Abstract
Aims
We evaluated if a dispersed left atrial (LA) contraction pattern was related to atrial fibrillation (AF) in patients with normal left ventricular (LV) function, and normal or mildly enlarged left atrium.
Methods and results
We included 61 patients with paroxysmal AF (PAF). Of these, 30 had not while 31 had recurrence of AF after radiofrequency ablation (RFA). Twenty healthy individuals were included for comparison. Echocardiography was performed in patients in sinus rhythm the day before RFA. LA volume was calculated. Peak negative longitudinal strain was assessed in 18 LA segments during atrial systole. Contraction duration in 18 LA segments was measured as the time from peak of the P wave on electrocardiogram to maximum myocardial shortening in each segment. The standard deviation of contraction durations was defined as LA mechanical dispersion (LA MD). LA size was rather preserved in patients with PAF (LA volume 25 ± 10 mL/m2). LA MD was more pronounced in patients with recurrence of AF after RFA compared with those without recurrence and controls (38 ± 14 ms vs. 30 ± 12 ms vs. 16 ± 8 ms, both P < 0.001). LA MD was a predictor of PAF [OR 7.84 (95%CI 2.15–28.7), P < 0.01, per 10 ms increase] adjusted for age, LA volume, e’, and LA function. LA function by strain was reduced in both patients with and without recurrent AF after RFA compared with controls (−14 ± 4% vs. −16 ± 3% vs. −19 ± 2%, both P < 0.05).
Conclusion
LA MD was pronounced, and LA deformation was reduced in patients with PAF with apparently normal LV structure and function, and normal or mildly enlarged LA. LA MD may be useful as a predictor of AF recurrence after RFA.
Keywords: Atrial fibrillation, Echocardiography, Strain, Dispersion, Prediction
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, with an estimated prevalence of 0.4–1% in the general population, increasing with age to 9% in those above 80 years.1 Paroxysmal and permanent AF may lead to reduced quality of life and has significant health economic consequences. Furthermore, asymptomatic AF increases risk for the development of stroke.2 In spite of this, confirming the aetiology of stroke even after extensive cardiologic assessment is often difficult.
For patients with symptomatic drug-refractory AF, radiofrequency ablation (RFA) has become an important therapy option. However, arrhythmia recurrence after RFA is reported to be as high as 40–50%.3 Redo procedures increase success rate, but are costly and include risk of adverse events. Consequently, careful patient selection prior to the procedure is pivotal. Increased left atrial (LA) size is a useful parameter to predict AF recurrence4–11 and values >5.0–5.5 cm indicate limited success.4 In patients with normal or only mildly increased LA size, prediction of arrhythmia recurrence is more difficult.
AF is most frequently related to coronary artery disease, structural heart disease, hypertension, diabetes mellitus, hyperthyroidism, or simply due to aging. However, in the population with no cardiovascular disease or other known causal factors, the mechanism of AF is not fully understood. A possible explanation is increased myocardial fibroses in the LA, promoting AF.12–14 Fibrosis may lead to micro-re-entry circuits that consequently lead to electrical dispersion, which is a known arrhythmogenic factor.15 Importantly, these subtle alterations are undetectable by traditional imaging methods.
For these reasons, an echocardiographic marker of paroxysmal AF (PAF) and a predictor of recurrence of AF after RFA in patients without apparent cardiac pathology would be of great clinical value. Strain echocardiography can accurately assess regional myocardial function and timing, and ventricular strain has been shown to be a marker of cardiac prognosis in several publications.16,17 Furthermore, recent studies have shown that inhomogeneous timing of contractions, presented as mechanical dispersion (MD), is a marker of ventricular arrhythmias.18–21 Atrial strain measurements are feasible22 and have been reported to predict recurrence of AF.23 However, studies including time measurements of atrial deformation are sparse.24
We hypothesized that pronounced LA MD by myocardial strain is associated with susceptibility to atrial arrhythmias in patients with PAF and, therefore, may be an additional tool in arrhythmia risk stratification. Furthermore, we aimed to investigate the extent of decrease in LA function assessed by strain echocardiography in patients with PAF.
Methods
From January 2012 to March 2014, 258 patients with PAF underwent RFA at our centre. Indications for RFA were symptomatic drug-refractory AF or intolerance to at least one Class I or III antiarrhythmic medication. Of these, 127 (45%) had recurrence of AF after first RFA and were accepted for redo RFA, while 158 (55%) did not have recurrence of AF. The echocardiographic recordings of these patients were reviewed and included in the study if they had normal systolic (EF >50%) and diastolic LV functions,25 and normal or only mildly increased LA size (LA volume < 42 mL/m2).26 Exclusion criteria were age <18 years, pregnancy, structural heart disease, history of previous myocardial infarction, percutaneous coronary intervention and open chest surgery, bundle branch block, moderate to severe valvular dysfunction, moderate to severe left ventricular (LV) hypertrophy and ongoing AF or other sustained arrhythmias. Echocardiography was performed 1 day prior to the first RFA therapy. A subset of 61 patients fulfilled inclusion criteria and were retrospectively included in this study. The echocardiographic data were analysed blinded to all clinical information. The medication received at the time of the echocardiographic study was recorded (Table 1).
Table 1.
Clinical characteristics
| Healthy individuals (n = 20) | Patients without recurrence of AF after RFA (n = 30) | Patients with recurrence of AF after RFA (n = 31) | P-value | |
|---|---|---|---|---|
| Age (years) | 54 ± 16 | 56 ± 10 | 55 ± 8 | 0.8 |
| Male, n (%) | 15 (75) | 23 (77) | 24 (77) | 1.0 |
| Heart rate (bpm) | 64 ± 10 | 57 ± 9 | 57 ± 11 | <0.05 |
| Height (cm) | 179 ± 9 | 180 ± 10 | 181 ± 9 | 0.8 |
| Weight (kg) | 74 ± 12 | 89 ± 13* | 91 ± 14* | <0.001 |
| BSA (m2) | 1.7 ± 0.6 | 2.1 ± 0.2* | 2.1 ± 0.2* | <0.001 |
| BMI (kg/m2) | 23 ± 2 | 28 ± 4* | 28 ± 5* | <0.001 |
| Clinical characteristics in patients with and without recurrent AF after RFA | ||||
| Years with PAF | 6.3 ± 6.1 | 7.3 ± 6.4 | 0.6 | |
| Hypertension, n (%) | 10 (33) | 6 (19) | 0.2 | |
| SBP (mmHg) | 130 ± 16 | 128 ± 15 | 0.7 | |
| DBP (mmHg) | 79 ± 11 | 80 ± 10 | 0.8 | |
| Diabetes mellitus, n (%) | 1 (3) | 2 (7) | 0.6 | |
| Coronary artery disease, n (%) | 0 (0) | 1 (3) | 0.3 | |
| Hypercholesterolemia, n (%) | 0 (0) | 2 (7) | 0.2 | |
| Smoking, n (%) | 5 (17) | 4 (13) | 0.7 | |
| CHA2DS2-VASC score for AF stroke risk27 | ||||
| Score 0, n (%) | 13 (43) | 17 (55) | 0.4 | |
| Score 1, n (%) | 10 (33) | 9 (29) | 0.7 | |
| Score 2, n (%) | 6 (20) | 4 (13) | 0.5 | |
| Score 3, n (%) | 1 (3) | 1 (3) | 1.0 | |
| Medication | ||||
| Beta blockers, n (%) | 16 (53) | 17 (55) | 0.9 | |
| Ca antagonist for frequency regulation, n (%) | 2 (7) | 5 (16) | 0.2 | |
| Flecainide, n (%) | 14 (47) | 17 (55) | 0.5 | |
| Sotalol, n (%) | 1 (3) | 1 (3) | 1.0 | |
| Amiodarone, n (%) | 3 (10) | 5 (16) | 0.5 | |
| Dronedarone, n (%) | 4 (13) | 4 (13) | 1.0 | |
| Warfarin, n (%) | 25 (83) | 30 (97) | 0.8 | |
| Dabigatran, n (%) | 3 (10) | 1 (3) | 0.3 | |
| Rivaroxaban, n (%) | 2 (7) | 0 (0) | 0.1 | |
| Acetylsalicylic acid, n (%) | 1 (3) | 1 (3) | 1.0 | |
| ACE inhibitors, n (%) | 0 (0) | 2 (7) | 0.2 | |
| AT II receptor antagonists, n (%) | 7 (23) | 2 (7) | 0.6 | |
| Anti-diabetic medication, n (%) | 1 (3) | 0 (0) | 0.3 | |
| Statins, n (%) | 9 (30) | 4 (13) | 0.1 | |
| Diuretics, n (%) | 7 (23) | 3 (10) | 0.2 | |
| Ca antagonist for hypertension, n (%) | 0 (0) | 1 (3) | 0.3 | |
Mean ± SD, numbers. Right column shows P-values for ANOVA and χ2 tests. Flags for significance are obtained from the post hoc pair-wise comparison using the Bonferroni correction when comparing the three groups.
ANOVA, analysis of variance; ACE, angiotensin-converting enzyme; AT, angiotensin; AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; RFA, radiofrequency ablation; SBP, systolic blood pressure.
*P < 0.05 compared with healthy individuals.
The control group consisted of 20 healthy individuals from the hospital staff, matched for age and sex with the AF patients. All individuals in the control group had normal electrocardiogram (ECG), physical examination, echocardiographic study, and were free from disease with potential impact on the cardiovascular system.
Written informed consent was given by all study participants. The study complies with the Declaration of Helsinki and is approved by the Regional Committee for Medical Research Ethics.
Two-dimensional transthoracic echocardiography
Patients and control subjects underwent an echocardiographic study (Vivid 7 and E9, GE, Vingmed, Horten, Norway). Cineloops from three standard apical views (four chamber, two chamber, and apical long axis) were recorded using greyscale harmonic imaging. Data were digitally stored for offline analysis (EchoPac, GE, Vingmed). From 2D echocardiography, the following parameters were assessed: LV end-diastolic and end-systolic diameters, volumes and ejection fraction by Simpson's biplane method using manual tracing of digital images, LV diastolic function parameters including mitral E and A velocities, E/A ratio, deceleration time, e’, E/e’, LA parameters including diameter, and area in two- and four-chamber views. LA volume was calculated using the bi-plane area length method.26 Frame rates were 59 ± 9 Hz for greyscale imaging.
Two-dimensional strain echocardiography and mechanical dispersion
The endocardial borders were manually traced using a point-and-click technique in the 2D images from the three apical views for longitudinal LA and LV myocardial deformation. All patients were in sinus rhythm during echocardiography. Speckles were tracked frame by frame throughout the LA wall during the atrial systole and the LV wall during ventricular systole. Segments that failed to track were manually adjusted by the operator. Any segments that subsequently failed to track were excluded. Peak negative longitudinal myocardial strain by 2D speckle tracking echocardiography was assessed in 18 LA and LV segments and averaged to LA (Figure 1) and LV global longitudinal strain (LA GLS and LV GLS, respectively). Contraction duration (Figure 2) was measured as time from peak of the P wave on ECG to maximum LA shortening by strain. Standard deviation of contraction durations in 18 LA segments was defined as LA MD.
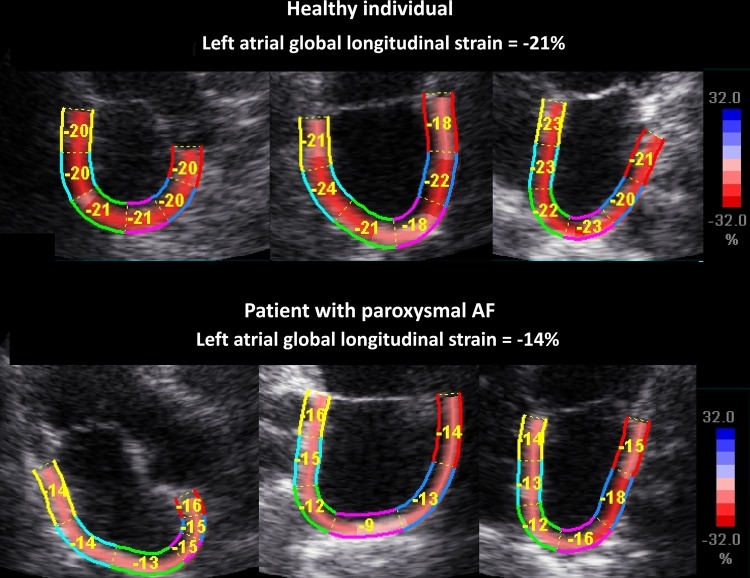
Figure 1.
Upper and lower panels demonstrating apical long-axis views (apical long-axis, two-chamber, and four-chamber views) with the left atrium as region of interest. Upper panels show a healthy individual with normal atrial deformation, while lower panels demonstrate reduced atrial deformation during atrial systole in a patient with PAF but in sinus rhythm during the study. AF, atrial fibrillation.
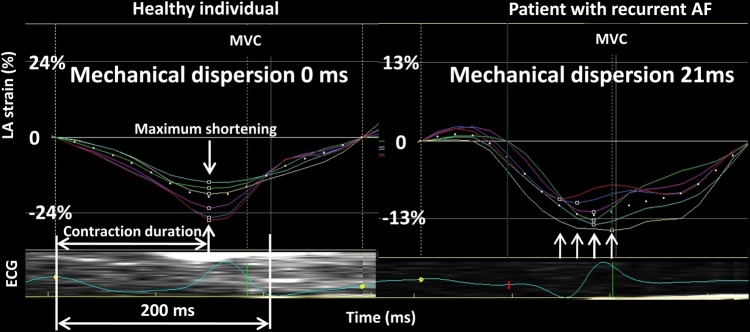
Figure 2.
Strain curves and MD from the left atrium during atrial systole in a healthy individual (left panel) and a patient with PAF (right panel) but in sinus rhythm during the study. Horizontal white arrow indicates contraction duration defined as the time from peak of the atrial P wave on ECG to maximum myocardial shortening. Vertical arrows indicate the timing of maximum myocardial shortening in each LA segment. Right panel shows more pronounced MD in the patient with PAF. AF, atrial fibrillation; LA, left atrial; MVC, mitral valve closure.
Electrophysiological study and radiofrequency ablation
A 6-F decapolar catheter (Biosense-Webster, Inc., CA, USA) was positioned via the right femoral vein into the coronary sinus. A double transseptal puncture was performed with the introduction of two SL0 sheaths (St. Jude Medical, MN, USA) into the LA. A Lasso circular mapping catheter (Biosense-Webster, Inc., CA, USA) and a 3.5-mm tip irrigated ablation catheter (Navistar Thermocool, Biosense-Webster, Inc., CA, USA) were introduced into the LA. A 3D electroanatomical map (FAM-map) was created using the Carto-3 system (Biosense-Webster, Inc., CA, USA). The ablation strategy was encircling of ipsilateral pairs of pulmonary vein antra using a maximum power of 30–35 watts. If needed to complete pulmonary vein isolation, additional lesions were given at the carina. The end point of the procedure was pulmonary vein isolation including exit block verified by the Lasso catheter.
Clinical follow-up
Patients were assessed at 3, 6, and 12 months after RFA, including outpatient visits with clinical evaluation, serial ECG, and 24-h Holter monitoring to detect asymptomatic AF episodes.4 Antiarrhythmic medication was continued for at least 3 months after RFA and then discontinued if no AF recurrences were detected and no subjective AF episodes occurred. Oral anticoagulation was continued for at least 6 months; further continuation of anticoagulation therapy was based on CHA2DS2-VASc cardioembolic risk score.4 The procedure was considered successful if there was no recurrence of AF lasting >30 s during the follow-up, after a blanking period of 3 months.
Statistical analyses
Data were presented as mean ± SD, and numbers, and percentages. The χ2 test (categorical variables) and unpaired Student's t-test (continuous variables) were used to determine differences between two groups. Comparisons of means for normally distributed variables were performed by analysis of variance (ANOVA) with the Bonferroni post hoc correction for multiple comparisons (SPSS version 21, SPSS, Inc., Chicago, IL).
Logistic regression analysis was performed to determine the independent prognostic value of LA MD for predicting PAF in the whole study population (n = 81). Age, LA volume, e’, LA GLS, and LA MD were selected for inclusion in a multivariate logistic regression analysis. The selection of variables was based on statistical significance of P < 0.05 in the univariate logistic regression analysis in addition to age and LA volume. The area under the receiver-operating characteristic (ROC) curve (AUC) was calculated for LA GLS and LA MD. The value closest to the upper left corner of the ROC curve determined optimal sensitivity and specificity for the ability of the parameters to discriminate between those with and without arrhythmic events.
Reproducibility was expressed as intra-class correlation coefficient. P-values were two tailed, and values below 0.05 were considered significant.
Results
In total, 61 patients with symptomatic drug-refractory PAF were included (Table 1). Of these, 30 had no recurrence of AF during the follow-up, while 31 had recurrent AF after one RFA therapy. There was no difference in stroke risk according to CHA2DS2-VASc score27 (Table 1), procedure time, total RFA time, radiation time, and radiation dose (Table 2) between the two groups. The first recurrence of AF after RFA occurred at 4 ± 2 months in patients who later on were accepted for a redo RFA. Total follow-up for patients without recurrence of AF was 16 ± 7 months.
Table 2.
RFA characteristics
| Patients without recurrence of AF after RFA (n = 30) | Patients with recurrence of AF after RFA (n = 31) | P-value | |
|---|---|---|---|
| Procedure time (min) | 228 ± 61 | 239 ± 53 | 0.5 |
| Total RFA time (s) | 2730 ± 1028 | 2619 ± 935 | 0.7 |
| Radiation time (min) | 40 ± 20 | 50 ± 26 | 0.08 |
| Radiation dose (uGym2) | 6831 ± 5310 | 6907 ± 4718 | 1.0 |
Mean ± SD. Right column shows P-values for Student's t-test.
AF, atrial fibrillation; RFA, radiofrequency ablation.
Patients with PAF had higher body weight, greater body mass index and body surface area, and lower heart rate compared with healthy controls (Table 1). No differences in age, sex, weight, height, body mass index, co-morbidity, or medication were observed between those with and without recurrence of AF after RFA (Table 1).
Echocardiographic findings
Patients with PAF had significantly greater LA MD and impaired LA function by GLS compared with healthy controls (both P < 0.001; Table 3). Importantly, patients with recurrence of AF after RFA showed greater LA MD compared with patients without recurrence (P < 0.05) and controls (P < 0.001; Table 3). Furthermore, patients without recurrence of AF after RFA showed significantly increased LA MD compared with controls (P < 0.001).
Table 3.
Echocardiographic results
| Healthy individuals (n = 20) | Patients without recurrence of AF after RFA (n = 30) | Patients with recurrence of AF after RFA (n = 31) |
P-value ANOVA F-test |
|
|---|---|---|---|---|
| LA MD (ms) | 16 ± 8 | 30 ± 12* | 38 ± 14*,** | <0.001 |
| LA GLS (%) | −19 ± 2 | −16 ± 3* | −14 ± 4* | <0.001 |
| LA diameter/BSA (cm/m2) | 1.7 ± 0.6 | 1.8 ± 0.2 | 1.8 ± 0.2 | 0.3 |
| LA area/BSA (cm2/m2) | 8 ± 3 | 9 ± 1 | 9 ± 1 | 0.3 |
| LA volume/BSA (mL/m2) | 29 ± 6 | 27 ± 4 | 28 ± 3 | 0.6 |
| LV GLS (%) | −22.5 ± 2.4 | −21.7 ± 2.2 | −21.8 ± 1.9 | 0.4 |
| LV EF (%) | 62 ± 4 | 63 ± 4 | 61 ± 4 | 0.4 |
| LV EDV/BSA (mL/m2) | 56 ± 23 | 64 ± 8 | 65 ± 10 | 0.052 |
| LV ESV/BSA (mL/m2) | 21 ± 9 | 24 ± 4 | 25 ± 5 | 0.6 |
| IVSd (mm) | 9 ± 2 | 9 ± 2 | 9 ± 2 | 0.7 |
| LVIDd/BSA (mm/m2) | 24 ± 9 | 27 ± 3 | 25 ± 3 | 0.2 |
| LVPWd (mm) | 8 ± 2 | 9 ± 2 | 9 ± 2 | 0.8 |
| E velocity (cm/s) | 70 ± 20 | 60 ± 20 | 70 ± 20 | 0.7 |
| DT (ms) | 206 ± 46 | 196 ± 39 | 206 ± 55 | 0.7 |
| A velocity (cm/s) | 50 ± 20 | 60 ± 10 | 50 ± 10 | 0.1 |
| E/A ratio | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.4 ± 0.5 | 0.3 |
| e’ (cm/s) | 10 ± 3 | 9 ± 2 | 9 ± 2 | 0.09 |
| E/e’ | 7 ± 2 | 8 ± 2 | 8 ± 3 | 0.2 |
Mean ± SD. Right column shows P-values for ANOVA test. Flags for significance are obtained from the post hoc pair-wise comparison using the Bonferroni correction when comparing the three groups.
AF, atrial fibrillation; ANOVA, analysis of variance; BSA, body surface area; DT, deceleration time; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; GLS, global longitudinal strain; IVSd, interventricular septum diastole; MD, mechanical dispersion; LA, left atrial; LV, left ventricle; LVIDd, left ventricular internal diameter diastole; LVPWd, left ventricular posterior wall diastole; RFA, radiofrequency ablation.
*P < 0.05 compared with healthy individuals.
**P < 0.05 compared with patients without recurrence of AF after RFA.
Patients with PAF showed significantly reduced LA function assessed by longitudinal myocardial strain compared with controls (P < 0.01). However, there was no difference in LA function by longitudinal strain between patients with and without recurrence of AF after RFA (Table 3).
LA MD and LA GLS were independent predictors of PAF in a multivariate logistic regression analysis (n = 81) (P < 0.05; Table 4).
Table 4.
Predictors of arrhythmia in patients with PAF
| Univariate logistic regression |
Multivariate logistic regression |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (years) | 1.01 | 0.97–1.06 | 0.6 | 1.02 | 0.89–1.17 | 0.8 |
| LA volume/BSA (mL/m2) | 0.95 | 0.84–1.07 | 0.4 | 0.76 | 0.57–1.03 | 0.8 |
| e’ (cm/s) | 0.77 | 0.60–0.99 | 0.04 | 0.76 | 0.34–1.69 | 0.5 |
| LA GLS (%) | 1.40 | 1.15–1.71 | 0.001 | 1.62 | 1.10–2.39 | 0.02 |
| LA MD (per 10 ms increase) | 4.67 | 2.16–10.1 | <0.001 | 7.84 | 2.15–28.7 | 0.002 |
BSA, body surface area; CI, confidence interval; GLS, global longitudinal strain; MD, mechanical dispersion; LA, left atrial; OR, odds ratio.
The ROC analysis demonstrated that LA MD (AUC = 0.88) of 24 ms was the optimal cut-off value to discriminate patients with PAF (n = 61) from healthy individuals (n = 20) with a sensitivity of 77% and a specificity of 85%, while LA GLS (AUC = 0.79) at a cut-off value of −18% had a sensitivity and specificity of 75%, respectively for discriminating patients with PAF (Figure 3).
Figure 3.
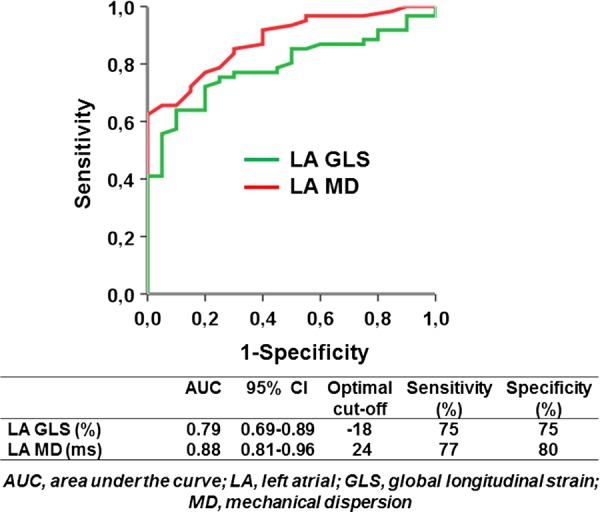
ROC curve analyses for the ability of LA MD and LA GLS to discriminate patients with PAF (n = 61) from healthy individuals (n = 20).
There were no differences in LV dimensions or volumes, LV diastolic function parameters including mitral E and A velocities, E/A ratio, deceleration time, e’, E/e’, or LA volumes between the three groups (Table 3). LV ejection fraction and LV GLS were within normal range in all groups and with no significant differences (Table 3).
Assessment of longitudinal strain and MD could be performed in 1199 (82%) LA segments.
Intra-observer and inter-observer intra-class correlation was performed in 10 patients and was 0.92 (P < 0.001) and 0.73 (P < 0.05) for LA GLS measurements and 0.92 (P < 0.01) and 0.84 (P < 0.01) for LA MD measurements, respectively.
Discussion
In this study, we utilized a new method for prediction of recurrence of AF by strain echocardiography. LA MD could discriminate patients with PAF with no apparent cardiac dysfunction assessed by traditional imaging methods from healthy individuals. Furthermore, LA MD was more pronounced in patients with recurrence of AF after RFA therapy compared with patients without recurrence.
MD assessed in the LV has been shown to predict ventricular arrhythmias in patients with cardiomyopathies and after myocardial infarction.18–21 The aim of this study was to further develop and apply a similar method to predict the risk of recurrence of AF in general and after RFA in particular. We wished to examine if MD, a method primarily developed to assess risk of ventricular arrhythmias, also could predict atrial arrhythmias.
It is well known that heart failure, structural heart disease, and increased LA size5–11 are predictors of AF recurrence after RFA and are associated with increased LA scarring.28 However, normal cardiac function, a structurally normal heart, and normal LA size do not exclude the occurrence of AF. Therefore, we studied a population practically without cardiovascular disease, and normal or only mildly enlarged LA, in order to explore if MD could predict AF recurrence in these patients where risk stratification tools are lacking.
Mechanical dispersion
Myocardial velocities and myocardial strain based on echocardiography have been introduced as quantitative parameters for regional myocardial motion and deformation. Minor degrees of myocardial contraction heterogeneity and dyssynchrony can be demonstrated by these techniques. We studied myocardial LA deformation to characterize global LA function (Figure 1) and timing of deformation, which reflects contraction heterogeneity (Figure 2).
Tsai et al. have shown previously that LA function by peak negative strain during atrial systole was impaired in patients with PAF and could predict new AF episodes.23 Furthermore, a recent study has used the same mathematical formula as our study to assess dyssynchrony in the LA.24 However, Providencia et al. focused on dispersion of peak positive LA strain and stiffness during the atrial reservoir phase. To quantify LA dyssynchrony, standard deviation of time to peak positive LA regional strains was calculated. During peak positive deformation, the LA is stretched mainly due to the venous return from the lungs and functions as a reservoir without active contractions. In our study, similarly to Tsai et al., we examined the LA during active atrial systole when depolarization of the LA takes place, and therefore electrical dispersion is possible. Furthermore, the primary aim of the LA conduit phase dyssynchrony study was to predict atrial stasis, whereas our study focuses on risk prediction of AF recurrence.
Even though ROC curve analysis for LA MD and LA GLS were similar in our study, there was a tendency towards greater sensitivity and specificity of LA MD in the prediction of AF. Furthermore, we found difference in LA MD between patients with and without recurrence of AF after RFA, while there was no difference in LA GLS between these groups. Finally, in a multivariate analysis, LA MD had an almost five times greater odds ratio in the prediction of AF compared with LA GLS. The analyses described above indicate additional information given by LA MD and supports LA MD as a more sensitive parameter than LA GLS.
Recent results from an animal model subjected to exercise showed an increase in the synthesis of mRNA markers of fibrosis, an increased turnover of matrix proteins, and the deposition of collagen in both atria and the right ventricle.12 Likewise, Verma et al. demonstrated that LA scarring assessed by extensive voltage mapping of the LA is a powerful, independent predictor of AF recurrence after pulmonary vein antrum isolation.28 Finally, an inverse relationship between LA fibrosis by late-enhancement magnetic resonance imaging (MRI) and LA strain by echocardiography was related to AF burden in studies by Kuppahally et al.13,14 We suggest that LA MD may reflect the burden of atrial fibrosis and therefore reflect the mechanism for increased risk of recurrence of AF after RFA.
In our study population, due to the wide age range (34–72 years) with most patients in their 50s, the mechanism of LA fibrosis is most likely secondary to a variety of causes including hypertension, diabetes mellitus, coronary artery disease, and probably also endurance training.12,29,30 According to our hypothesis, LA fibrosis regardless of aetiology, promotes AF. Fibrosis leads to atrial micro-re-entry circuits,31,32 which consequently lead to electrical dispersion during depolarization. Ultimately, electrical dispersion triggers arrhythmias. Electrical dispersion also leads to MD, which can be detected by strain echocardiography. A simple, bedside, non-invasive method that could predict recurrence of AF in general and after RFA in particular would be of great clinical interest.
Clinical implications
Identification of non-invasive markers of LA dysfunction with prognostic implications for patients with PAF could have major clinical implications with regard to the intensity of medical and invasive treatment in these patients.
By early detection of atrial dysfunction in patients with PAF, larger clinical trials might clarify whether earlier and more aggressive treatment might slow the progression of atrial dysfunction, reduce AF recurrence, and prevent the development of permanent AF.
Finally, a recent study demonstrated that asymptomatic AF increases the risk of stroke.2 However, confirming the aetiology of a stroke is often difficult, even after extensive cardiologic assessment. MD in the LA might be able to demonstrate susceptibility to AF. This could have major clinical implications in the case of asymptomatic PAF without electrocardiographic proof, as anticoagulation is often indicated in order to prevent stroke in these patients.
Prediction of development of paroxysmal and permanent AF is pivotal to all cardiologists, due to the inherent risk of embolization and fatal stroke. However, current methods to foresee AF and recurrence of AF are limited. The echocardiographic methods described in this study can be performed with nearly all last generation echocardiographic scanners and can be learned and utilized easily in a clinical setting.
Study limitations
The retrospective design and the low sample size are limitations of the current study.
Follow-up of patients without recurrence of AF was 12 months, which is relatively short as AF may reoccur years later.
We did not include patients with heart failure, structural heart disease, and increased LA size. Clinical implications of our finding in these patients remain to be determined.
Our patients had had PAF for 7 ± 6 years. Oslo University Hospital is a tertiary hospital for AF ablation, and therefore our population did not include patients at first diagnosis, and hence we do not have echocardiographic data from this time point. Consequently, we could not study LA function at an early stage of PAF.
We assessed global longitudinal strain but not strain rate or radial strain. This measure was chosen because longitudinal strain has been best validated, measurements are reproducible and easily obtained with only a minor increase in procedure duration. Reproducibility of strain rate and radial strain is generally poor. In addition, radial strain from the LA is difficult to obtain due to the thin atrial wall.
The reservoir and conduit phases of the LA were not assessed as we focused on the active atrial systole when depolarization and active contraction occur.
The magnitude of MD in the LA is considerably smaller compared with dispersion in the ventricles, potentially reflecting difference in the structures for propagation of electrical impulses. Consequently, it is more difficult to differentiate between pathological and normal dispersion in the LA.
Lateral resolution might be a factor limiting the delineation of delicate structures. This might be exaggerated in the LA segments that are relatively far from the transducer, and consequently the density of echo beams is lower.
No late-enhancement MRI or voltage mapping was performed in order to quantify atrial fibrosis.
Conclusions
We found a dispersed LA contraction pattern and reduced LA deformation in patients with PAF and normal or only mildly enlarged LA, and apparently normal LV structure and function when comparing with healthy individuals. LA MD before RFA treatment was most pronounced in AF patients who experienced recurrence of AF after RFA. We propose that LA MD by strain echocardiography may be useful as a marker of PAF and as a predictor of AF recurrence after RFA.
Conflict of interest: None declared.
Funding
This work was supported by the South-Eastern Norway Regional Health Authority and by the Bergesen Foundation. Funding to pay the Open Access publication charges for this article was provided by the Research Council of Norway.
References
- 1.Freedman JE, Gersh BJ. Atrial fibrillation and stroke prevention in aging patients: what's good can be even better. Circulation 2014;130:129–31. [DOI] [PubMed] [Google Scholar]
- 2.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 3.Knecht S, Sticherling C, von Felten S, Conen D, Schaer B, Ammann P et al. . Long-term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: a propensity score matched analysis. Int J Cardiol 2014;176:645–50. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA et al. . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 2012;9:632–96.22386883 [Google Scholar]
- 5.Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, Santos KR et al. . Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 2009;11:1289–94. [DOI] [PubMed] [Google Scholar]
- 6.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M et al. . Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007;28:836–41. [DOI] [PubMed] [Google Scholar]
- 7.Hof I, Chilukuri K, Arbab-Zadeh A, Scherr D, Dalal D, Nazarian S et al. . Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol 2009;20:1005–10. [DOI] [PubMed] [Google Scholar]
- 8.Lo LW, Lin YJ, Tsao HM, Chang SL, Udyavar AR, Hu YF et al. . The impact of left atrial size on long-term outcome of catheter ablation of chronic atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:1211–6. [DOI] [PubMed] [Google Scholar]
- 9.Montserrat S, Gabrielli L, Borras R, Poyatos S, Berruezo A, Bijnens B et al. . Left atrial size and function by three-dimensional echocardiography to predict arrhythmia recurrence after first and repeated ablation of atrial fibrillation. Eur Heart J Cardiovasc Imaging 2014;15:515–22. [DOI] [PubMed] [Google Scholar]
- 10.Shin SH, Park MY, Oh WJ, Hong SJ, Pak HN, Song WH et al. . Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation. J Am Soc Echocardiogr 2008;21:697–702. [DOI] [PubMed] [Google Scholar]
- 11.Sohns C, Sohns JM, Vollmann D, Luthje L, Bergau L, Dorenkamp M et al. . Left atrial volumetry from routine diagnostic work up prior to pulmonary vein ablation is a good predictor of freedom from atrial fibrillation. Eur Heart J Cardiovasc Imaging 2013;14:684–91. [DOI] [PubMed] [Google Scholar]
- 12.Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC et al. . Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 13.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S et al. . Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231–9. [DOI] [PubMed] [Google Scholar]
- 14.Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, Kholmovski E et al. . Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 2010;160:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amlie JP. Dispersion of repolarization. A basic electrophysiological mechanism behind malignant arrhythmias. Eur Heart J 1997;18:1200–2. [DOI] [PubMed] [Google Scholar]
- 16.Sarvari SI, Gjesdal O, Gude E, Arora S, Andreassen AK, Gullestad L et al. . Early postoperative left ventricular function by echocardiographic strain is a predictor of 1-year mortality in heart transplant recipients. J Am Soc Echocardiogr 2012;25:1007–14. [DOI] [PubMed] [Google Scholar]
- 17.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64. [DOI] [PubMed] [Google Scholar]
- 18.Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J 2009;30:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A et al. . Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 2012;25:667–73. [DOI] [PubMed] [Google Scholar]
- 20.Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T et al. . Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 2010;3:247–56. [DOI] [PubMed] [Google Scholar]
- 21.Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E et al. . Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089–96. [DOI] [PubMed] [Google Scholar]
- 22.Sirbu C, Herbots L, D'Hooge J, Claus P, Marciniak A, Langeland T et al. . Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr 2006;7:199–208. [DOI] [PubMed] [Google Scholar]
- 23.Tsai WC, Lee CH, Lin CC, Liu YW, Huang YY, Li WT et al. . Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation. Echocardiography 2009;26:1188–94. [DOI] [PubMed] [Google Scholar]
- 24.Providencia R, Faustino A, Ferreira MJ, Goncalves L, Trigo J, Botelho A et al. . Evaluation of left atrial deformation to predict left atrial stasis in patients with non-valvular atrial fibrillation—a pilot-study. Cardiovasc Ultrasound 2013;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2009;2:10–5. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 27.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 28.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S et al. . Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol 2005;45:285–92. [DOI] [PubMed] [Google Scholar]
- 29.Calvo N, Brugada J, Sitges M, Mont L. Atrial fibrillation and atrial flutter in athletes. Br J Sports Med 2012;46:37–43. [DOI] [PubMed] [Google Scholar]
- 30.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J et al. . Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace 2008;10:618–23. [DOI] [PubMed] [Google Scholar]
- 31.Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C et al. . Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res 2002;54:390–6. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]