Abstract
In reptiles, habitat selection is the process whereby suitable habitat is selected that optimizes physiological functions and behavioral performance. Here, we used the brown forest skink (Sphenomorphus indicus) as a model animal and examined whether the frequency of active individuals, environmental temperature, illumination of activity area, and habitat type vary with different age classes. We surveyed the number of active individuals and measured environmental variables at Baiyunshan Mountain in Lishui, Zhejiang, China. We found no difference in the activity frequency of adult and juvenile S. indicus; the activity pattern of active individuals was bimodal. The mean environmental temperature selected by adults was higher than that selected by juveniles. The environmental temperature of active areas measured at 0900-1000 h and 1100-1200h was higher than at 1400-1500h; illumination of the active area at 1000-1200h was also higher than at 1400h-1600 h. The number of active individuals, the environmental temperature and illumination of activity areas showed pairwise positive correlation. There was a difference in habitat type between juveniles and adults whereby juveniles prefer rock habitats. We predict that active S. indicus select optimal habitats with different environmental temperatures and types to reach the physiological needs particular to their age classes.
Keywords: Sphenomorphus indicus, Age-related selection difference, Habitat selection, Temperature, Illumination, Habitat type, Activity frequency
INTRODUCTION
Habitat selection is the process of animals choosing optimal habitat (Buckland et al, 2014; Johnson, 1980). Studies on habitat selection can help us understand animal behavior, population dynamics, propagation, survival and wildlife conservation (Buckland et al, 2014; Manly et al, 2002; Strickland & McDonald, 2006). Because habitat selection variation occurs with spatial dimensional change, we can acquire information about habitat selection by examining animal distributions at specific spatial scales (De La Cruz et al, 2014; Hódar et al, 2000; Oppel et al, 2004). Studies on habitat selection have mainly focused on the three levels of population, home range and microhabitat (Thomas & Taylor, 1990). However, studies at different levels are important in determining the factors vital to habitat utilization and effective conversation measures (Beasley et al, 2007; Buckland et al, 2014; Razgour et al, 2011).
Habitat selection in most ectotherms is determined by specific thermal preferences, social interactions, predator avoidance and other factors (Angilletta et al, 2002; Dochtermann et al, 2012; Downes & Shine, 1998; Hall et al, 1992). Ectotherms are able to optimize their physiological and functional performance through habitat selection, and studies at the microhabitat level are important in understanding the mechanisms of selection. As a representative species and ectothermic group, reptiles not only need to avoid predators during activity, but more importantly, their behavior and physiological capabilities are significantly affected by temperature. It is vital that reptiles find suitable heterogeneous thermal environments to improve fitness (Angilletta et al, 2002; Castilla et al, 1999; Huey, 1982; Webb, 1996). During microhabitat selction, the activities of a reptile are under the influence of other repetiles or other species within the environment (Manicom & Schwarzkopf, 2011; Stamps & Tanaka, 1981; Wasko & Sasa, 2012). Sometimes, competition over environmental resources occurs (e.g., food, thermal resources) (Amarasekare & Coutinho, 2014; Marler et al, 1995; Hart & Marshall, 2012) and differences in intraspecies or interspecies habitat selection will follow.
The brown forest skink (Sphenomorphus indicus) is a member of the family Scincidae and mainly distributed across sourthen China in dark and wet grass, rock piles or cracks in cliffs (Huang, 1999). They are active away from their caves from early April to late October, and are more likely to be observed in shady areas during the morning and afternoon, and occasionally at noon. Here, we used line transects to investigate the quantity and type of habitat used by S. indicus in hilly road areas of Baiyunshan Mountain, Lishui, China. Environmental temperatures and illumination intensity were measured to explore characteristics and underlying mechanisms of habitat selection by S. indicus of different ages. Our results will aid the conservation of wild populations of this species.
MATERIALS AND METHODS
Experimental design
From May to early July 2014 we used line transect methods to investigate S. indicus and their habitat at Baiyunshan Mountain, Lishui, Zhejiang, China (N27°49′17.50′′, E120°32′14.89′′). The full length of the line transect was 4 km, and the altitude at the start and end points was 140 m and 360 m, respectively. During the survey observers proceeded at an even pace along hilly road. Active individuals sighted within 2 m of the road along the cliff (width=2 m) and environmental parameters of habitat were recorded. Seventeen surveys were randomly carried out during 0900h-1230h (10 surveys) and 1230h-1600h (7 surveys) over 6 days. The weather and temperature during surveys are shown in Table 1. Active individuals were determined as adults (SVL> 65 mm) or juveniles (SVL<65 mm) (Ji & Du, 2000) by visually estimating body size. The snout-vent length (SVL) of randomly captured individuals was measured by digital square caliper (0.01 mm, Mitutoyo, Japan) and the accuracy of visual estimation was verified. Before being released to the original capture area, the 1st toe on the left hind legs was clipped in individuals to avoid repeated sampling. Because sex can not be determined by visual estimation, gender-related differences were not considered here. The habitat of observed S. indicus was determined whereby grass or leaf litter layer was categorized as grass habitat and bare rock or ditch was categorized as rock habitat. An infrared radiation thermometer (UT301A, Uni-T, China) was used to measure the temperature of the spot where a skink was located, defined by a circle with a 10 cm diameter around the animal; the environmental temperature of this habitat was the average value of 10 measurements. A sensitive probe was planted at the center of this circle to measure illumination intensity (lux).
Table 1.
Weather conditions during field surveys
| Date | Time | Weather | Temperature (ºC) |
|---|---|---|---|
| 16/05 | 1230h-1600h | Sunny | 22.8 (20.7-26.5) |
| 21/05 | 0900h-1230h | Sunny | 24.1 (17.1-31.8) |
| 24/05 | 0900h-1230h | Cloudy | 24.8 (20.4-31) |
| 25/05 | 0900h-1230h | Sunny | 26.4 (23.2-32) |
| 27/05 | 0900h-1600h | Overcast to cloudy | 24.1 (22.3-29.4) |
| 01/06 | 0900h-1600h | Sunny | 26.6 (22.7-32.3) |
| 07/06 | 0900h-1600h | Overcast to cloudy | 26.9 (22.0-34.3) |
| 25/06 | 0900h-1600h | Overcast to sunny | 25.4 (20.8-32) |
| 06/07 | 0900h-1230h | Showers to cloudy | 29.6 (26.0-34.0) |
| 07/07 | 0900h-1600h | Showers to cloudy | 26.4 (26.0-36.0) |
| 09/07 | 0900h-1600h | Cloudy to sunny | 29.6 (26.0-36.0) |
Statistical analysis
Data were analyzed using Statistica (v6.0). The normality and homogeneity of variance of all data were checked by Kolmogorov-Simirnov tests and Bartlet tests, respectively; no parameters required transformation before statistical analyses. The percentage of active S. indicus during a survey was equal to the numbers of observed active S. indicus during a survey divided by the total numbers of observed S. indicus during a survey, multiplied by 100. Contingency tables and G-tests were used to analyze age-related activity patterns and habitat type, and activity frequencies during each time period. Two-factor ANOVA was used to determine the effects of age and survey time on environmental temperature and illumination intensity of the active area. One-factor ANOVA was used to determine the effects of age-related individual differences and habitat type on environmental temperature and illumination intensity of the active area. Partial correlation was used to check correlations between numbers of active individuals during a survey period, environmental temperature and illumination intensity of the active area. Group differences were compared using Tukey’s post hoc test. All data were expressed as mean±SE and statistical significance was taken at α=0.05.
RESULTS
We collected 404 S. indicus (211 juveniles and 193 adults), and acquired parameters for habitat type, environmental temperature and illumination intensity in active areas. Body size was measured in 96 individuals (31 juveniles and 65 adults). The SVL of adults (74.7± 0.6 mm, 65.8-91.3 mm) was longer than that of juveniles (60.5±0.7 mm, 46.0-64.7 mm) (F1, 94=205.82, P<0.001) and no overlap was found between the SVL ranges of adults and juveniles. The activity patterns of adults and juveniles during each survey period were comparable (G=9.04, df=6, P=0.171) (Figure 1A). However, when data from adults and juveniles were grouped together, differences in the proportions of active individuals during each survey period were found (G=178.22, df=6, P<0.001). For example, two activity peaks occurred at 1000-1200 h and 1300-1500 h, and individuals were more active during 1000-1200 h (Figure 1B).
Figure 1.
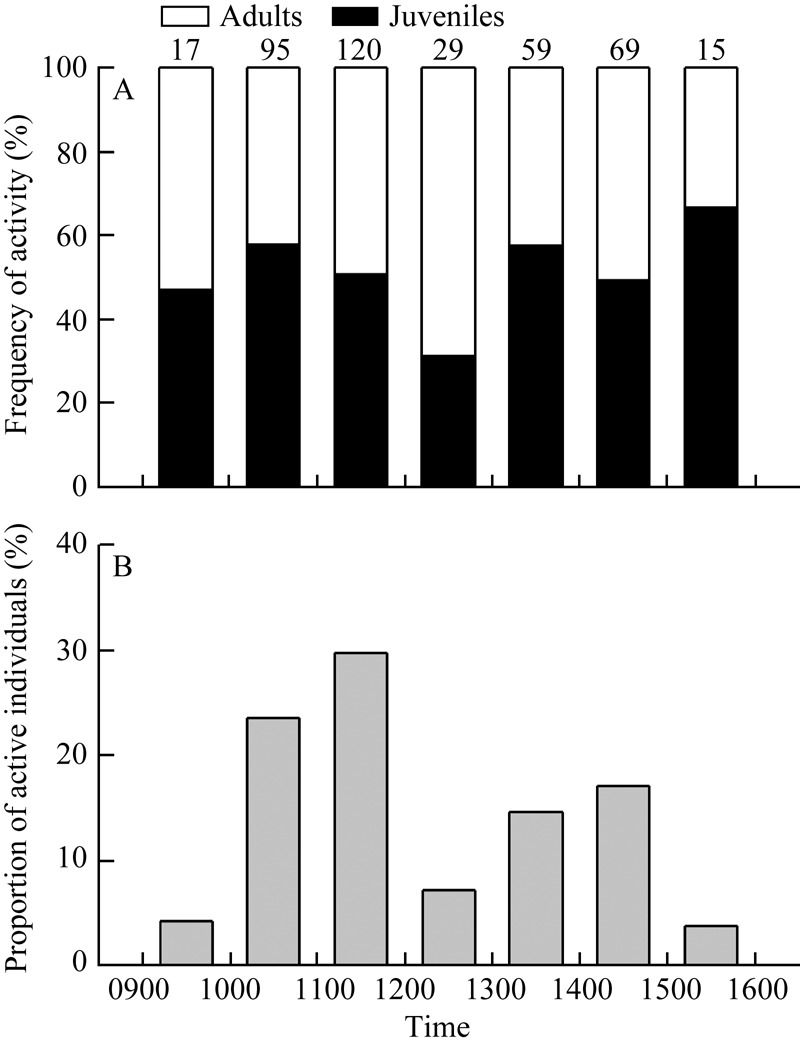
Activity frequency of juvenile and adult Sphenomorphus indicus (A) and proportion of active individuals at survey times (B) Numbers above bars indicate sample size for each time.
Both age and survey time influenced the environmental temperature of areas of activity in S. indicus, but the age × urvey time interaction did not. The environmental temperature of areas of adult activity (24.8±0.2 °C) was higher than for juveniles (24.1±0.2 °C) (Tukey’s test, P<0.001). The environmental temperatures during 0900-1000 h and 1100-1200 h were both higher that during 1400-1500 h (Tukey’s test, both P<0.03); no differences were found across other survey periods (Tukey’s test, all P>0.05).
Survey time influenced illumination intensity of the areas frequented by active S. indicus, but age and the age× survey time interaction did not. The illumination intensity during 1000-1200 h was higher than during 1400-1600 h (Tukey’s test, all P<0.04); no differences were found among other survey periods (Tukey’s test, all P>0.05) (Figure 2, Table 2). During each survey period, the number of active individuals, environmental temperature and illumination intensity of the active area were positively correlated (all P<0.04).
Figure 2.
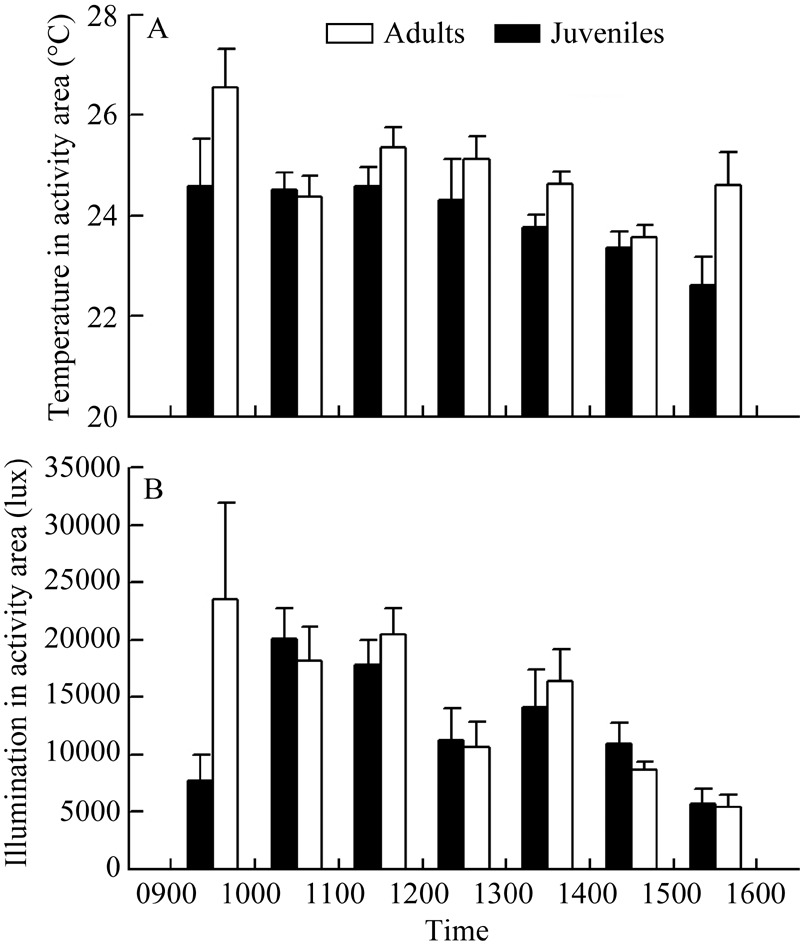
Environmental temperature (A) and illumination intensity (B) of areas where Sphenomorphus indicus are active over different survey times
Table 2.
Statistical results of environmental temperature and illumination in areas where Sphenomorphus indicus are active
| Environmental temperature | Illumination intensity | |||||
|---|---|---|---|---|---|---|
| F | df | P | F | df | P | |
| Age | 3.88 | 1, 390 | <0.001 | 1.10 | 1, 390 | 0.295 |
| Measurement time | 8.00 | 6, 390 | <0.01 | 4.58 | 6, 390 | <0.001 |
| Interaction | 0.92 | 6, 390 | 0.480 | 0.97 | 6, 390 | 0.448 |
Habitat type in the activity areas did not affect either environmental temperature (F1,402=0.05, P=0.829) or illumination intensity (F1,402=2.49, P=0.115). Habitat selection between adults and juveniles was different (G=4.70, df=1, P<0.04) (Figure 3).
Figure 3.
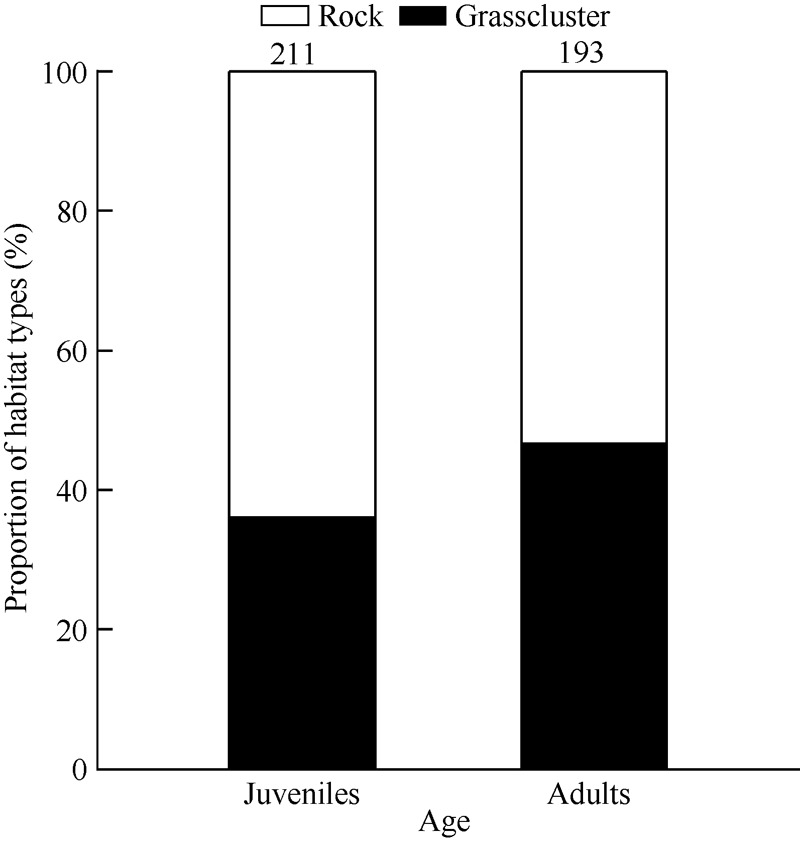
Proportion of habitat types selected by active juvenile and adult Sphenomorphus indicus
DISCUSSION
The activity patterns of reptiles is affected by many biotic and abiotic factors (Cloudsley-Thompson, 1961; Oishi et al, 2004), including age-related energy requirements among different individuals (Liu et al, 2008; Mautz & Nagy, 1987; Oishi et al, 2004; Paulissen, 1987; ). For example, the daily activity of common lizard (Lacerta vivipara) in juveniles is started later and shorter than in adults (Liu et al, 2008). Here, activity patterns were similar between adult and juvenile S. indicus (Figure 1A), indicating that because the habitat of S. indicus is relatively shady, wet and closed with high food abundance, skinks are less likely to compete with each other over food. It appears that niche separation over activity time distribution is not induced among individuals of different ages in this species and this is consistent with other studies on diurnal activity rhythms in other diurnal lizards (Liang et al, 2006; Liu et al, 2008; Wen & Zou, 2002;). Activity rhythms with double peaks were found in S. indicus during summer. The highest percentage of active individuals occurred during 1000-1200 h, and this decreased during 1200-1300 h and increased again during 1300-1500 h (Figure 1B). Differences between our results and those reported by Wang (1964) on S. indicus in Hangzhou are probably the result of local weather and geographic conditions.
As typical ectotherms, the biological function and behavioral performance of reptiles are significantly influenced by the thermal environment (Angilletta et al, 2002; Angilletta, 2009; Shu et al, 2010; Vermunt et al, 2014). Extreme temperatures can be harmful to individuals and good fitness requires a suitable temperature range (Clusella-Trullas & Chown, 2014; Shen et al, 2013). We found that the mean environmental temperature of the active areas frequented by adult S. indicus was 0.7 °C higher than the areas used by juveniles (Figure 2); a similar phenomenon has been observed in other thermal biology studies on the Iberian wall lizard (Podarcis hispanica atrata), the Mongolia racerunner (Eremias argus) and the multi-ocellated racerunner (Eremias multiocellata) (Castilla & Bauwens, 1991; Tang et al, 2013; Xu & Ji, 2006). These age-related differences in body temperature and environmental temperature of habitat may be correlated with body size because a small body size is usually characterized by higher thermo-conductivity but weaker heat storage capacity (Castilla & Bauwens, 1991; Tang et al, 2013; Xu & Ji, 2006). Moreover, the differences in feeding activity and predator avoidance during development may induce variation in thermal environments (Christian & Bedford, 1995; Hertz et al., 1993). The similar illumination intensities among habitats of individuals at different ages (Figure 2) indicate that illumination intensity does not affect habitat selection. However, temporal rhythms were found in both adult and juvenile S. indicus, i.e., the environmental temperature and illumination intensity of the active areas were both higher in mornings (0900-1200 h) than afternoons (1400-1600 h) (Figure 2), indicating that the rhythms of S. indicus choosing environmental factors are consistent with diurnal activity patterns. Positive correlations among the number of active individuals, environmental temperature and illumination intensity also indicate that the activity rhythms of S. indicus are interwoven with environmental thermal and illuminative factors.
Reptiles choose optimal habitats to obtain more thermal resources and a better chance of obtaining food (Wang, 1964; Strickland & McDonald, 2006; Buckland et al, 2014). Our study found that juvenile S. indicus preferred rock habitats (64%) during diurnal activity (Figure 3), consistent with Wang (1964). However, neither the environmental temperature nor illumination intensity differed between rock habitat and grass habitat, suggesting that the reason juveniles choose rock habitat is not based on acquiring more heat and illumination. The optimal physiological function and behavioral performance of lizards changes during development (Tang et al, 2013; Xu & Ji, 2006), therefore, lizards at different ages should choose different habitats to meet their physiological requirements and avoid predators (Irschick et al, 2000). For example, two species of anole lizards (Anolis lineatopus and Anolis gundlachi) in India choose habitats located in low and narrow areas to avoid predators (Irschick et al, 2000). Because the dark brown body color of S. indicus (Huang, 1999) is close to the color of rocks, we assume that juveniles prefer moving on rocks so as to avoid predators. We therefore predict that active S. indicus select optimal habitats with different environmental temperatures and types to reach the physiological needs particular to their age classes.
ACKNOWLEDGEMENTS
We are grateful to Shi-Liang QIN, Yu YU, Xiao-Wei WANG, Jian-Yang XIE, Li-Shuang ZHANG, Qiong-Lu ZHANG, Xue-Ya ZHANG, Xiao-Ning ZHOU for their help during this research.
Funding Statement
This work was supported by the Open Research Fund program of Laboratory of Lishui University (2014-26-10); the Scientific Research Foundation of Ph.D.in Lishui University (QD1301);the Science and Technology Planning Project of Lishui (20110426) and the Project of Summer Work for Undergraduates in Lishui University (2014-245-23)
Footnotes
The authors have declared that no competing interests exist.
Contributor Information
Qi-Ping ZHU, College of Ecology, Lishui University, Lishui 323000, China.
Meng-Yao ZHU, College of Ecology, Lishui University, Lishui 323000, China.
Ying-Chao HU, College of Ecology, Lishui University, Lishui 323000, China.
Xue-Ya ZHANG, College of Ecology, Lishui University, Lishui 323000, China.
Guo-Hua DING, College of Ecology, Lishui University, Lishui 323000, China.
Zhi-Hua LIN, College of Ecology, Lishui University, Lishui 323000, China.
REFERENCES
- [1].Amarasekare P, Coutinho RM.2014. Effects of temperature on intraspecific competition in ectotherms.American Naturalist, 184(3): E50-E65. [DOI] [PubMed] [Google Scholar]
- [2].Angilletta MJ Jr. 2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press. [Google Scholar]
- [3].Angilletta MJ Jr, Niewiarowski PH, Navas CA.2002. The evolution of thermal physiology in ectotherms.Journal of Thermal Biology, 27(4): 249-268. [Google Scholar]
- [4].Beasley JC, Devault TL, Retamosa MI, Rhodes OE.2007. A hierarchical analysis of habitat selection by raccoons in northern Indiana.Journal of Wildlife Management, 71(4): 1125-1133. [Google Scholar]
- [5].Buckland S, Cole N C, Godsall B, Rodríguez-Pérez JR, Gallagher LE, Henshaw SM, Harris S.2014. Habitat selection of the Mauritian lowland forest day gecko at multiple spatial scales: a baseline for translocation.Global Ecology and Conservation, 1: 71-79, doi: 10.1016/j.gecco.2014.06.001. [Google Scholar]
- [6].Castilla AM, Bauwens D.1991. Thermal Biology, microhabitat selection, and conservation of the insular lizard Podarcis hispanica atrata.Oecologia, 85(3): 366-374. [DOI] [PubMed] [Google Scholar]
- [7].Castilla AM, Van Damme R, Bauwens D.1999. Field body temperatures, mechanisms of thermoregulation and evolution of thermal characteristics in Lacertid lizards.Natura Croatica, 8(3): 253-274. [Google Scholar]
- [8].Christian KA, Bedford GS.1995. Seasonal changes in thermoregulation by the frillneck lizard, Chlamydosaurus kingii, in tropical Australia.Ecology, 76(1): 124-132. [Google Scholar]
- [9].Cloudsley-Thompson JL.1961. Rhythmic Activity in Animal Physiology and Behaviour. New York: Academic Press. [Google Scholar]
- [10].Clusella-Trullas S, Chown SL.2014. Lizard thermal trait variation at multiple scales: a review.Journal of Comparative Physiology B, 184(1): 5-21. [DOI] [PubMed] [Google Scholar]
- [11].De La Cruz SEW, Eadie JM, Miles AK, Yee J, Spragens KA, Palm EC, Takekawa JY.2014. Resource selection and space use by sea ducks during the non-breeding season: implications for habitat conservation planning in urbanized estuaries.Biological Conservation, 169: 68-78. [Google Scholar]
- [12].Dochtermann NA, Jenkins SH, Swartz MJ, Hargett AC.2012. The roles of competition and environmental heterogeneity in the maintenance of behavioral variation and covariation.Ecology, 93(6): 1330-1339. [DOI] [PubMed] [Google Scholar]
- [13].Downes S, Shine R.1998. Heat, safety or solitude? Using habitat selection experiments to identify a lizard’s priorities.Animal Behaviour, 55(5): 1387-1396. [DOI] [PubMed] [Google Scholar]
- [14].Hall CAS, Stanford JA, Hauer FR.1992. The distribution and abundance of organisms as a consequence of energy balances along multiple environmental gradients.Oikos, 65(3): 377-390. [Google Scholar]
- [15].Hart SP, Marshall DJ.2012. Advantages and disadvantages of interference-competitive ability and resource-use efficiency when invading established communities.Oikos, 121(3): 396-402. [Google Scholar]
- [16].Hertz PE, Huey RB, Stevenson R.1993. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question.The American Naturalist, 142(5): 796-818. [DOI] [PubMed] [Google Scholar]
- [17].Hódar JA, Pleguezuelos JM, Poveda JC.2000. Habitat selection of the common chameleon (Chamaeleo chamaeleon) (L.) in an area under development in southern Spain: implications for conservation.Biological Conservation, 94(1): 63-68. [Google Scholar]
- [18].Huang QY. 1999. Sphenomorphus indicus (Gray, 1853). In: Zhao EM, Zhao KT, Zhou KY. Fauna Sinica, Reptile Vol. 2, Squamata, Lacertilia. Beijing: Science Press, 340-349. (in Chinese) [Google Scholar]
- [19].Huey RB.1982. Temperature, physiology, and the ecology of reptiles. In: Gans C C, Pough FH. Biology of the Reptilia, Vol. 12. New York: Academic Press, 25-91. [Google Scholar]
- [20].Irschick DJ, Macrini TE, Koruba S, Forman J.2000. Ontogenetic differences in morphology, habitat use, behavior, and sprinting capacity in two West Indian Anolis lizards.Journal of Herpetology, 34(3): 444-451. [Google Scholar]
- [21].Ji X, Du WG.2000. Sexual dimorphism in body size and head size and female reproduction in a viviparous skink, Sphenomorphus indicus.Zoological Research, 21(5): 349-354. (in Chinese) [Google Scholar]
- [22].Johnson DH.1980. The comparison of usage and availability measurements for evaluating resource preference.Ecology, 61(1): 65-71. [Google Scholar]
- [23].Liang WB, Zhang YX, Su P, Long Q, Huang JQ.2006. Observation on time budget of Shinisaurus crocodilurus in captivity.Sichuan Journal of Zoology, 25(2): 264-266. (in Chinese) [Google Scholar]
- [24].Liu P, Liu ZT, Li DW, Zhao WG.2008. Diurnal activity rhythm and time budget of Lacerta vivipara in simulated habitat.Chinese Journal of Ecology, 27(12): 2146-2152. (in Chinese) [Google Scholar]
- [25].Manicom C, Schwarzkopf L.2011. Diet and prey selection of sympatric tropical skinks.Austral Ecology, 36(5): 485-496. [Google Scholar]
- [26].Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP.2002. Resource Selection by Animals: Statistical Design and Analysis for Field Studies. 2nd ed Netherlands: Kluwer Academic Publishers. [Google Scholar]
- [27].Marler CA, Walsberg G, White ML, Moore M.1995. Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation.Behavioral Ecology and Sociobiology, 37(4): 225-231. [Google Scholar]
- [28].Mautz WJ, Nagy KA.1987. Ontogenetic changes in diet, field metabolic rate, and water flux in the herbivorous lizard Dipsosaurus dorsalis.Physiological Zoology, 60(6): 640-658. [Google Scholar]
- [29].Oishi T, Nagai K, Harada Y, Naruse M, Ohtani M, Kawano E, Tamotsu S.2004. Circadian rhythms in amphibians and reptiles: ecological implications.Biological Rhythm Research, 35(1-2): 105-120. [Google Scholar]
- [30].Oppel S, Schaefer HM, Schmidt V, Schröder B.2004. Habitat selection by the pale-headed brush-finch (Atlapetes pallidiceps) in southern Ecuador: implications for conservation.Biological Conservation, 118(1): 33-40. [Google Scholar]
- [31].Paulissen MA.1987. Optimal foraging and intraspecific diet differences in the lizard Cnemidophorus sexlineatus.Oecologia, 71(3): 439-446. [DOI] [PubMed] [Google Scholar]
- [32].Razgour O, Hanmer J, Jones G.2011. Using multi-scale modelling to predict habitat suitability for species of conservation concern: the grey long-eared bat as a case study.Biological Conservation, 144(12): 2922-2930. [Google Scholar]
- [33].Shen JW, Meng FW, Zhang YP, Du WG.2013. Field body temperature and thermal preference of the big-headed turtle Platysternon megacephalum.Current Zoology, 59(5): 626-632. [Google Scholar]
- [34].Shu L, Zhang QL, Qu YF, Ji X.2010. Thermal tolerance, selected body temperature and thermal dependence of food assimilation and locomotor performance in the Qinghai toad headed lizard, Phrynocephalus vlangalii.Acta Ecologica Sinica, 30(8): 2036-2042. (in Chinese) [Google Scholar]
- [35].Stamps JA, Tanaka S.1981. The relationship between food and social behavior in juvenile lizards.Copeia, 1981(2): 422-434. [Google Scholar]
- [36].Strickland MD, McDonald LL.2006. Introduction to the special section on resource selection.Journal of Wildlife Management, 70(2): 321-323. [Google Scholar]
- [37].Tang XL, Yue F, He JZ, Wang NB, Ma M, Mo JR, Chen Q.2013. Ontogenetic and sexual differences of thermal biology and locomotor performance in a lacertid lizard, Eremias multiocellata.Zoology, 116(6): 331-335. [DOI] [PubMed] [Google Scholar]
- [38].Thomas DL, Taylor EJ.1990. Study designs and tests for comparing resource use and availability.Journal of Wildlife Management, 54(2): 322-330. [Google Scholar]
- [39].Vermunt A, Hare KM, Besson AA.2014. Unusual change in activity pattern at cool temperature in a reptile (Sphenodon punctatus).Journal of Thermal Biology, 42: 40-45. [DOI] [PubMed] [Google Scholar]
- [40].Wang PC.1964. Ecology of four lizards in Hangzhou (1): distribution, activity rhythm and food habits.Chinese Journal of Zoology, 6(2): 70-76. (in Chinese) [Google Scholar]
- [41].Wasko DK, Sasa M.2012. Food resources influence spatial ecology, habitat selection, and foraging behavior in an ambush-hunting snake (Viperidae: Bothrops asper): an experimental study.Zoology, 115(3): 179-187. [DOI] [PubMed] [Google Scholar]
- [42].Webb J.1996. Ecology of A Threatened Snake Species, Hoplocephalus bungaroides (Elapidae). Ph. D. thesis, University of Sydney, Sydney. [Google Scholar]
- [43].Wen CY, Zou PZ.2002. Preliminary studies on population ecology and diet of Eumeces chinensis in the north of Guangdong province.Journal of Guangzhou University(Natural Science Edition), 1(3): 19-22. (in Chinese) [Google Scholar]
- [44].Xu XF, Ji X.2006. Ontogenetic shifts in thermal tolerance, selected body temperature and thermal dependence of food assimilation and locomotor performance in a lacertid lizard, Eremias brenchleyi.Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 143(1): 118-124. [DOI] [PubMed] [Google Scholar]


