Abstract
The adequate time to perform physical activity (PA) to maintain optimal circadian system health has not been defined. We studied the influence of morning and evening PA on circadian rhythmicity in 16 women with wrist temperature (WT). Participants performed controlled PA (45 min continuous-running) during 7 days in the morning (MPA) and evening (EPA) and results were compared with a no-exercise-week (C). EPA was characterized by a lower amplitude (evening: 0.028 ± 0.01 °C versus control: 0.038 ± 0.016 °C; p<0.05) less pronounced second-harmonic (power) (evening: 0.41 ± 0.47 versus morning: 1.04 ± 0.59); and achrophase delay (evening: 06:35 ± 02:14 h versus morning: 04:51 ± 01:11 h; p>0.05) as compared to MPA and C. Performing PA in the late evening might not be as beneficial as in the morning.
Keywords: Circadian ambulatory monitoring, exercise, free-living conditions, rhythms, timing
INTRODUCTION
The potential role of physical activity (PA) as an external synchronizer of our circadian rhythm has been investigated for decades fostered by pioneering work during the seventies linking the diurnal variation in blood pressure to PA in human subjects (Krönig et al., 1976; Mann et al., 1979). Since then, and supported by accumulating evidence, PA has been consolidated as a key nonphotic synchronizer of rhythmicity (Back et al., 2007). A significant body of evidence has been obtained in experimental animal models (Bobrzynska & Mrosovsky, 1998; Redlin & Mrosovsky, 1997), as well as in humans under strict and highly controlled laboratory conditions (Edwards et al., 2002; Klerman et al., 1998). However, we do not really know to what extent the outcomes of some of the experimental approaches can be extrapolated to individuals living their daily routines and further studies should be conducted in humans under normal living conditions to understand the real impact of different synchronizers to our every-day circadian rhythms.
Structured, regular exercise is recommended to maintain or achieve optimal health. However, exercise takes many forms and varies in type, intensity, duration and frequency; and it is unclear which combination results in the best health benefits for the individual. Moreover, a frequently ignored variable that may affect both health outcomes and circadian rhythm is timing of exercise. Most previous studies have focused on the effects of timing on sports performance (Drust et al., 2005) and whether training time should match competition time in order to achieve maximum performance during critical times (Hill et al., 1989).
However, to our knowledge, none of the previous studies has analyzed the synchronizing effect of PA on the circadian system depending on the time of the day in free-living subjects. Moreover, we do not know which time of the day is the most appropriate to perform PA in order to maintain a healthy circadian rhythm, considered as the one with high amplitude, high regularity (high inter-day stability) and low fragmentation (low intraday rhythmicity) (IV) (Blazquez et al., 2012; Corbalán-Tutau et al., 2011).
A practical marker to evaluate circadian rhythm and to identify its disorders is skin temperature. This easily obtained measure has been shown to be useful to characterize biorhythms in a variety of population groups including babies (Zornoza-Moreno et al., 2011) and young people (Martinez-Nicolas et al., 2011; Ortiz-Tudela et al., 2010; Sarabia et al., 2008), as well as to identify the chronodisruption associated with different pathologies such as hypertension (Blazquez et al., 2012), metabolic syndrome and obesity (Corbalán-Tutau et al., 2011). Moreover, it has been shown that wrist skin temperature (WT) rhythm has a distinct endogenous component, even in the presence of multiple external influences. More importantly, WT phase indexes are capable of accurately detecting the phase of the circadian system in subjects recorded under normal living conditions as compared to Dim Light Melatonin Onset (DLMO) (Bonmati-Carrion et al., in press). Therefore, WT has been proposed as an informative and minimally invasive technique to measure circadian rhythm in free-living subjects (Martinez-Nicolas et al., 2013).
Therefore, in order to close some of the current knowledge gaps, we have investigated using measures of WT whether the quality of the circadian rhythm is affected by the timing of PA in healthy women exposed to their usual routines.
MATERIAL AND METHODS
Subjects
The study was conducted in 16 normal-weight women (Age: 23 ± 4; BMI: 23.2 ± 2.9 kg/m2) who signed a written informed consent before participating in the study, which was approved by the University of Murcia ethical committee (Portaluppi et al., 2010).
The participants performed controlled PA outdoors (45 min of continuous running) during 7 days in the morning (09:00 h) and night (21:00 h) in two alternative weeks and results were compared with a no exercise week (control week). WT was measured every 10 min during 7 days/per week and in 3 consecutive weeks.
Determination of WT rhythm
WT rhythm was assessed using a temperature sensor (Thermochron iButton DS1921H; Dallas, Maxim, Dallas, TX) with a sensitivity of 0.125 ± 1 °C and programmed to collect information every 10 min. It was attached to a double-sided cotton sport wrist band, and the sensor surface was placed over the inside of the wrist on the radial artery of the non-dominant hand, as described previously by Sarabia et al. (2008). The information stored in the iButton was transferred through an adapter (DS1402D-DR8; Dallas, Maxim) to a personal computer using iButton Viewer v. 3.22 (Dallas Semiconductor Maxim software provided by the manufacturer). Data were recorded during the months of March–May, with environmental temperatures ranging between 17.7 ± 4.1 °C (data obtained from the Center for Statistics of Murcia, Spain) to minimize the influence of extreme environmental temperatures on WT and with no significant changes in environmental temperature between both weeks of experiments. Rhythmic parameters were obtained using an integrated package for temporal series analysis “Circadianware® (Murcia, Spain)”.
Statistical methods and variables obtained
To characterize WT, activity and position rhythms, we calculated the following parameters using parametric and non-parametric methods in the seven recording days.
Cosinor's analysis
– Amplitude: Difference between the maximum (or minimum) value of the cosine function and mesor.
– Acrophase: Timing of the maximum value of the cosine function.
– Fourier analysis: we calculated the first 12 harmonics – (from 24 to 2 h periods) and the circadian index as the division between the powers of the first harmonic divided by the sum of the first 12 harmonics. This index represents the relative strength of the first harmonic as compared to the other ultradian harmonics.
– The second harmonic (P2) that gives the postpran-dial rise in WT.
Non-parametric analysis (Van Someren et al., 1999)
These non-parametrical parameters are used to define the waveform in those cases of not having a strictly sinusoidal pattern as is the case of WT, activity and position. Moreover, the circadian rhythms of WT in humans are not symmetric with respect to 12/12. They are not 12 hour of decrease and 12 h of rising. Instead, they are 2/3 of decrease and 1/3 of rising.
– Interdaily stability: The similarity of the 24 h pattern over days. It varied between 0 for Gaussian noise and 1 for perfect stability, where the pattern repeated itself exactly day after day.
– Intradaily variability (IV), which characterizes the rhythm fragmentation. Its values oscillated between 0, when the wave was perfectly sinusoidal, and 2, when the wave was as Gaussian noise.
– Average of the 5 consecutive hours of maximum values (M5) of WT and its timing (TM5): hourly average during the 10 consecutive hours of minimum values (L10) of WT and its timing (TL10)
– Relative amplitude, it was calculated by the difference between M5 and L10 divided by the sum of M5 and L10.
All these rhythmic parameters were obtained using an integrated package for temporal series analysis “Circadianware” (Chronobiology Laboratory, University of Murcia, Spain, 2010).
Morning–evening questionnaire
Women completed the morningness/eveningness (M/E) questionnaire (MEQ) 19-item scale of Horne & Ostberg (1976). M/E typology is a way to characterize subjects depending on individual differences of wake/sleep patterns and the time of day people feel or perform best. Some people are night “owls” and like to stay up late at night and sleep late in the morning (evening type), while others are early birds and prefer to go to bed at an early hour and arise with the break of dawn (Morning types). The majority of people is in between and categorized as “Neither types”. Evening types were considered as scoring under 41 and morning types above 59. All subjects within the range of 42–58 were classified as neither type (Adan & Almirall, 1990).
Sleep and feeding diary
Subjects were instructed to keep a sleep and feeding diary designed by the Murcia University Chronobiology Laboratory (Sarabia et al., 2008). The following data were obtained for every subject on a daily basis: time the subject went to bed, time of lights off, nocturnal awakenings lasting more than 10 min, sleep offset, time the subject got up, time and duration of naps and the onset time, duration and nutrient composition of the three main meals (breakfast, lunch and dinner) and of any snacks.
Statistical analyses
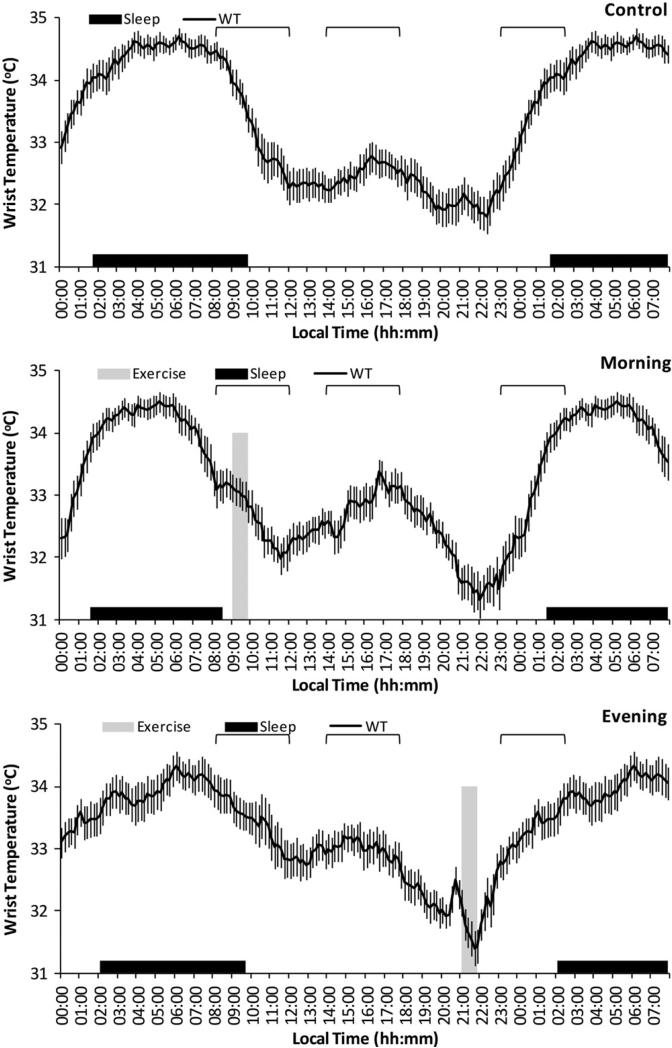
To assess statistical differences between control, morning and evening PA WT waveforms, a repeated-measures analysis of variance was performed for the studied women (global analysis of variance, p for timing of PA influence), the kinetics of the response (p for time of WT) and the interaction of both factors (timing of PA × time of WT). When statistical differences were found by the repeated-measures analysis of variance, a multiple-comparison test, adjus f ted by the least significant difference, was applied to identify differences between groups or each time point. Statistical differences among control, morning and evening PA are represented in Figure 1 in brackets.
FIGURE 1.
WT means waveform. Daily evolution of WT (black line) in the three different conditions (control: without PA; morning: with scheduled activity between 09:00 and 09:45; evening: with scheduled activity between 21:00 and 21:45 in the upper, middle and bottom part, respectively). The time of PA is marked with the grey bar and the averaged sleep pattern with the black area for all experimental subjects (n = 16). The data shown have been obtained by averaging all individual mean waveforms. All variables are expressed as mean ± SEM and the three signaled areas are awakening (from 8:30 to 12:00), postprandial (from 14:00 to 17:50) and to fall asleep (from 23:00 to 02:50) from left to right and represents those sections of the graph with significant differences (p<0.05). Note that the last 8 h of the figure (from 00:00 to 07:50) are repeated in terms of clarity.
RESULTS AND DISCUSSION
This study was performed to elucidate whether the time at which PA is performed influences the quality of the habitual circadian pattern and its relation with health status. Along these lines, our data support the notion that performing PA during the evening impairs circadian rhythmicity and may counteract some of the health benefits associated with PA.
Average waveforms for WT during seven consecutive days of control and PA of 45 min running in the morning (09:00 h) or in the evening (21:00 h), are shown in Figure 1. Signaled areas are awakening (from 8:30 to 12:00), postprandial (from 14:00 to 17:50) and to fall asleep (from 23:00 to 02:50) from left to right and represents those sections of the graph with significant differences among control, morning PA and evening PA.
During the control week, daily WT patterns of the participating women were characterized by the expected increase of temperature before the time of sleep onset, a nocturnal steady high temperature and a pronounced drop after arising in the morning. There was a secondary peak during the afternoon hours, a period traditionally associated with napping, and a dip between 20:00 h and 22:00 h a period of minimum temperature already known as the “wake maintenance zone” (Lavie, 1985).
Compared to the control week, we found more stable and less fragmented WT pattern when PA was performed in the morning, with a more pronounced drop after arising in the morning, a more marked second harmonic (postprandial peak) and also a more marked “wake maintenance zone” as assessed by ANOVA (p<0.05) (Figure 1). Furthermore, significant differences were found in the parameters obtained from Cosinor's analysis such as the power of the second harmonic P2 (control: 0.49 ± 0.48 versus morning: 1.04 ± 0.59) (= 0.007) and the circadian index (control: 66.3 ± 13.48 versus morning: 35.97 ± 24.86)(p<0.0001). These factors have been previously considered as indicators of a healthier pattern (Blazquez, 2012; Corbalán-Tutau et al., 2011; Van Someren, 1993) and as a consequence these results suggest that morning exercise improves the circadian pattern as compared to the control period.
Conversely, when exercise was performed during the evening hours (21:00 h), we found a significantly flattened and irregular pattern in the evening as compared to the control and morning week after an ANOVA analysis (Figure 1 sections highlighted in parenthesis), suggesting that circadian rhythmicity was impaired. Indeed, the relative amplitude was significantly diminished as compared to the control week (°C) (evening: 0.028 ± 0.01 versus control: 0.0381 ± 0.016) (p<0.05), although statistic did not reach significance when compared with morning exercise for this specific variable.
The slower elevation of temperature at the beginning of the sleep in the evening PA curve, as assessed by the scope of the curve (°C/h) (control: 0.55 ± 0.09a versus morning: 0.77 ± 0.06b versus evening: 0.28 ± 0.09c) (p<0.001) suggests a decrease in the sleep deepness. These results could be explained by different causes: (a) performing intense PA during the evening is able to maintain the sympathetic nervous system activated which could impair vasodilation and as a consequence difficult the increment of temperature that is necessary for the onset of a deep sleep; (b) the accumulation of pro-oxidants and inflammatory metabolites that occurs during vigorous exercise could also hinder the temperature increase; (c) changes in other synchronizers such as light or food intake could also explain these results. In fact, although light was not measured in this study, women ran outdoors in the morning and they were exposed to natural light and this could have additive or synergistic actions over the PA. This did not happen during evening period. Furthermore, when women ran in the evening hours, they had changes in the sleep characteristics and in the timing of dinner (Table 1) that could influence WT patterns. Indeed, evening PA was associated with a latter nap onset, while morning PA was related to shorter sleep duration. Under free-living conditions, this is an important aspect to consider because the timing of exercise may influence the circadian rhythms by affecting other external synchronizers.
TABLE 1.
Sleep and meal time variables for each experimental situation.
| Sleep | Nap | Breakfast | Lunch | Dinner | |
|---|---|---|---|---|---|
| Onset (hh:mm) | |||||
| Control | 01:42 ± 00:14a | 16:30 ± 00:19ab | 10:05 ± 00:14a | 14:66 ± 00:07a | 21:58 ± 00:06a |
| Morning | 01:31 ± 00:09a | 15:44 ± 00:23a | 09:24 ± 00:15a | 14:44 ± 00:06a | 22:35 ± 00:07b |
| Evening | 02:01 ± 00:12a | 17:13 ± 00:22b | 09:53 ± 00:12a | 14:39 ± 00:04a | 22:35 ± 00:07b |
| p | 0.211 | 0.023 | 0.098 | 0.840 | 0.000 |
| Offset (hh:mm) | |||||
| Control | 09:52 ± 00:16a | 17:28 ± 00:23a | 10:20 ± 00:14a | 15:20 ± 00:09a | 22:32 ± 00:08a |
| Morning | 08:33 ± 00:15b | 17:10 ± 00:21a | 09:38 ± 00:26a | 15:15 ± 00:07a | 23:13 ± 00:07b |
| Evening | 09:47 ± 00:16a | 18:17 ± 00:21a | 10:07 ± 00:13a | 15:11 ± 00:06a | 23:02 ± 00:08b |
| p | 0.001 | 0.091 | 0.114 | 0.657 | 0.000 |
| Duration (hh:mm) | |||||
| Control | 08:10 ± 00:09a | 00:59 ± 00:14a | 00:14 ± 00:02a | 00:41 ± 00:05a | 00:33 ± 00:27a |
| Morning | 07:02 ± 00:13b | 01:26 ± 00:11a | 00:14 ± 00:02a | 00:31 ± 00:02a | 00:39 ± 00:05a |
| Evening | 07:47 ± 00:19ab | 01:04 ± 00:08a | 00:14 ± 00:02a | 00:32 ± 00:04a | 00:27 ± 00:03a |
| p | 0.005 | 0.204 | 0.974 | 0.128 | 0.117 |
| Frequency (%) | |||||
| Control | 100.00 ± 0.00a | 21.85 ± 5.89a | 86.61 ± 6.96a | 100.00 ± 0.00a | 97.32 ± 1.94a |
| Morning | 100.00 ± 0.00a | 47.90 ± 5.61a | 93.75 ± 5.37a | 100.00 ± 0.00a | 99.11 ± 0.89a |
| Evening | 100.00 ± 0.00a | 32.77 ± 6.68a | 85.71 ± 3.45a | 99.11 ± 0.89a | 99.54 ± 1.71a |
| p | 1.000 | 0.140 | 0.526 | 0.376 | 0.289 |
Onset, offset, duration and frequency of meals (breakfast, lunch and dinner) and sleep periods (night sleep and daytime naps) for morning exercise, evening exercise and control weeks. Onset, offset and duration are expressed as hours and minutes and frequency as a percentage (Mean ± SEM). Different letters indicate significant differences between experimental situations in each variable.
Interestingly, the timing of the 5 consecutive hours of maximum temperature (TM5), which coincide with the deepest and more restoring sleep, showed a phase delay of approximately 2 h during the evening training period (morning: 04:51 ± 01:11 h versus evening: 06:35 ± 02:14 h) (p = 0.009) (Figure 1). Our group has previously demonstrated that WT phase indexes are capable of accurately detecting the phase of the circa-dian system in subjects recorded under normal living conditions as compared to DLMO. Indeed, WT phase indexes were capable to detect the phase of the circadian system as compared to DLMO showing a strong correlation, a high prediction capacity and a temporal coincidence with DLMO. This phase delay is rather common among young people nowadays (Roenneberg et al., 2003, 2007) and has been previously seen in laboratory conditions (Van Reeth et al., 1994). Therefore, more information is needed about the overall benefits of exercising late in the evening, considering the previously noted phase delay and the potentially negative effects associated with this chronodisruption (Escames et al., 2012).
Changes in the nocturnal temperature, which characterized the evening PA, could explain the higher temperature values achieved during the morning, particularly during the range from 08:00 h to 12:00 h (ANOVA, p<0.05) (Figure 1). Higher temperatures have been related to diurnal skin vasodilation, parasympathetic activation and sleepiness (Buijs & Kalsbeek, 2001; Cajochen et al., 2005). Moreover, the pronounced drop of temperature after arising in the morning that characterized the control week practically disappeared during the evening week. Conversely, this drop was accentuated during the morning exercise week (ANOVA)(p<0.05) (Figure 1).
Another important difference was related to the ultradian rhythms (from 12 to 2 h periods) and with the circadian index. Evening waveforms were characterized by a decrease in the second harmonic (Power) (morning: 1.04 ± 0.59 versus evening: 0.41 ± 0.47) (p = −0.001) with a less pronounced peak (ANOVA) (p<0.05) (Figure 1), and with an increased circadian index (morning: 35.97 ± 24.86 versus evening: 52.90 ± 19.58; p = 0.001).
Previously, our group has shown that the absence of this second circadian harmonic peak (P2) is a marker of chronodisruption, metabolic dysfunction (García-Prieto et al., 2007) and obesity (Corbalán-Tutau et al., 2011). Differences in food intake, and its specific dynamic action, or differences in short periods of napping could be related to these changes (Sarabia et al., 2008). Indeed, the results from the sleep diary show significant differences in the timing of naps’ onset and offset between evening and morning PA weeks, although frequency and duration of naps were similar. More importantly, differences were found in the intake of proteins that was significantly lower in the evening than in the control week (kcal) (evening: 113.6 ± 3.28 versus control: 143.16 ± 26.12), while no significant differences were found for morning and control weeks. We should underscore that dietary protein is the macronutrient with the highest specific dynamic action.
One of the objectives of this study was to define whether evening-type women who could be potentially better adapted to exercise in the evening presented fewer circadian system alterations when performing intense PA in the evening than their morning-type counterparts. However, our results show that when performing PA during the evening, evening-type women had similar changes in the WT circadian pattern than morning-type women. Moreover, they had a significantly higher circadian index with a higher predominance of P1 as compared to P2 and ultradian peaks (60.62 ± 18.71; 46.90 ± 19.07) (p = 0.02), a significantly lower position CIF (0.229 ± 0.11, 0.249 ± 0.12) (p = 0.028) and a similar trend in WT CIF (0.42 ± 0.06; 0.44 ± 0.07) in evening type than morning-type subjects, suggesting that evening exercise may not be beneficial regardless of the chronotype.
One limitation of the current study is that although data indicate that the timing of exercise affects the circadian system status of a subject considered as the combination of its endogenous and exogenous components, it is not possible to distinguish weather the effects are due to (a) direct effect on the endogenous component, (b) desyncrony between the internal clock and exercise as an external synchronizer or (c) changes in other external synchronizers (timing of food intake, sleep or light) associated to the changes in the timing of exercise. Moreover, we only have data about circadian rhythmicity patterns, but we lack information about the actual impact of these changes on the pathophysiology of the individual. On the other hand, this is a short-term study and we do not know about the circadian and metabolic consequences of habitual PA during the evening versus the morning hours.
Previous studies have analyzed the effect of the time of the day in sports performance; however, to the best of our knowledge, this is the first study to report differences about the synchronizing effect of PA on the human circadian system in relation to the timing of the exercise. The results of the current experiment suggest that, based on the observed effects on the circadian rhythm, performing intense PA in the late evening might not be as beneficial as doing it in the morning.
Acknowledgments
This study was supported by grants from the Tomás Pascual and Pilar Gómez-Cuétara Foundations, the Spanish Government of Science and Innovation (BFU2011-24720 and BFU2010-21945-C02-01) and the Séneca Foundation from the Government of Murcia (15123/PI/10). National Heart, Lung, and Blood Institute grants HL-54776, National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number DK075030 and by contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research.
Footnotes
DECLARATION OF INTEREST
Authors fully declare any financial or other potential conflict of interest.
REFERENCES
- Adan A, Almirall H. Adaptation and standardization of a Spanish version of the morningness–eveningness questionnaire: Individual differences. Pers Indivd Differ. 1990;11:1223–30. [Google Scholar]
- Back FA, Fortes FS, Santos EHR, et al. Sincronização nãofótica: o efeito do exercício físico aeróbio:[revisão]: Non-photic synchronization: The effect of aerobic physical exercise:[review]. Rev Bras Med Esporte. 2007;13:138–42. [Google Scholar]
- Blazquez A, Martinez-Nicolas A, Salazar FJ, et al. Wrist skin temperature, motor activity and body position as determinants of the circadian pattern of blood pressure. Chronobiol Int. 2012;29:747–56. doi: 10.3109/07420528.2012.679328. [DOI] [PubMed] [Google Scholar]
- Bobrzynska KJ, Mrosovsky N. Phase shifting by novelty-induced running: Activity dose-response curves at different circadian times. J Comp Physiol A. 1998;182:251–8. doi: 10.1007/s003590050175. [DOI] [PubMed] [Google Scholar]
- Bonmati-Carrion MA, Middleton B, Revell V, et al. Circadian phase assessment by ambulatory monitoring in humans: Correlation with dim light melatonin onset. Chronobiol Int.; In press. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–6. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrino Metab. 2005;90:1311–16. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Corbalán-Tutau MD, , Madrid JA, Ordovás JM, et al. Differences in daily rhythms of wrist temperature between obese and normal-weight women: Associations with metabolic syndrome features. Chronobiol Int. 2011;28:425–33. doi: 10.3109/07420528.2011.574766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust B, Waterhouse J, Atkinson G, et al. Circadian rhythms in sports performance – An update. Chronobiol Int. 2005;22:21–44. doi: 10.1081/cbi-200041039. [DOI] [PubMed] [Google Scholar]
- Edwards B, Waterhouse J, Atkinson G, Reilly T. Exercise does not necessarily influence the phase of the circadian rhythm in temperature in healthy humans. J Sports Sci. 2002;20:725–32. doi: 10.1080/026404102320219437. [DOI] [PubMed] [Google Scholar]
- Escames G, Ozturk G, Baño-Otálora B, et al. Exercise and melatonin in humans: Reciprocal benefits. J Pineal Res. 2012;52:1–11. doi: 10.1111/j.1600-079X.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- García-Prieto MD, Tébar FJ, Nicolás F, et al. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: Relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf) 2007;66:185–91. doi: 10.1111/j.1365-2265.2006.02705.x. [DOI] [PubMed] [Google Scholar]
- Hill DW, Cureton KJ, Collins MA. Circadian specificity in exercise training. Ergonomics. 1989;32:79–92. doi: 10.1080/00140138908966069. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Klerman EB, Rimmer DW, Dijk DJ, et al. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:991–6. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- Krönig B, Knappen F, Dufey K, Wolff HP. Blood pressure response to physical activity in hypertensive subjects at different times of the day. Clin Sci Mol Med Suppl. 1976;3:677s–80s. doi: 10.1042/cs051677s. [DOI] [PubMed] [Google Scholar]
- Lavie P. Ultradian rhythms: Gates of sleep and wakefulness. Exp Brain Res. 1985;12:148–64. [Google Scholar]
- Mann S, Craig MW, Melville DI, et al. Physical activity and the circadian rhythm of blood pressure. Clin Sci (Lond) 1979;57:291s–4s. doi: 10.1042/cs057291s. [DOI] [PubMed] [Google Scholar]
- Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiol Int. 2011;28:617–29. doi: 10.3109/07420528.2011.593278. [DOI] [PubMed] [Google Scholar]
- Martinez-Nicolas A, Ortiz-Tudela E, Rol MA, Madrid JA. Uncovering different masking factors on wrist skin temperature rhythm in free-living subjects. PLoS One. 2013;8:e61142. doi: 10.1371/journal.pone.0061142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Tudela E, Martinez-Nicolas A, Campos M, et al. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comp Biol. 2010;6:e1000996. doi: 10.1371/journal.pcbi.1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;25:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Exercise and human circadian rhythms: What we know and what we need to know. Chronobiol Int. 1997;14:221–9. doi: 10.3109/07420529709001157. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects: A candidate of new index of the circadian system. Physiol Behav. 2008;95:570–80. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Sturis J, Byrne MM, et al. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol. 1994;266:E964–74. doi: 10.1152/ajpendo.1994.266.6.E964. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer's disease: Chronobiological perspectives. Behav Brain Res. 1993;57:235–53. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Van Someren EJW, Swaab DF, Colenda CC, et al. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16:505–18. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- Zornoza-Moreno M, Fuentes-Hernandez S, Sanchez-Solis M, et al. Assessment of circadian rhythms of both skin temperature and motor activity in infants during the first 6 months of life. Chronobiol Int. 2011;28:330–7. doi: 10.3109/07420528.2011.565895. [DOI] [PubMed] [Google Scholar]