Abstract
Aims
Exercise limitation is common post-Fontan. Hybrid X-ray and magnetic resonance imaging (XMR) catheterization allows haemodynamic assessment by means of measurement of ventricular volumes and flow in major vessels with simultaneous invasive pressures. We aim to assess haemodynamic response to stress in patients with hypoplastic left heart syndrome (HLHS) post-Fontan.
Methods and results
Prospective study of 13 symptomatic children (NHYA 2) with HLHS post-Fontan using XMR catheterization. Three conditions were applied: baseline (Stage 1), dobutamine at 10 µg/kg/min (Stage 2), and dobutamine at 20 µg/kg/min (Stage 3). Seven consecutive patients received inhaled nitric oxide (iNO) at peak stress. Control MRI data were from normal healthy adults. In the HLHS patients, baseline mean pulmonary vascular resistance (PVR) was 1.51 ± 0.59 WU m2 and aortopulmonary collateral flow was 17.7 ± 13.6% of systemic cardiac output. Mean right ventricular end-diastolic pressure was 6.7 ± 2.5 mmHg which did not rise with stress. Cardiac index (CI) increased at Stage 2 in HLHS (40%) and controls (61%) but continued to increase at Stage 3 only in controls (19%) but not in HLHS. The blunted rise in CI in HLHS was due to a continuing fall in end-diastolic volume throughout stress, with no significant change in PVR or CI at peak stress in response to iNO.
Conclusion
Cardiac output post-Fontan in HLHS at peak stress is blunted due to a limitation in preload which is not responsive to inhaled pulmonary vasodilators in the setting of normal PVR.
Keywords: Fontan, dobutamine, MRI, hypoplastic left heart syndrome, pulmonary vascular resistance
Introduction
Hypoplastic left heart syndrome (HLHS) accounts for 2–3% of all forms of congenital heart disease1 and is uniformly fatal unless patients undergo staged surgical palliation leading to a Fontan. The Fontan circulation, however, fails over time, and patients have a burden of morbidity and reduced life expectancy.2–4 A predominant symptom is effort intolerance which matches the reduced exercise capacity measured in children post-Fontan compared with healthy peers,5–8 with a gradual decline with age.2–4
Routine assessment of these patients under resting conditions using magnetic resonance imaging (MRI) or cardiac catheterization may fail to fully disclose haemodynamic issues within the circulation.9–11 Dobutamine stress MRI studies have been successfully performed in children with a good safety profile at low doses.11–15 Hybrid X-ray and MRI catheterization (XMR) catheterization provides accurate anatomical and haemodynamic assessment of the Fontan pathways, ventricular pressures, and calculation of vascular resistance in a single procedure.16,17 We prospectively applied a pharmacological stress protocol using high-dose dobutamine during XMR catheterization to simultaneously evaluate the anatomy and haemodynamics of symptomatic patients post-Fontan.
We hypothesized that preload augmentation during stress is limited in HLHS post-Fontan and that reduction in pulmonary vascular resistance would result in an augmentation in cardiac output.
Methods
Study population
All patients with HLHS post-Fontan procedure referred for clinical evaluation with MRI catheterization due to effort intolerance (NYHA ≥ 2) were eligible for inclusion. These patients had undergone a staged approach to surgical palliation involving the classical Norwood procedure, Hemifontan, and total cavopulmonary connection with a fenestrated lateral tunnel. All procedures had been performed by two surgeons and employed similar surgical techniques. Specific exclusion criteria consisted of (i) patient or parental refusal to participate in the study, (ii) arrhythmias, (iii) end-stage renal failure, or (iv) patients with ferromagnetic implants who cannot undergo MRI. To serve as controls, healthy adult subjects were recruited for MRI assessment of ventricular function in response to high-dose dobutamine without invasive studies. Studies in controls were performed without anaesthesia.
XMR catheterization
All HLHS patients underwent XMR catheterization in a specifically designed suite with combined MRI (1.5T Philips Achieva) and X-ray facilities (BV Pulsera cardiac X-ray unit, Philips Medical Systems, Best, The Netherlands) as previously described.18 All studies were performed under general anaesthesia (GA) employing inhaled sevofluorane and a low-dose remifentanyl infusion.
The fraction of inspired oxygen (FiO2) was maintained at 0.3 and arterial pCO2 maintained at 4.0–4.5 kPa. All patients received a 20–30 mL/kg fluid bolus prior to catheterization. β-Blockers and ACE inhibitors were omitted 24 h prior to the study.
Femoral arterial and femoral venous or internal jugular venous puncture was followed with 4–6 Fr short vascular sheaths. Standard anticoagulation was administered. During XMR catheterization, a full diagnostic catheter study was performed at baseline with catheters positioned using guidewires and X-Ray guidance where necessary. Invasive haemodynamic pressures were recorded on a haemodynamic tracer system (EP Tracer 102, CardioTek B.V, Maastricht, The Netherlands) at end expiration without positive end expiratory pressure. Pressures were measured in the inferior vena cava (IVC), lateral tunnel, superior vena cava (SVC), right pulmonary artery (RPA), left pulmonary artery (LPA), LA, or PCW, systemic right ventricle, ascending aorta, and descending aorta. The transpulmonary gradient was the difference between mean pressures in the PA and LA or PCW pressure.
Once diagnostic catheterization was completed, MRI compatible catheters (Wedge catheter, Arrow, Reading, PA, USA) were left in place in the PA and systemic ventricle for continuous haemodynamic pressure monitoring, and the patient was transferred into the MRI scanner on a sliding table.
MRI protocol
A two-element phase array cardiac surface coil (Flex M or Flex L depending on patient size; Philips) was used for XMR, and a 32-channel coil (Philips) for healthy adult controls who were studied in a standard 1.5T CMR scanner (Achieva, Philips).
Anatomy
The institutional MRI protocol for patients with HLHS has been described previously.19 Briefly, the sequences included selected ‘black blood’ images, balanced SSFP cine images of the cardiac long-axis and short-axis stack, a free-breathing 3D balanced SSFP volume, and 3D contrast-enhanced magnetic resonance angiogram to characterize the heart and vessels.
The Nakata index20 was applied to objectively measure the cross-sectional area of the branch PAs from the systolic phase of 3D SSFP images. The sites for measurement of the branch PAs are as previously described.19
Ventricular volumes function and flow
Ventricular volumes, stroke volume (SV), and ejection fraction (EF) were calculated from short-axis slices of the ventricle obtained using retrospective ECG-gated two-dimensional balanced SSFP cine sequences.19
Standard two-dimensional (2D) phase contrast flow imaging was acquired in IVC, SVC, RPA, LPA, ascending, and descending aorta. Flow was measured perpendicular to the long-axis planes of the vessel with free-breathing flow-sensitive segmented k-space fast field echo sequence (approximate echo time 3 ms, approximate repetition time 5 ms, matrix 128 × 256, field of view 250–350 mm, flip angle 15°, number of signal averages 3, retrospective gating, acquired temporal resolution 40 phases per average heart beat), targeted velocity encoded value 60–300 m/s.
Dobutamine stress protocol
All standard imaging was done under baseline conditions. For each subsequent haemodynamic stage, only selected phase contrast flow images of the neoaorta and a short-axis cine stack covering the ventricle were performed. Continuous ECG and invasive haemodynamic pressure monitoring was recorded. There were three stages to the protocol as listed below.
Stage 1: Baseline
Stage 2: Dobutamine at 10 µg/kg/min
Stage 3: Dobutamine at 20 µg/kg/min
Dobutamine was infused for 10 min or until there was a plateau in heart rate and blood pressure, and discontinued if there was a significant arrhythmia or haemodynamic compromise.
Inhaled nitric oxide (iNO) was administered at 20 ppm with 100% oxygen following peak stress in the last seven consecutive patients to assess the effects of pulmonary vasodilation on preload and cardiac output. Flow in the branch pulmonary arteries was remeasured in these patients during the different stages of dobutamine stress and with addition of iNO.
Data processing
Raw MRI data including phase contrast flow and short-axis cine data for volumetric assessment were analysed on an Extended MR Workspace version 2.6.3.4 (Philips Medical System).
Calculations
Pulmonary vascular resistance (PVR) expressed as woods units normalized to body surface area (WU m2) was calculated as:
Systemic vascular resistance (SVR):
Flow calculations through vessels that cannot be measured directly were made using the following formulae after exclusion of systemic to pulmonary venous collaterals on contrast-enhanced angiography:
Statistics
Statistical data were analysed using SPSS Version 20.0. Mean values and standard deviations are described for normally distributed data, and median values with ranges for skewed distributions. Comparison of volumes and flows from one level of stress to the next was evaluated using a one-way ANOVA followed by paired Student's t-test, for subgroup analysis with P < 0.05 level of significance and a Bonferroni correction for multiple testing.
Inter-user variability of the right ventricular volume and aortic flow measurements was quantified using an intra-class correlation coefficient two-way model with absolute agreement. Each baseline measurement in the HLHS group was assessed by two authors (K.P., J.W.).
Results
There were 114 patients with HLHS post-Fontan in our centre at the time of recruitment. All the patients referred for investigation because of exercise intolerance or signs of Fontan failure were included in the study. Thirteen consecutive HLHS patients with exercise intolerance with a median age of 9.2 years (3.5–11.6 years) and 10 healthy controls with a median age 40.6 years (23.9–51.8 years) were recruited. All patients were in NYHA class II with a median time from Fontan of 6.2 years (0.5–8.9 years). In addition to exercise intolerance, two patients had a peripheral oedema, one had protein-losing enteropathy and one had plastic bronchitis. Median resting saturations were 95% (85–98%). All patients were on ACE inhibitors. Mean radiation dose for XMR catheterization in HLHS patients was 0.5 Gy cm2 (0.09 mSv).
The control patients had an equal sex distribution with a median age of 40.6 years (23.9–51.8). Mean right ventricular (RV) EF was 59 ± 4%; RV indexed end-diastolic volume (iEDV), 76.6 ± 14.3 mL/m2; RV indexed end-systolic volume (iESV), 21.3 ± 8.6 mL/m2; left ventricular (LV) EF, 57 ± 4%; LV iEDV, 76.9 ± 12.5 mL/m2; LV iESV, 33.4 ± 7.5 mL/m2
Table 1 summarizes baseline anatomical, volumetric, and haemodynamic values of HLHS patients. Only one patient had bilateral SVCs. Inter-observer variability of MRI analysis was good. Intra-class coefficient (95th confidence interval) was 0.97(0.86–0.99) for aortic stroke volume, 0.97(0.80–0.99) for EDV, 0.95(0.82–0.980 for ESV, 0.95(0.82–0.98) for SV, and 0.89(0.58–0.97) for EF.
Table 1.
HLHS anatomical data and baseline haemodynamics
| Patient | LV | RV iEDV | TVRF (%) | Nakata index | LPA: RPA flow | % APC of iCO | RV EDP (mmHG) | Fontan pressure (mmHg) |
|---|---|---|---|---|---|---|---|---|
| 1 | MA/AA | 67 | 0 | 167 | 0.4 | 31 | 5 | 8 |
| 2 | MA/AA | 88 | 13 | 256 | 0.32 | 0 | 10 | 13 |
| 3 | MA/AA | 87 | 16 | 229 | 0.39 | 19 | 6 | 10 |
| 4 | MA/AA | 66 | 0 | 301 | 0.35 | 5 | 10 | 13 |
| 5 | MS/AS | 64 | 0 | 304 | 0.42 | 25 | 11 | 13 |
| 6 | MS/AS | 62 | 0 | 323 | 0.57 | 28 | 5 | 11 |
| 7 | MA/AA | 70 | 0 | 178 | 0.35 | 38 | 7 | 11 |
| 8 | MS/AS | 56 | 10 | 411 | 0.30 | 15 | 3 | 8 |
| 9 | MA/AA | 61 | 10 | 290 | 0.29 | 36 | 4 | 8 |
| 10 | MA/AA | 90 | 32 | 393 | 0.31 | 4 | 5 | 9 |
| 11 | MS/AS | 57 | 15 | 259 | 0.50 | 30 | 8 | 10 |
| 12 | MS/AS | 72 | 12 | 247 | 0.40 | 7 | 8 | 11 |
| 13 | MA/AA | 85 | 14 | 245 | 0.48 | 0 | 5 | 8 |
| Mean | 71.2 | 9.4 | 223 | 0.40 | 17.7 | 6.7 | 10.2 | |
| Standard deviation | 12.2 | 9.4 | 48.1 | 0.09 | 13.6 | 2.5 | 2.0 |
MA/AA, mitral atresia/aortic atresia; MS/AS, mitral stenosis/aortic atresia or aortic stenosis; TVRF, tricuspid valve regurgitant fraction; RVEDP, right ventricular end-diastolic pressure; % APC, percentage of aortopulmonary collateral flow; iCO, indexed cardiac output.
All patients had an unobstructed Fontan pathway with patent branch pulmonary arteries on MRI and no pullback gradients >1 mmHg within the lateral tunnel or branch PAs during MRI catheterization. The Nakata index of the PAs was good in the majority of patients and <200 in only two patients (167 and 178). There were no systemic to pulmonary venous collaterals identified from contrast-enhanced angiography. Calculated fenestration flow was <10% for all patients. Mean baseline right ventricular EDP in HLHS patients was 6.7 ± 2.5 mmHg. The mean resting transpulmonary gradient was 3.5 ± 1.10 mmHg and the mean PVR was 1.51 ± 0.59 WU m2. Baseline SVR was 22.2 WU m2.
One patient in the HLHS group only completed Stage 2 of the protocol due to an elevated blood pressure response. This was taken as the peak response to pharmacological stress for this patient. There were no adverse events.
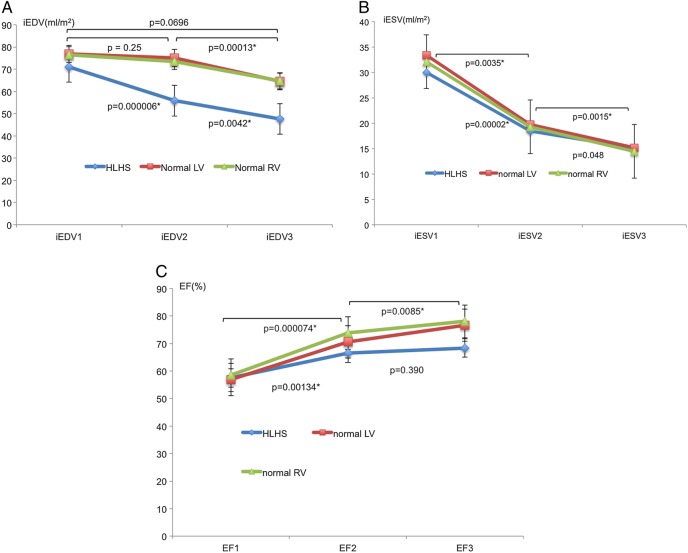
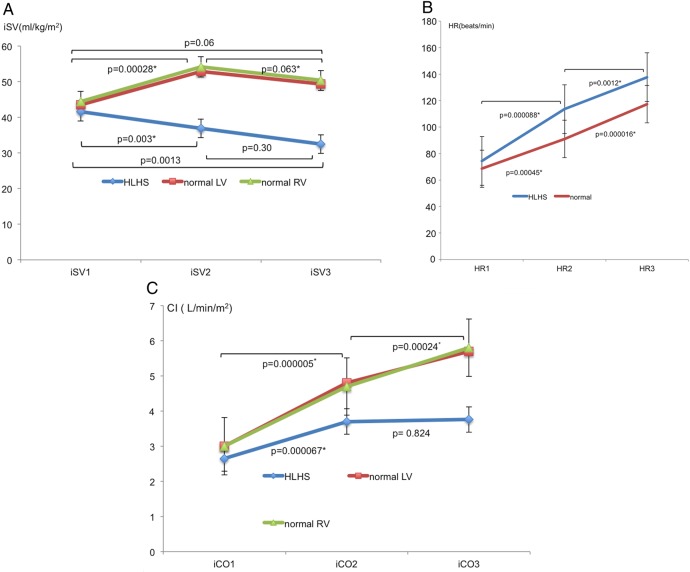
The iEDV was preserved at Stage 2 in controls, and only fell significantly at Stage 3, while in HLHS patients, there was a significant fall at Stage 2 and a further fall at Stage 3 (Figure 1A). The difference in change of iEDV between groups was significant from Stage 1 to Stage 2 (P < 0.001) but not from Stage 2 to Stage 3 (P = 0.477). iESV reduced similarly in both groups at all stages (P = 0.628) (Figure 1B) with an associated increase in EF (Figure 1C). There was an overall reduction in indexed stroke volume (iSV) significant at Stage 2 in HLHS patients (Figure 2A) as opposed to controls where there was a rise at Stage 2 with no significant change overall (P = 0.18). This difference in change of iSV between groups was significant from Stage1 to Stage 2 (P < 0.001) but not from Stage 2 to Stage 3 (P = 0.11). The total pulmonary artery (PA) flows in the last seven patients having serial PVR measurements are presented in Figure 3. This shows a rise in total PA flow only at Stage 1.
Figure 1.
(A) Mean ± SD of iEDV changes with pharmacological stress. (B) Mean ± SD of iESV changes with pharmacological stress. (C) Mean ± SD of EF changes with pharmacological stress.
Figure 2.
(A) Mean ± SD of iSV changes with pharmacological stress. (B) Mean ± SD heart rate (HR) response to pharmacological stress. (C) Mean ± SD CI response to pharmacological stress.
Figure 3.
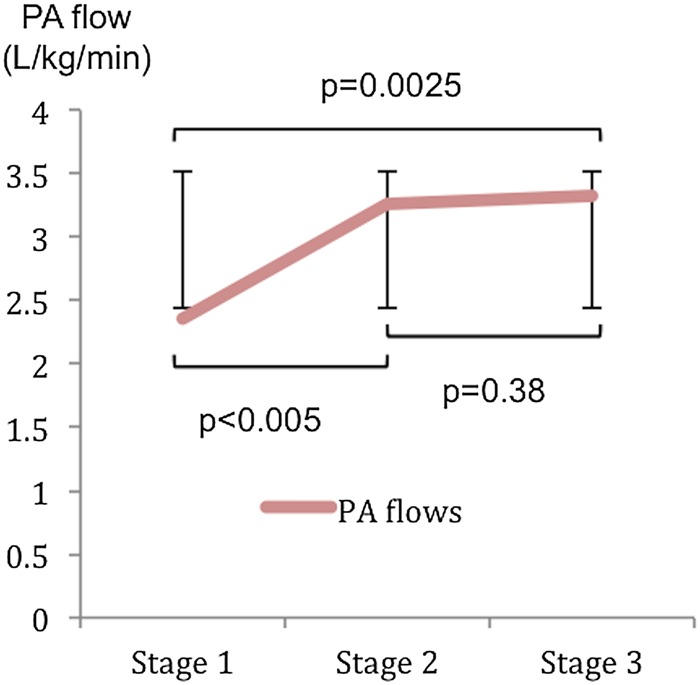
Total PA flow as measured from combined RPA and LPA flows during pharmacological stress.
Mean HR increased by 85% from baseline (P < 0.001) in HLHS vs. 72% (P < 0.001) in controls (Figure 2B) with a significant difference between groups only from Stage 2 to Stage 3 (P < 0.001). Mean cardiac index (CI) increased in HLHS (Figure 2C) from 2.65 ± 0.36 to 3.71 ± 0.41 L/min/m2 (P < 0.001) at Stage 2 but remained static at 3.76 ± 0.55 L/min/m2 (P = 0.824) at Stage 3. However, controls were able to continue to significantly increase CI at all stages from 2.96 ± 0.55L/min/m2 at baseline to 4.76 ± 0.77 L/min/m2 at Stage 2 (P < 0.001) and 5.67 ± 0.78 L/min/m2 (P < 0.001) at Stage 3. The change in CI between Stage 1 and Stage 2 was not significantly different between the two groups (P = 0.162). However, there was a significant difference in the percentage increase of CI between groups from Stage 2 to Stage 3 (P < 0.001). The ANOVA P-values for comparisons of HR, iEDV, iESV, iSV, EF, and CI were all <0.05.
Mean baseline right ventricular EDP in HLHS did not rise with stress (6.69 ± 2.5 to 6.3 ± 3.7 mmHg; P = 0.50). There was no change in oxygen saturations (93.6 ± 3.7 to 93.9 ± 3.8%; P = 0.30) with pharmacological stress indicating no increase in shunt through the fenestration in these patients.
There was a fall in SVR in both groups. This fell from 33.4 ± 7.2 to 20.1 ± 3.5 WU m2 (P < 0.001) in controls and from 22.2 ± 6.2 to 15.3 ± 2.7 WU m2 (P = 0.0039) in HLHS. Baseline APC flow accounted for 17.7 ± 13.6% of systemic CI. Effective systemic blood flow, expressed as the APC flow deducted from the total aortic CI, is represented in Figure 4, with APC flow measured at Stage 1 and extrapolated to Stages 2 and 3 assuming APC flow remained constant.
Figure 4.
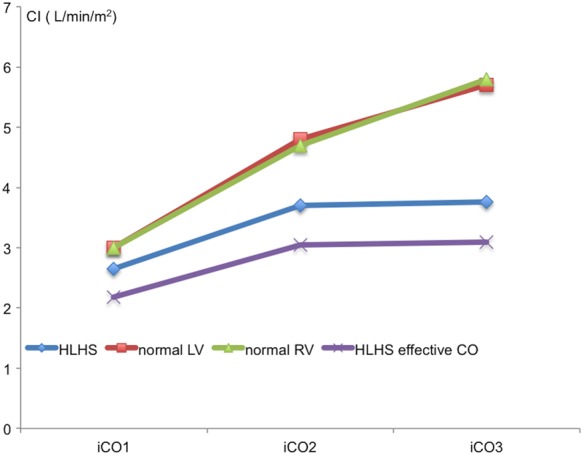
Effective cardiac output in view of APCs.
The mean PVR at peak stress in the last seven patients in the study did not change with 20 ppm iNO and 100% oxygen (1.62 to 1.57 WU m2; P = 0.83) with no significant change in mean CI (3.76 to 3.47 L/min/m2; P = 0.32).
Discussion
Exercise intolerance is often the first sign in the gradual deterioration of HLHS Fontan patients. Understanding the physiological basis for this is instructive and could help devise new strategies for treatment.
Limitation of cardiac reserve
The cardiac reserve is the maximum percentage the CI can increase above baseline. A striking finding in this group of HLHS Fontan patients is that CI only increases moderately in response to 10 µg/kg dobutamine, with no further increase at peak stress. A similar limitation of the cardiac reserve has been reported at peak physiological stress using cardiopulmonary exercise tests (CPETs) in HLHS patients post-Fontan.21
We found that the rise in CI in the HLHS group from Stage 1 to Stage 2 was driven by an increase in HR in the setting of an inability to increase iSV because of a significant fall in iEDV. This was different in the control group between Stage 1 and Stage 2 where the iSV rose significantly and the iEDV did not fall. In the HLHS group, the CI plateaued at peak pharmacological stress as HR increase alone was unable to compensate for the abnormal fall in iEDV. However, in the controls between Stages 2 and 3, there is a significant further increase in CI, which is driven by a significant increase in HR, while the iSV is preserved. As HR increases, there is less time for diastolic filling and a fall in iEDV. When this reduction in iEDV can no longer be compensated by a corresponding fall in iESV, then iSV falls and negates the effect of the increased HR on CI. Other studies using low-dose dobutamine have also shown preservation in iSV,11,12 but our study is the first to also look at high-dose dobutamine and show the fall in iSV and plateauing of CI at peak stress.
We also found normal EDPs and low SVR in these patients throughout. One can therefore hypothesize that an inability to maintain adequate preload is responsible for the fall in iEDV and the resulting blunting of CI response. This is supported by the observed plateauing of directly measured PA flows, which represents Fontan circuit flow in response to stress.
Systolic and diastolic ventricular function
Using invasive studies, Schmitt et al.11 reported normal early diastolic relaxation with no significant rise in end-diastolic pressures with 10 µg/kg/min of dobutamine, despite abnormal calculated diastolic compliance. They suggested that the effect of diastolic compliance might be unmasked at higher pharmacological stress conditions. We did not observe a significant increase in the resting end-diastolic pressure in these patients with high-dose pharmacological stress, suggesting that diastolic dysfunction is not the rate-limiting factor in augmentation of cardiac output in our study group. This is in keeping with Goldstein et al.6 who also noted no increase in ventricular filling pressures during exercise despite baseline echocardiographic evidence of diastolic dysfunction.
The progressive fall in iESV with a rise in EF seen in HLHS patients suggests good systolic function in these patients. This may reflect the relatively selected population of young HLHS patients who have survived multiple staged surgical palliation procedures with an early attrition of those with significant systolic dysfunction.
The progressive fall in SVR in both groups in our study relate to systemic vasodilation from the β2 effects of high-dose dobutamine. However, unlike the biventricular controls, this afterload reduction did not result in an increase in cardiac output in the HLH Fontan patients. All patients in this study were on long-term ACE inhibitors as part of individual physician preference and not part of institutional policy. In the context of preload reduction and preserved systolic and diastolic function, utilization of ACE inhibitors may not actually be indicated.
Pulmonary vascular resistance
All our patients had an unobstructed lateral tunnel Fontan with a baseline PVR of 1.51 ± 0.59 WU m2. Previous studies of PVR in Fontan11,22 have reported slightly higher values ranging from 2.11 ± 0.79 to 2.7 ± 1.0 WU m2. The lower PVR in our population may reflect the increased accuracy of PVR estimation by XMR catheterization,17 but also their younger age with a shorter duration of exposure to the adverse effects of lack of pulsatile flow.22,23
Despite a normal PVR, there are a number of studies investigating the use of pulmonary vasodilator therapy in this patient population. Khambadkone et al.22 have shown a fall in PVR of 24% in response to iNO which was more pronounced in the subgroup of patients palliated with a PA band and therefore exposed to pulsatile flow in the early stages of surgical palliation. Importantly, they noted no significant reduction in PVR in those with a baseline PVR of < 2WU m2. In the subgroup of our patients given iNO and 100% oxygen during peak stress, there was no significant fall in PVR or change in CI.
Sildenafil, a pulmonary vasodilator, has been shown to improve respiratory efficiency during CPET performance in Fontan patients24,25 but without any real improvement in indirect measures of cardiac output. However, our study suggests that there is no strong physiological basis for attempting to lower PVR in patients with normal baseline PVR. This is supported by a recent randomized control trial of an endothelin-1 receptor blocker, bosentan, in adults with Fontan circulations where no therapeutic benefit was seen after 6 months.26
The effect of collaterals on CI
MRI has been used to accurately quantify APC flow.11,27 We quantified APC flow at baseline but not serially during dobutamine stress, as this would have lengthened the study protocol. We assumed that the amount of APC flow would remain constant during stress, although others have shown that it is likely to rise with an inverse relationship of the amount of APC flow to CPET performance.11 Although an increase in APC flow may serve to increase preload, blood from APCs return to the pulmonary vascular bed and bypass the systemic circulation leading to a further reduction of effective systemic blood flow. This may contribute to symptoms of exercise intolerance in these patients. We found that the mean effective systemic blood flow in HLHS patients at peak stress only just matches baseline values of controls.
Fontan pathways and energy loss
Our data clearly demonstrate that PVR and ventricular function are not the rate-limiting steps in the reduced ability of HLH Fontan patients increasing their CI during stress. This points to an inherent lack of recruitable preload due to the Fontan circuit and the lack of the driving force of a subpulmonary ventricle.
Previous studies have demonstrated that the energy loss within the Fontan circuit, despite unobstructed pathways, may compound the limitations in preload.28 Simulations studying the effects of exercise have also shown that power loss within the caval component the Fontan increases in a non-linear fashion with physiological increases in flow.29 Furthermore, this is compounded when there is non-balanced differential flow to each lung at rest and with exercise in the Fontan.29 This is relevant to our patients who demonstrated a similar degree of preferential flow to the RPA.
Limitations
The small sample size is a limitation of the study. Patients were survivors with HLHS post-Fontan and therefore may reflect a self-selected cohort of the HLHS spectrum undergoing staged surgical palliation.
An ideal protocol would involve non-anaesthetized patients assessed under physiological stress with a supine MRI compatible bike and invasive pressure monitoring. However, a protocol of this nature is not ethically acceptable in this age group.
Ethical constraints also led to the control pharmacological stress data being obtained from healthy adults. There are clearly differences in adult and paediatric responses to exercise. The key difference relates to the smaller size of children and their hearts, which results in smaller stroke volumes, work rate, and peak oxygen consumption compared with adults. This difference in stroke volume is eliminated when expressed in reference to BSA.30 A study of normal values from CPETs in children found no difference in peak oxygen consumption (VO2)/kg with age, but there was an age-related difference in heart rate and peak work (WR).31 Importantly, the VO2/WR slope remained constant throughout all ages indicating a constant O2 utilization within the tissues. It would seem acceptable to compare adult and paediatric data where the rates of change in haemodynamic parameters are of interest and not absolute values.
All patients in this group were on ACE inhibitors and despite being discontinued, there may still be a small residual effect given the washout period.32 General anaesthetic agents impose some vasodilatory changes on the circulation resulting in a lower SVR in our Fontan group at baseline compared with controls, which is expected to be elevated in Fontan patients.
Being under anaesthesia, patients were supine with no contribution of the peripheral muscle pump effect.33,34 Our observed increase in cardiac output of 40% at the first stage of pharmacological stress matches previously reported increase of 30% with 7.5 µg/kg/min dobutamine and12 and 50% with 10 µg/kg/min dobutamine11 in non-anaesthetized patients. This suggests that our protocol controlled for some of the potential errors related to dobutamine stress testing under general anaesthesia.
Flows in the Fontan pathways were measured using standard ECG gating35 as opposed to respiratory gating which is more appropriate for the respiratory-dependent passive flow in the Fontan. As such APC flow may be overestimated based on systematic underestimation of Fontan flows.
Conclusions
Our findings suggest that there is an inherent lack of recruitable preload within the Fontan circuit in the young patient with otherwise favourable Fontan haemodynamics. The clinical benefit from pulmonary vasodilation to increase preload in this setting remains to be seen, but we found no acute benefit from pulmonary vasodilation.
Funding
The Division of Imaging Sciences receives also support as the Centre of Excellence in Medical Engineering (funded by the Wellcome Trust and EPSRC; grant number WT 088641/Z/09/Z) as well as the BHF Centre of Excellence (British Heart Foundation award RE/08/03). Further support is received from the Medical Research Council (MRC) Centre for Transplantation, King's College London, UK—MRC grant no. MR/J006742/1. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Acknowledgements
The authors thank the staff of the magnetic resonance imaging department at Evelina Children's Hospital.
Conflict of interest: None declared.
References
- 1.Barron DJ, Kilby MD, Davies B, Wright JGC, Jones TJ, Brawn WJ. Hypoplastic left heart syndrome. Lancet 2009;374:551–64. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous fontan surgery. Pediatr Cardiol 2009;31:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuirk SP. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart 2005;92:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation 2000;102:III-136–141. [DOI] [PubMed] [Google Scholar]
- 5.Joshi VM, Carey A, Simpson P, Paridon SM. Exercise performance following repair of hypoplastic left heart syndrome: a comparison with other types of Fontan patients. Pediatr Cardiol 1997;18:357–60. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BH, Connor CE, Gooding L, Rocchini AP. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving fontan palliation. Am J Cardiol 2010;105:1169–75. [DOI] [PubMed] [Google Scholar]
- 7.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99–107. [DOI] [PubMed] [Google Scholar]
- 8.d'Udekem Y, Cheung MMH, Setyapranata S, Iyengar AJ, Kelly P, Buckland N et al. How good is a good Fontan? Quality of life and exercise capacity of Fontans without arrhythmias. ATS 2009;88:1961–9. [DOI] [PubMed] [Google Scholar]
- 9.Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N et al. Cardiac rest and reserve function in patients with fontan circulation. J Am Coll Cardiol 2006;47:2528–35. [DOI] [PubMed] [Google Scholar]
- 10.Senzaki H, Naito C, Masutani S, Nogaki M, Ohono A, Kobayashi J et al. Hemodynamic evaluation for closing interatrial communication after fenestrated Fontan operation. J Thorac Cardiovasc Surg 2001;121:1200–2. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt B, Steendijk P, Ovroutski S, Lunze K, Rahmanzadeh P, Maarouf N et al. Pulmonary vascular resistance, collateral flow, and ventricular function in patients with a fontan circulation at rest and during dobutamine stress. Circulation 2010;3:623–31. [DOI] [PubMed] [Google Scholar]
- 12.Robbers-Visser D, Jan Ten Harkel D, Kapusta L, Strengers JL, Dalinghaus M, Meijboom FJ et al. Usefulness of cardiac magnetic resonance imaging combined with low-dose dobutamine stress to detect an abnormal ventricular stress response in children and young adults after fontan operation at young age. AJC 2008;101:1657–62. [DOI] [PubMed] [Google Scholar]
- 13.Robbers-Visser D, Luijnenburg SE, van den Berg J, Roos-Hesselink JW, Strengers JL, Kapusta L et al. Safety and observer variability of cardiac magnetic resonance imaging combined with low-dose dobutamine stress-testing in patients with complex congenital heart disease. Int J Cardiol 2011;147:214–8. [DOI] [PubMed] [Google Scholar]
- 14.Strigl S, Beroukhim R, Valente AM, Annese D, Harrington JS, Geva T et al. Feasibility of dobutamine stress cardiovascular magnetic resonance imaging in children. J Magn Reson Imaging 2009;29:313–9. [DOI] [PubMed] [Google Scholar]
- 15.Parish V, Valverde I, Kutty S, Head C, Qureshi SA, Sarikouch S et al. Dobutamine stress MRI in repaired tetralogy of Fallot with chronic pulmonary regurgitation A comparison with healthy volunteers. Int J Cardiol 2013;166:96–105. [DOI] [PubMed] [Google Scholar]
- 16.Razavi R, Hill DLG, Keevil SF, Miquel ME, Muthurangu V, Hegde S et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet 2003;362:1877–82. [DOI] [PubMed] [Google Scholar]
- 17.Muthurangu V, Taylor A, Andriantsimiavona R, Hegde S, Miquel ME, Tulloh R et al. Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow. Circulation 2004;110:826–34. [DOI] [PubMed] [Google Scholar]
- 18.Tzifa A, Schaeffter T, Razavi R. MR imaging-guided cardiovascular interventions in young children. Magn Reson Imaging Clin N Am 2012;20:117–28. [DOI] [PubMed] [Google Scholar]
- 19.Bellsham-Revell HR, Tibby SM, Bell AJ, Witter T, Simpson J, Beerbaum P et al. Serial magnetic resonance imaging in hypoplastic left heart syndrome gives valuable insight into ventricular and vascular adaptation. J Am Coll Cardiol 2013;61:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M et al. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J Thorac Cardiovasc Surg 1984;88:610–9. [PubMed] [Google Scholar]
- 21.Möller P, Weitz M, Jensen K-O, Dubowy K-O, Furck AK, Scheewe J et al. Exercise capacity of a contemporary cohort of children with hypoplastic left heart syndrome after staged palliation. Eur J Cardiothorac Surg 2009;36:980–5. [DOI] [PubMed] [Google Scholar]
- 22.Khambadkone S, Li J, De Leval M, Cullen S, Deanfield J, Redington A. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation 2003;107:3204–8. [DOI] [PubMed] [Google Scholar]
- 23.Presson RG, Baumgartner WA, Peterson AJ, Glenny RW, Wagner WW. Pulmonary capillaries are recruited during pulsatile flow. J Appl Physiol 2002;92:1183–90. [DOI] [PubMed] [Google Scholar]
- 24.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J 2008;29:1681–7. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation 2011;123:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuuring MJ, Vis JC, van Dijk APJ, van Melle JP, Vliegen HW, Pieper PG et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail 2013;15:690–8. [DOI] [PubMed] [Google Scholar]
- 27.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or fontan completion. Circulation 2009;2:219–25. [DOI] [PubMed] [Google Scholar]
- 28.Bove EL, de Leval MR, Migliavacca F, Guadagni G, Dubini G. Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the norwood procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2003;126:1040–7. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation 2007;116:I-165–71. [DOI] [PubMed] [Google Scholar]
- 30.Turley KR, Wilmore JH. Cardiovascular responses to treadmill and cycle ergometer exercise in children and adults. J Appl Physiol 1997;83:948–57. [DOI] [PubMed] [Google Scholar]
- 31.Harkel Ten ADJ, Takken T, Van Osch-Gevers M, Helbing WA. Normal values for cardiopulmonary exercise testing in children. Eur J Cardiovasc Prev Rehabil 2010;18:1–54. [DOI] [PubMed] [Google Scholar]
- 32.Beermann B. Pharmacokinetics of lisinopril. Am J Med 1988;85:25–30. [DOI] [PubMed] [Google Scholar]
- 33.Hjortdal VE, Emmertsen K, Stenbøg E, Fründ T, Schmidt MR, Kromann O et al. Effects of exercise and respiration on blood flow in total cavopulmonary connection: a real-time magnetic resonance flow study. Circulation 2003;108:1227–31. [DOI] [PubMed] [Google Scholar]
- 34.Cordina RL, O'Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol 2013;168:780–8. [DOI] [PubMed] [Google Scholar]
- 35.Grosse-Wortmann L, Drolet C, Dragulescu A, Kotani Y, Chaturvedi R, Lee K-J et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg 2012;144:1329–36. [DOI] [PubMed] [Google Scholar]