Abstract
Frailty is an important construct in aging which allows for the identification of the most vulnerable subset of older adults. At least two conceptual models of frailty have been developed that have in turn facilitated the development of multiple frailty screening tools. This has enabled the study of populations of frail and nonfrail older adults, and facilitated the risk assessment for adverse health outcomes. In addition, using the syndromic approach to frailty, numerous biological hypotheses have been tested, which have identified chronic inflammatory pathway activation, hypothalamic-pituitary-adrenal axis activation, and sympathetic nervous system activity as important in the development of frailty. In addition, age-related molecular changes related to autophagy, mitochondrial decline, apoptosis, senescent cell development, and necroptosis likely contribute to the heterogeneous phenotype of frailty. The recent development of a frail mouse model with chronic inflammatory pathway activation has helped to facilitate further whole organism biological discoveries. The following article attempts to create an understanding of the connections between these age-related biological changes and frailty.
Introduction
Frailty has long been recognized by health care providers as a syndrome of vulnerability that marks a subset of older adults as being at high risk for adverse health care outcomes, such as functional decline, disability, worsening chronic illnesses, and mortality. Over the past several years, research interest in frailty has grown exponentially as has the interest in integrating frailty into clinical practice models. In addition, marked progress in understanding age-related biological changes has helped to unravel the biology that likely underlies frailty and related biological vulnerability. The purpose of this chapter is to provide a state-of-the-art conceptual overview of frailty and to describe the known physiological and molecular changes that associate with and drive the development of frailty. Further research in this area will help to (1) identify those older adults at highest risk of adverse outcomes, (2) facilitate improved understanding of the biological underpinnings of frailty-related vulnerability, and (3) foster the development of improved preventive and intervention strategies aiming to improve health outcomes and quality of life of older adults.
Frailty Conceptualization
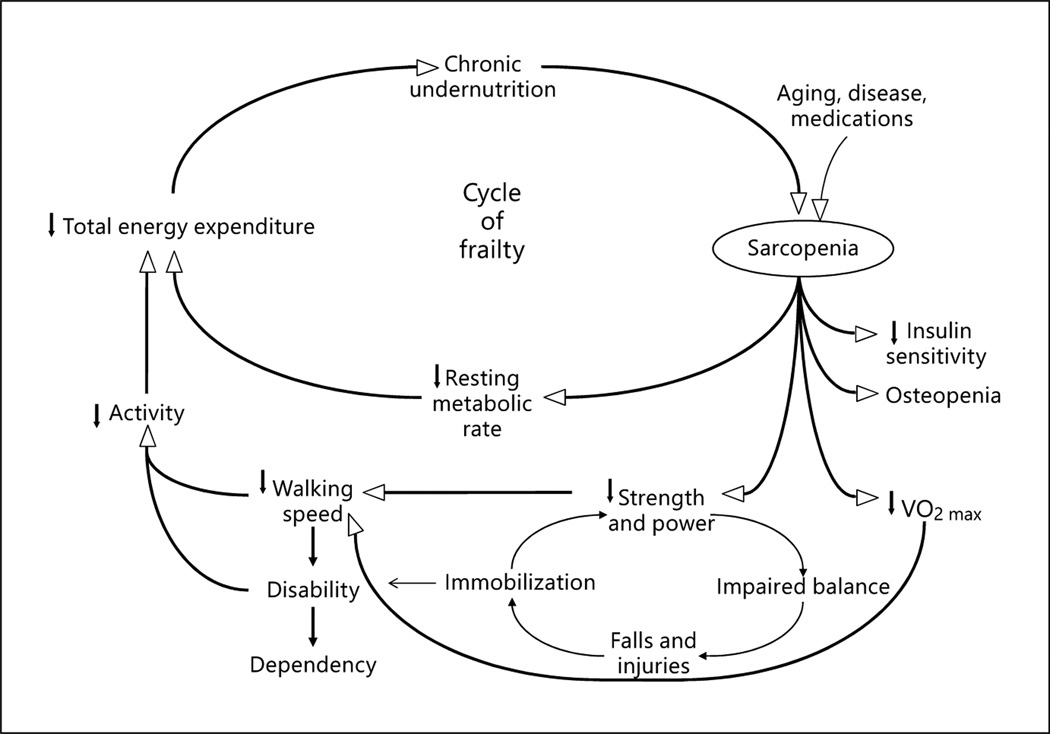
Over the past 20 years, the definitions of frailty have evolved from generalized observations by clinicians that frail individuals were vulnerable, weak, and failing to multiple definitions that have helped to bring scientific rigor to the field of frailty research. At present, two major conceptualizations of frailty have emerged in the literature. The most commonly utilized conceptualization is that frailty is an age-related syndrome with a deep biological basis that becomes manifest during periods of stress [1]. This concept has been further refined into a cycle of decline in energy, skeletal muscles and nutrition that can be triggered by disease, medications, or environmental stressors ( fig. 1). This cycle of decline, further influences medical illnesses, mobility, functionality, and cognition. Importantly, this conceptualization has been operationalized into the most commonly utilized frailty screening tool [1] and has allowed for hypothesis testing related to etiology and for intervention development period.
Fig. 1.
Cycle of frailty that underlies one of the primary conceptualizations of frailty, demonstrating a reinforcing downward physiological spiral that facilitates the development of adverse health outcomes. Reproduced with permission from Walston and Fried [26].
In addition to this biological conceptualization, a cumulative burden conceptualization of frailty has evolved. In this model, frailty is defined by a number of related and unrelated health, biological, social, functional, and cognitive variables that are tallied in an index. Those that are most frail are those with the highest tally of variables [2]. This model has been widely tested for predictive validity and has been shown to capture vulnerability as well as the biological model described above. However, frailty index was not constructed with an underlying biological theory in mind, and hence has not been extensively utilized to test biological hypotheses [2]. Further, because it was not conceptualized around a potential etiology, targeted preventive and intervention strategies have not been developed or tested to date using this model.
Frailty Measurement
Dozens of studies have been published over the past two decades that detail specific frailty measurement tools [3]. The vast bulk of these tools have been developed to study the risk of adverse outcomes in populations of older adults, and for the most part were developed utilizing aggregate measures of physical function, fatigue, and activity [3]. To date, no clear gold standard for the measurement of frailty has emerged and it is becoming increasingly apparent that none of these tools is adequate for all of the emerging clinical practice, risk assessment, biological research, and intervention development purposes that require frailty screening tools [4]. To date, most of the tools that have been developed can be divided into two groups that match the conceptualization of frailty articulated in the section above, i.e. either tools that attempt to capture essence of a biologically based, syndromic frailty or those that work to capture a tally of deficits or problems that in sum define a level of frailty. A brief summary of commonly utilized measurement tools are discussed below.
Fried et al. [1] previously developed a screening tool that consists of measured grip strength, walking speed, and weight loss along with subjective reporting of activity and fatigue levels. A score of 0–5 is generated based on a series of cutoff points for each measure, with 0 being robust, 1–2 being prefrail, and 3–5 being frail. This tool and adaptations to this tool have been used in population studies of community dwelling older adults to determine demographic information about older adults, and to identify individuals that are at significantly higher risk of adverse health outcomes such as disability, hospitalization, functional decline, and mortality compared to age-matched nonfrail individuals. In addition to this commonly utilized tool, many others have been developed that have a similar construct around syndromic frailty with weight loss, physical function, and skeletal muscle weakness measures most often included in these adaptations. While many have been validated by their ability to predict adverse outcomes in older adults, few have been utilized to test biological hypotheses or interventions.
A second common approach to detecting and measuring frailty comes from Rockwood and Mitnitski [2]. Building on the conceptualization of frailty as a condition that arises from cumulative declines, the tool consists of up to 71 measures of function, illness, cognition, and social mobility. A tally is taken, and those with the highest number of tallies are deemed most frail, and those with the lowest number of tallies are deemed least frail. This tool or similar indexing approaches do not require de novo measurements as information can be abstracted from medical records. Hence, it has been most commonly utilized to assess the risk of an adverse outcome, especially mortality in older adults. Importantly, numerous studies have attempted to include social or cognitive variables into screening tools in order to better capture frailty and its incumbent risk for adverse outcomes in broader population contexts. This will continue to be an important area of development in frailty research [5].
The Biology That Underlies Frailty
In order to better understand the etiology of frailty and late-life vulnerability, and in order to facilitate the development of treatment and prevention strategies, an improved understanding of the connections between age-related biological changes and frailty is required. As mentioned above, the most commonly utilized frailty construct was conceptualized around age-related biological and physiological changes with potential influence from poor nutrition and medical conditions [1]. In older adults, it is apparent that these changes do not exist in isolation; rather they exist together in a variety of constellations, which in sum contribute to frailty and the inherent biological vulnerability observed in frail, older adults [6]. We and others have helped to develop model pathways that have helped to conceptualize the interconnections between age-related biological changes, physiological system changes, and disease states with frailty and adverse outcomes [6]. The following sections detail studies of chronic disease, physiology, and biology that have been performed related to frailty.
Disease States and Frailty
Multiple investigators have attempted to identify important relationships between disease states and frailty in older adults. Early work identified a strong correlation between glucose intolerance and type 2 diabetes mellitus and frailty in older adults [7]. In addition, vascular disease, especially congestive heart failure, has been shown to be highly related to frailty in older adults [8]. Although associations between frailty and disease states do not prove causality, they suggest that a common underlying biological mechanism ties some diseases and frailty together. For example, an important biological commonality that has been identified in frailty and chronic disease is the chronic activation of inflammatory pathways and the strong relationship between inflammation and frailty as detailed below. Other studies have shown heightened activation of clotting pathways and more clotting events in frail older adults, further supporting biological commodity [7, 9]. Many studies demonstrate a substantial overlap between inflammation, frailty, and chronic disease states, including congestive heart failure, hypertension, and diabetes mellitus [7, 8, 10].
Physiological System Dysregulation and Frailty
Dysfunction in multiple physiological systems is thought to play an important role in late-life vulnerability. As biological understanding rapidly evolved, the distinction between physiological and molecular biological systems is blurred. For the purposes of this text, we will refer to physiological changes as those related to stress response systems that bridge between organs and tissues. The multiple chronic disease states including diabetes, vascular disease, chronic obstructive pulmonary disease, depression, and congestive heart failure that often coexist in frail, older adults likely contribute to the underlying biological vulnerability of many tissues and organs in older adults [11]. Many of these disease states have been demonstrated to chronically activate physiological systems, including the innate immune system, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis.
The identification of major physiological systems also thought to drive late-life vulnerability comes in part from the literature on frailty. Altered heart rate variability is a key measure of dysregulated sympathetic nervous system activity and is associated with aging, frailty, and cardiac arrhythmias [12]. Significantly higher levels of salivary cortisol during the afternoon nadir period have been observed in frail older adults compared to the nonfrail, suggesting chronically increased activity of the hypothalamic-pituitary-adrenal axis [13]. Multiple studies demonstrate a consistent and strong relationship between multiple inflammatory cytokines, C-reactive protein, and adverse outcomes of functional decline, frailty, chronic disease, and mortality [7]. The inflammatory cytokine interleukin (IL)-6 is most often used as a serum marker of inflammation in research studies involving older adults. Both of these measures are highly biologically active molecules and are the best predictors of mortality in at least two large population studies of older adults. Chronic, long-term exposure to IL-6 likely negatively impacts stem and satellite cells, which in turn may influence the development of chronic anemia and age-related declines in skeletal muscle (sarcopenia) and bone mass (osteopenia) commonly observed in frail, older adults [14].
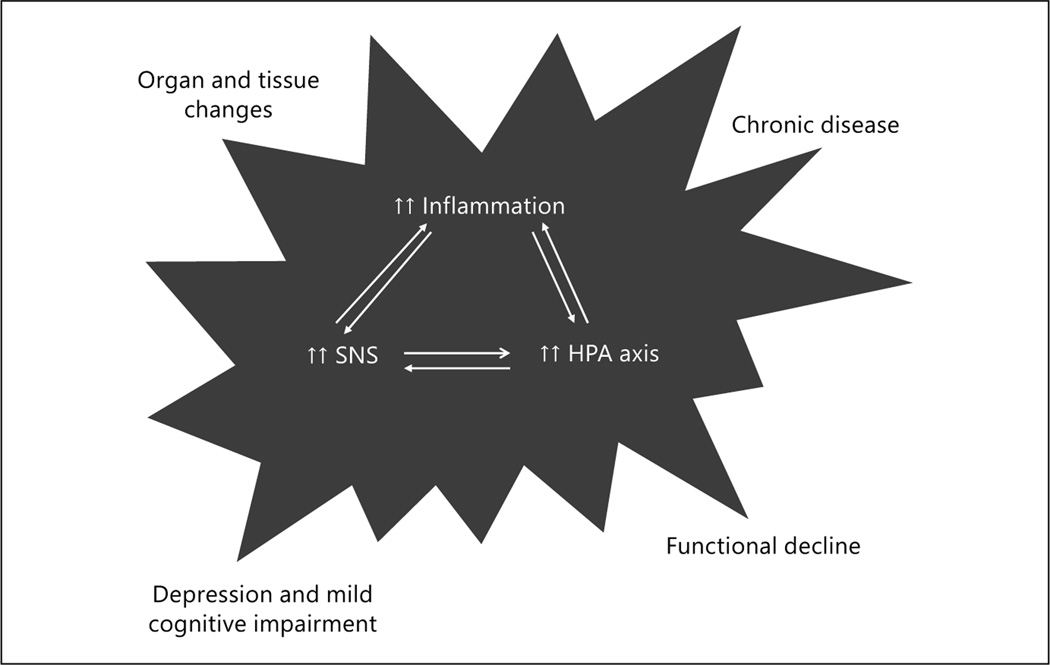
Importantly, the activation of each of these systems helps to reinforce the activation of chronic activation of the other stress response systems [14]. This in turn facilitates the local and systemic release of a broad array of bioactive molecules, such as cortisol, IL-6, and norepinephrine. Although each of these biomediators triggers critical stress responses and plays signaling roles that are vital for health and well-being, chronic activation of these systems is likely to have a negative impact on many organs, tissues, and stem cells that may replenish these organs and tissues. This in turn can further exacerbate aging-related clinical conditions such as osteoporosis and hypertension and increase vulnerability to other adverse health outcomes. Figure 2 illustrates the interacting stress response systems described above and chronically secreted bioactive mediators that may influence vulnerability late in life.
Fig. 2.
Model of interacting stress response systems that likely drive pathophysiological changes in frailty. HPA = Hypothalamic-pituitary-adrenal; SNS = sympathetic nervous system.
Age-Related Cellular and Molecular Changes and Frailty
Over the past decade, marked improvement in the understanding of age-related biological changes has been realized, including advances in the understanding of apoptosis, necroptosis, mitochondrial dysfunction, cell senescence, autophagy, and inflammatory pathway activation. This in turn has often facilitated the understanding of the role of these aging-related changes in the development of aging-related disease states, such as Parkinson’s disease and type 2 diabetes mellitus [15]. These biological changes are also likely highly relevant to the multisystem changes observed in frailty, as well as the multiple disease states that are highly associated with frailty. Like the physiological changes, the molecular changes likely do not exist in isolation but contribute across systems, organs, and tissues to frailty and its incumbent vulnerability to adverse outcomes such as functional decline, acute and chronic illness, and ultimately to mortality.
An example of this comes from attempts to understand the underlying etiology of the age-related activation of inflammatory pathways and its relationship to a host of disease states and functional decline [16]. The etiology of the activation of inflammatory pathway activation comes from several age-related biological sources. Increasing evidence suggests that senescent cell populations arise with increasing age, and may lead to alterations in tissue and immune system function that contribute to frailty. For example, fibroblasts and fat cells evolve towards a phenotype whereby reproduction and cell death are less likely to take place, and survival persists in an altered, less functional state [17]. These senescent cells no longer function normally and chronically secrete inflammatory cytokines and other bioactive molecules that negatively impact surrounding tissues [17]. Beyond this inflammatory impact, senescent T-cell populations also increase in number with increasing age, perhaps related to early-life cytomegalovirus or other viral exposure [18]. Age-related changes in mitochondrial function are also thought to play an important role in the development of activated inflammatory pathways through the increased production of proinflammatory free-radical by-products [19].
Many other altered molecular processes are also thought to contribute to broad systemic changes in older frail adults. Autophagy, an intracellular process responsible for the recycling of damaged or redundant organelles or proteins, becomes less effective with age [15]. This results in an intracellular accumulation of dysfunctional mitochondria and proteins, which in turn can trigger cellular dysregulation via increased levels of free radicals, lower mitochondrial energy production, and programmed cell death or apoptosis [19, 20]. Next, it is evident that some tissues become more sensitive to apoptosis or programmed cell death. Apoptosis is a normal cellular program that serves to kill and dissemble damaged or redundant cells in all tissues. However, it appears to accelerate and likely contributes to the vulnerability to chronic disease states such as Parkinson’s disease and congestive heart failure, or to the generalized loss of cells in many tissues [15]. Another potential molecular contributor to frailty and late-life vulnerability is increased TGF-β signaling, which likely plays an important role in the fibrotic changes that are observed in heart and lung tissue [21]. Importantly, these aging-related molecular and cellular changes are heterogeneous, and may contribute to frailty at different ages and through different disease states.
Building on the need to identify animal models to study aging-related physiological and biological changes and how they affect frailty and vulnerability to adverse outcomes, a frail mouse model was recently characterized [22]. Given the strong relationship between frailty and chronic activation of inflammatory pathways, the IL-10tm/tm mouse was chosen for further study because of the mild activation of inflammation associated with loss of the anti-inflammatory cytokine IL-10 [22]. Like frail humans, the IL-10tm/tm mouse has been found to develop modest elevations in serum inflammatory markers with increasing age, age-related declines in muscle strength and activity levels, and premature all-cause mortality compared to age- and gender-matched background control strain mice [22, 23]. In addition, the mouse develops decreased cardiac ejection fraction without substantial myocardial loss, consistent with the strong relationship noted between frailty and congestive heart failure in humans [8, 24]. The underlying molecular etiology that drives this multisystem decline has not yet been completely characterized. However, there is evidence for skeletal muscle mitochondrial dysfunction in the older IL-10tm/tm mouse through studies that demonstrate lower levels of ATP flux in frail compared to control mice [25]. Although characterization of this mouse model is not complete, early evidence suggests that it may be a good model for testing additional mechanistic and biological hypotheses related to aging, chronic inflammation and frailty, as well as a model to test intervention strategies.
In summary, frailty in older adults is a rapidly emerging clinical construct that likely has age-related biological underpinnings, and stress response systems and especially inflammation activation appear to drive declines in function and health. New discoveries related to apoptosis, necroptosis, cellular senescence, and mitochondrial decline are now being implemented in frailty research using novel cellular and animal models. Future interventions targeting specific biological pathways may slow these pathophysiological changes and improve health and well-being in older adults.
Acknowledgments
Disclosure Statement
This work was supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center of the US National Institute on Aging (award No. P30AG021334).
References
- 1.Fried LP, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M1–M11. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 3.Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr. 2013;13:64. doi: 10.1186/1471-2318-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment – a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Boland LL, Cushman M, et al. Frailty and risk of venous thromboembolism in older adults. J Gerontol A Biol Sci Med Sci. 2007;62:79–82. doi: 10.1093/gerona/62.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60:1701–1707. doi: 10.1111/j.1532-5415.2012.04099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JL, Boudreau RM, Fried LP, et al. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: the Cardiovascular Health Study. J Am Geriatr Soc. 2011;59:1581–1588. doi: 10.1111/j.1532-5415.2011.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varadhan R, Chaves PH, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64:682–687. doi: 10.1093/gerona/glp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadhan R, Walston J, Cappola AR, et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63:190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- 14.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang GC, Kao WH, Murakami P, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- 20.Ghavami S, Shojaei S, Yeganeh B, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 22.Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko F, Yu Q, Xue QL, et al. Inflammation and mortality in a frail mouse model. Age (Dordr) 2012;34:705–715. doi: 10.1007/s11357-011-9269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikka G, Miller KL, Steppan J, et al. Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp Gerontol. 2013;48:128–135. doi: 10.1016/j.exger.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akki A, Yang H, Gupta A, et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr) 2014;36:21–30. doi: 10.1007/s11357-013-9540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]