scholar]
- 25. De Vries E, Soerjomataram I, Houterman S, et al. Decreased risk of prostate cancer after skin cancer diagnosis: a protective role of ultraviolet radiation? Am J Epidemiol 2007; 165: 966–972. [DOI] [PubMed] [Google Scholar]
- 26. Grant WB, Garland CF, Holick MF. Comparisons of estimated economic burdens due to insufficient solar ultraviolet irradiance and vitamin D and excess solar UV irradiance for the United States. Photochem Photobiol 2005; 81: 1276–1286. [DOI] [PubMed] [Google Scholar]
- 27. Tuohimaa P, Pukkala E, Scélo G, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer 2007; 43: 1701–1712. [DOI] [PubMed] [Google Scholar]
- 28. Olsen CM, Green AC, Neale RE, et al. Cohort profile: The QSkin Sun and Health Study. Int J Epidemiol 2012; 41: 929–929-i. [DOI] [PubMed] [Google Scholar]
- 29. Morze CJ, Olsen CM, Perry SL, et al. Good test–retest reproducibility for an instrument to capture self-reported melanoma risk factors. J Clin Epidemiol 2012; 65: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 30. Souberbielle J-C, Body J-J, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 2010; 9: 709–715. [DOI] [PubMed] [Google Scholar]
- 31. Merlino LA, Curtis J, Mikuls TR, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum 2004; 50: 72–77. [DOI] [PubMed] [Google Scholar]
- 32. Van der Mei I. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 2007; 254: 581–590. [DOI] [PubMed] [Google Scholar]
- 33. Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab 2010; 95: 3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armstrong D, Meenagh G, Bickle I, et al. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol 2007; 26: 551–554. [DOI] [PubMed] [Google Scholar]
- 35. Scragg R, Holdaway I, Jackson R, et al. Plasma 25-hydroxyvitamin D and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol 1992; 2: 697–703. [DOI] [PubMed] [Google Scholar]
- 36. Chuang TY, Lewis D, Spandau D. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Br J Dermatol 2005; 153: 552–557. [DOI] [PubMed] [Google Scholar]
- 37. Jhappan C, Noonan FP, Merlino G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene 2003; 22: 3099–3112. [DOI] [PubMed] [Google Scholar]
- 38. Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2010; 128: 1414–1424. [DOI] [PubMed] [Google Scholar]
- 39. Avenell A, Gillespie W, Gillespie L, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. ACP J Club 2006; 144: 14. [DOI] [PubMed] [Google Scholar]
- 40. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 41. Knekt P, Kilkkinen A, Rissanen H, et al. Serum vitamin D and the risk of Parkinson disease. Arch Neurol 2010; 67: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 2008; 37: 113–119. [DOI] [PubMed] [Google Scholar]
- 43. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2010–11. General practice series no. 29, November 2011. Sydney, NSW, Australia: Sydney University Press. [Google Scholar]
- 44. Porter R, Kaplan J. The Merck manual online. http://www.merck.com/mmpe/index.html (accessed 1 June 2013).
- 45. Australian Government Department of Health, MBS Online Medical Benefits Schedule. http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Medicare-Benefits-Schedule-MBS-1 (accessed 1 June 2013).
- 46. Australian Government Department of Health, The Pharmaceutical Benefits Scheme. http://www.pbs.gov.au/pbs/home (accessed 1 June 2013).
- 47. Rowell D, Gordon LG, Olsen CM, et al. A reconstruction of a medical history from administrative data: with an application to the cost of skin cancer. Health Econ Rev 2015; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dobbinson SJ, Volkov A. 2010–11 National Sun Protection Survey: report 2. Australians’ sun protective behaviours and sunburn incidence on summer weekends, 2010–11 and comparison with 2003–04 and 2006–07 (unpublished).Melbourne, VIC, Australia: Centre for Behavioural Research in Cancer, Cancer Council Victoria, 2010. [Google Scholar]
- 49. Thompson BS, Olsen CM, Subramaniam P, et al. Medicare claims data reliably identify treatments for basal cell carcinoma and squamous cell carcinoma: a prospective cohort study. Aust N Z J Public Health. Epub ahead of print 11 November 2015. DOI: 10.1111/1753-6405.12478. [DOI] [PubMed] [Google Scholar]


