Abstract
There is much clinical interest in the development of a low cost and reliable test for diagnosing inflammatory bowel disease and irritable bowel syndrome, two very distinct diseases that can present with similar symptoms. The assessment of stool samples for the diagnosis of gastro-intestinal diseases is in principle an ideal non-invasive testing method. This paper presents an approach to stool analysis using headspace gas chromatography and a single metal oxide sensor coupled to artificial neural network (ANN) software. Currently the system is able to distinguish samples from patients with irritable bowel syndrome (IBS) from patients with inflammatory bowel disease (IBD) with a sensitivity and specificity of 76% and 88% respectively, with an overall mean predictive accuracy of 76%.
Introduction
Inflammatory bowel disease (IBD) is an inflammatory autoimmune disease, thought to be caused by an inappropriate response of the immune system to commensal gut microbes [1, 2]. There are two types of IBD, ulcerative colitis (UC) and Crohn’s disease (CD). UC affects the large bowel only, affecting variable lengths of the colon continuously from the rectum, with inflammation primarily in the mucosa. CD can affect any part of the GI tract, and is a transmural disease [3]. Common symptoms of IBD are severe abdominal pain, defecation urgency and diarrhoea, which can contain blood and pus.
Irritable bowel syndrome (IBS) is a functional disorder of the digestive tract. Functional disorders are characterized by their symptoms, with no physiological changes seen in the GI tract and include disorders such as IBS, functional vomiting and functional abdominal bloating. IBS can be diarrhoea predominant (IBS-D), constipation predominant (IBS-C) or can alternate between the two (IBS-A). Common symptoms include abdominal pain and cramps, bloating and flatulence, and unusual bowel habit. IBS has, as yet, no known cause. People with IBS show abnormal gut motility and hypersensitivity to pain in the GI tract [4]. Stress and anxiety are known to cause changes in gut motility [5] with these symptoms being common co-morbidities of IBS.
IBS can present with symptoms similar to those of IBD and other non-functional bowel conditions such as colon cancer. The diagnosis of IBS can be difficult and is often one of exclusion, where more serious bowel diseases, such as IBD or colon cancer and other functional disorders are ruled out.
The current gold standard for diagnosis of inflammatory bowel disease is endoscopic and histological testing; however, these investigations are both invasive and costly, and have associated risks [6]. Endoscopic procedures can be used to rule out more serious conditions during diagnosis of irritable bowel syndrome. The costs associated with functional bowel diseases are significant [7]. Of the patients referred for invasive investigation few actually have organic bowel disease [8], and unnecessary procedures account for a large proportion of the costs associated with functional bowel disease. A colonoscopy costs the UK NHS many hundreds of pounds, whereas less invasive tests are typically of much lower cost [9].
There are currently no known biomarkers of IBS. There are various biomarkers that have potential in the differentiation of functional from inflammatory gastrointestinal disease; non-invasive testing for biomarkers such as lactoferrin and calprotectin and others such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are all used to help distinguish functional from inflammatory bowel disorders and to diagnose IBD [10, 11]. Biomarkers such as antibodies to common bacterial and fungal antigens that can indicate an abnormal response to commensal microbes can also be useful in diagnosing IBD [12].
Although these biomarkers can be useful as part of the screening process when establishing a diagnosis [8, 13], these tests can have a slow turnaround and there is still a need to develop quicker, lower cost, less invasive testing for diagnosis of gastro-intestinal disease.
An emerging area in disease diagnosis technology is detection of volatile organic compounds (VOCs). Changes in the composition of faeces, breath and other bodily fluids can be associated with disease states and can be reflected in the VOCs emitted from a sample [14,15,16,17]. Much work has been undertaken to investigate VOC analysis for gastro-intestinal disease diagnosis often using gas chromatography – mass spectrometry (GC-MS) or selected ion flow tube – mass spectrometry (SIFT-MS) [18,19, 20, 21,22, 23]. A summary of mass-spectrometry techniques for investigation of IBS and IBD can be found in a recent review [24]. The use of electronic noses has also recently been investigated for gastro-intestinal disease diagnosis [25,26]. These techniques use compound identification to identify possible biomarkers. An emerging theme in biomarker research is the use of combinations of multiple biomarkers, or of biomarker patterns for more accurate prediction [27] [28]. Disease pathology is often complex and can lead to multiple physiological changes resulting in an altered biomarker profile.
This paper describes the assessment of an in-house developed gas-chromatograph coupled to a metal oxide sensor system with pattern recognition software as a rapid diagnostic test to distinguish IBS from IBD.
2. Materials and Methods
2.1. Patient consent and ethical approval
Patients attending the gastroenterology clinic at the Bristol Royal Infirmary were requested by letter to bring a faecal sample to the clinic, the letter also contained the patient information sheet describing the study. Patients who took part gave verbal consent to the physician during the clinic appointment. Ethical approval for the study was granted by Wiltshire research and ethics committee (NRES 06/Q2008/6).
2.2. Sample collection and patient information
182 Stool samples were obtained from patients with IBD and IBS between October 2010 and October 2011 (Table 1). IBS samples were obtained from patients with IBS-D, IBS-C and IBS-A. IBD samples were obtained from patients with both UC and CD. IBS was diagnosed according to the Rome II criteria [29].
Table 1.
Patient information, patients were diagnosed by their physician at the gastroenterology clinic at the Bristol Royal Infirmary. Active IBD was classified by the patients HBI score (CD) or SCCAI score (UC). IBS was diagnosed according to the Rome II criteria.
| CD | UC | IBS | Healthy | |
|---|---|---|---|---|
| Total Samples | 42 | 59 | 34 | 46 |
| Mean age (years) | 45.6 | 61.7 | 50.7 | 57.6 |
| Ratio male: female | 1 : 0.63 | 1 : 0.71 | 1 : 2.1 | 1 : 1.7 |
| Total Samples from active IBD patients | 26 | 19 | ||
| Mean age of active IBD patients (years) | 44.4 | 56.4 | ||
| Ratio male: female of active IBD patients | 1 : 0.6 | 1 : 0.85 | ||
IBD samples used in the final analysis came from patients whose IBD was active at the time the sample was given. IBD was diagnosed by the physician after endoscopy and histological tests, or by radiology in the case of small intestinal disease. Disease activity in the patients with ulcerative colitis was determined by their simple colitis clinical activity index (SCCAI) score [30] with a score more than or equal to three classifying the patient as active. Crohn’s disease patients were classified by their Harvey Bradshaw index (HBI) score [31] with a score more than or equal to four classifying the patient as active.
Healthy controls were collected from healthy relatives of those visiting the clinic and from healthy patients referred for early endoscopy/colonoscopy due to family history of upper GI/colon cancer. All patients were on an ad lib diet. Samples were collected and aliquoted into 10ml glass headspace vials (Supelco, Sigma Aldrich) within 6 hours of sample production and frozen at -20 °C.
2.3. Sample analysis by gas chromatography
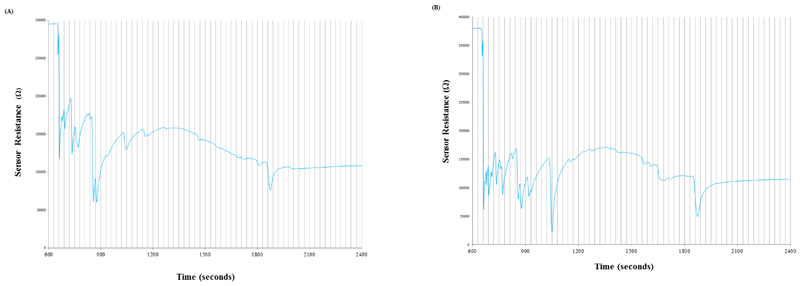
Headspace gas chromatography (GC) with a single metal oxide sensor as the detector was undertaken. Chromatograms were obtained for each sample, which were a trace of the sensors electrical resistance over time (Figure 1).
Figure 1.
Examples of typical chromatograms produced from a sample of an ulcerative colitis (a) and an IBS patient (b). Minor gridlines indicate the 30 second time bins divisions used in the ANN analysis.
The sensor was housed in a chamber and operated at 450 °C. Sensor substrates incorporating interdigitated gold electrodes with 8 interpenetrating bars, an electrode width 150 µm and electrode gap 100 µm were designed in–house, and screen-printed onto 3x3mm alumina substrates with a platinum heater on the reverse (ESL, Reading, UK). The gold electrodes were coated in-house with a tin and zinc oxide paste using a method previously described [32 ]. Gold wires were used to mount the sensor to a TO-39 four pin header (Eltek Semiconductors Ltd, Dartmouth UK).
The GC used was an SRI 8610C (SRI Instruments, CA, USA) fitted with a heated static headspace injector. The oven was held at 40°C for 13.4 minutes before heating to 100 °C using a temperature ramp of 5°C/minute, held at 100 °C for 30 minutes then cooled to 40 °C using a temperature ramp of 10°C/minute. The carrier gas was synthetic air (BOC, Bristol, UK) and the capillary column used was a SPB-1 sulphur, 30m, internal diameter 0.32 mm, 4 µm film thickness (Supelco, Sigma Aldrich). Each sample was heated to 50°C for ten minutes prior to sampling 2 cm3 of the headspace using the in-built autosampler. Samples were run for 65 minutes from the time of headspace injection, the majority of compounds eluted within 30 minutes and the remaining run time was used to ensure there was no interference from late eluting peaks with the next sample.
2.4. Artificial Neural Network (ANN) analyses
An artificial neural network (ANN) software platform, based on a multi-layer perceptron model, written in-house, was used to train and test a series of artificial neural networks, with the aim of diagnosing the different sample groups.
The 30 minute chromatograms (Figure 1) of the samples were first subjected to a time correction based on the analysis of a standard stool sample which had been run daily prior to sample analysis, in order to compensate for any drift in retention time during the study. The traces were then manipulated to produce a set of discrete inputs suitable for ANN analysis. The first derivative with respect to time (dR/dt) was taken to display the rate of change of the sensor resistance, resulting in a set of discrete peaks. The chromatogram trace was split into 60 x 30 second time bins highlighted in Figure 1. The values of each of the data points falling within each 30 second time bin were summed to give a value for each bin. Finally the 60 bin values were normalised such that the largest bin was assigned a value of one, the smallest zero and all others proportional in between (a full list of bin values for each chromatogram can be found in supplementary table A1). The 60 bin values for the chromatogram were then input into the ANN. The momentum (alpha) and learning rate (eta) were both set to 0.5 and all networks contained one hidden layer. Predicting the number of neurons to use in the hidden layer to achieve the optimum network (where an optimum network is defined as a network that gives the most accurate classification of samples in the validation set) is not straightforward. Therefore, for each training set, the in-house software permitted the generation and validation of a set of networks using an iterative process whereby the number of neurons in the hidden layer was initially the same as the number of inputs (60), but then the number of neurons in the hidden layer was decremented by one and a network generated and validated for each loop until the number of neurons in the hidden layer reached the number of outputs (2). The optimum networks could then be ascertained from these results.
Three different binary networks were trained and tested: one to differentiate IBS from IBD, one to differentiate IBD from controls and one to differentiate IBS from controls. Four-fold cross-validation was used with a training set: validation set (TS:VS) split of 75:25, to ensure that no dataset had the same chromatogram in both training and validation sets, and that each sample was validated once. The ratio of sample types i.e. control vs. IBD was 50:50 or as close as possible, allowing for the difference in sample numbers collected. The breakdown of ANN training sets and the validation sets used to test them are shown in Table 2. The overall percentage of correctly assigned chromatograms was then calculated in addition to the percentages of correctly classified samples in each group.
Table 2.
shows the number of chromatograms from each group (IBS/IBD/controls) that were included in each of the training and validation sets of the four datasets used to train and validate each of the different ANNs.
| Training set | Validation set | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dataset | Controls | IBS | IBD | Subtotal | Controls | IBS | IBD | Subtotal | |
| IBS Vs IBD | 1 | 25 | 34 | 59 | 9 | 11 | 20 | ||
| 2 | 25 | 34 | 59 | 9 | 11 | 20 | |||
| 3 | 26 | 34 | 59 | 8 | 11 | 19 | |||
| 4 | 26 | 33 | 59 | 8 | 12 | 20 | |||
| IBS Vs Controls | 1 | 34 | 26 | 60 | 12 | 8 | 20 | ||
| 2 | 34 | 26 | 60 | 12 | 8 | 20 | |||
| 3 | 34 | 26 | 60 | 11 | 9 | 20 | |||
| 4 | 35 | 26 | 61 | 11 | 9 | 20 | |||
| IBD Vs Controls | 1 | 34 | 34 | 68 | 12 | 11 | 23 | ||
| 2 | 34 | 34 | 68 | 12 | 11 | 23 | |||
| 3 | 35 | 34 | 69 | 11 | 11 | 22 | |||
| 4 | 35 | 33 | 68 | 11 | 12 | 23 | |||
Results
The mean accuracy, sensitivity and specificity were calculated for each type of network, using the data from the four optimum networks trained for the particular group (Table 3). The sensitivity was calculated as the proportion of disease positive samples that were correctly identified as such, and the specificity calculated as the proportion of disease negative samples correctly diagnosed as being negative. In the case of differentiating IBS from IBD we have considered IBD to be the disease negative group [33].
Table 3: showing the ANN results of the best (optimum) network for each of the four network validations. For each validation set the percentages of correctly assigned ‘diagnosed’ chromatograms are shown, along with the percentage of correctly assigned chromatograms within each group. The final column indicates the number of units in the hidden layer of those networks which produced the results.
Table 3(a).
Results of the ANNs used to differentiate IBS from IBD
| Data Set | % All | % IBS | % IBD | Number of units in networks giving this result |
|---|---|---|---|---|
| 1 | 97 | 92 | 100 | 2-4, 6-60 |
| 2 | 55 | 44 | 64 | 24, 36-60 |
| 3 | 74 | 62 | 82 | 3-60 |
| 4 | 80 | 75 | 83 | 43-47, 52-60 |
| Mean | 76 | 68 | 82 | |
When using the system to differentiate each disease from controls, it was able to distinguish IBD from controls with 79% mean accuracy, identifying 80% of the controls and 78% of the IBD samples correctly (Table 3(b)). This method was much less able to distinguish IBS from controls with a mean accuracy of only 54%, identifying on average 58% of controls and 46% of the IBS samples (Table 3(c)). When using the system to differentiate IBS from IBD samples (Table 3(a)), the sensitivity and specificity are 76% and 88% respectively.
Table 3(b).
Results of the ANNs used to differentiate IBD from controls
| Data Set | % All | % IBD | % Controls | Number of units in the networks giving this result |
|---|---|---|---|---|
| 1 | 91.3 | 91 | 92 | 31, 33 |
| 2 | 87 | 73 | 100 | 5, 36-46, 54-60 |
| 3 | 63.6 | 73 | 55 | 6, 9, 29-35, 40-44 |
| 4 | 73.9 | 75 | 73 | 9-19, 21 |
| Mean | 79 | 78 | 80 | |
Table 3(c).
Results of the ANNs used to differentiate IBS from controls
| Data Set | % All | % IBS | % controls | Number of units in networks giving this result |
|---|---|---|---|---|
| 1 | 50 | 12 | 75 | 4, 12, 36, 52, 54, 56, 57 |
| 2 | 62 | 56 | 67 | 31 |
| 3 | 60 | 78 | 45 | 5, 38, 39, 42, 46-49 |
| 4 | 42 | 38 | 45 | 17, 33-35, 42-45, 47-49, 51-59 |
| Mean | 54 | 46 | 58 | |
Discussion
The GC-sensor ANN method to differentiate irritable bowel syndrome and inflammatory bowel disease was able to diagnose IBD with a sensitivity and specificity of 86% and 88% respectively and with a mean accuracy of 76%. These results are on a par with the reported sensitivities and specificities of other faecal tests used such as calprotectin and lactoferrin [10, 34].
When comparing samples from patients with IBD to control samples, mean diagnostic accuracy rises to 79%. This may be due to the greater difference in stool consistency and composition between people affected with IBD and healthy people. Differentiating IBS from controls only gave a mean accuracy of 54%. This lower accuracy implies similar volatile profiles between healthy people and those with IBS and may be due to the fact that IBS is a functional disease and so changes in the VOC composition of the faeces are not as great. Another possibility is that some of the healthy controls may have had unreported IBS, as data used for the NICE 2008 report on the cost of IBS [35] estimated that only 56% of people with IBS consulted a healthcare professional. This could also have affected the results when differentiating IBD from apparently healthy controls.
Other considerations that need to be addressed are the possibility that differences in the VOCs being detected arise not from changes in the gastrointestinal tract due to disease state but are a result of factors such as dietary changes or medication. Of the participants in this study most were being prescribed drugs containing 5-aminosalicylic acid (5-ASA), glucocorticoid steroids or a combination of the two. The effects of 5-ASA on the bacterial colonisation of the mucosa was investigated by Swidsinki et al. [36] who found that treatment with 5-ASA did not significantly alter the concentration of mucosal bacteria compared to untreated IBD. A recent study by Ahmed et al. [37] used GC-MS to identify the VOCs from the stool of IBD patients. No derivatives of these drugs were detected in the headspace of the patient’s stool, which indicates that the similarities found between groups do not arise directly from excreted medicinal metabolites. Another possibility is the sensing of dietary changes between groups rather than VOC alterations in disease. As no detailed dietary information was collected from the participants it is difficult to distinguish whether this is a factor and a larger study would need to be undertaken to rule out this possibility.
This being said, the method used for this study was able to distinguish differences in samples from IBS and IBD patients, showing a definite proof of principle for applying this diagnostic method to IBS and IBD and with further optimisation the method has much potential. The use of a VOC test to screen patients at the point of care would be very useful. In 2010 a meta-analysis of six studies by Van Rheenen et al. [13] found that screening patients by their faecal calprotectin levels would have reduced the number of endoscopies performed by 67%, with diagnosis delayed in only 6% of patients. Screening using biomarkers is of much benefit both to the patient, as it can rule out the need for unnecessary procedures and lower the burden of functional GI disease on the healthcare system. The VOC detection method is potentially more cost effective and could be completed in half an hour at the point of care, giving it significant potential in this setting.
Conclusion
Previous work using sophisticated and expensive analytical equipment has shown that the VOC analysis approach for IBD and IBS diagnosis is a promising way forward [18, 20, 23, 26, 38, 39]. The work described here builds on this, demonstrating that a low cost device based on the principle of VOC analysis, which can be operated at the point of care (POC), is of potential use in IBS and IBD diagnosis and differentiation. The evidence is sufficient to merit further study and development of the technique, which if successful, i.e. by producing results exceeding current commercial methods, would add VOC analysis for diagnosing IBS and IBD to the growing number of medical tests that use VOC analysis.
Supplementary Material
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salzman NH, Bevins CL. Negative Interactions with the Microbiota: IBD. Advances in Experimental Medicine and Biology. 2008;635:67–78. doi: 10.1007/978-0-387-09550-9_6. [DOI] [PubMed] [Google Scholar]
- 3.Geboes K. Inflammatory bowel disease. 4th. Churchill Livingstone; Elsevier; 2003. Histopathology of Crohn’s disease and ulcerative colitis; pp. 255–76. [Google Scholar]
- 4.Murray CD, et al. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 6.Green J. [cited 9th October 2013];Complications of gastrointestinal endoscopy [internet BSG Guidelines in Gastroenterology. 2006 available from: http://www.bsg.org.uk/pdf_word_docs/complications.pdf.
- 7.Maxion-Bergemann S, et al. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kok L. Diagnostic accuracy of point-of-care faecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: the cost-effectiveness of a decision rule for abdominal complaints in primary care (CEDAR) study. Clinical chemistry. 2012;58:989–998. doi: 10.1373/clinchem.2011.177980. [DOI] [PubMed] [Google Scholar]
- 9. [cited 2013 September 24th];Irritable bowel syndrome: NICE costing report [internet] 2008 Available from: http://www.nice.org.uk/nicemedia/live/11927/39597/39597.pdf.
- 10.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflammatory Bowel Diseases. 2009;15:746–1754. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 11.Angriman I, et al. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clinica chimica acta. 2007;381:63–68. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein CN, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflammatory Bowel Diseases. 2010;16:112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 13.Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clinica Chimica Acta. 2004;347:25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 15.de Lacy Costello B, Ratcliffe NM. Volatile organic compounds (VOCs) found in urine and stool. In: Amann A, Smith D, editors. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine. 1st. Elsevier; 2013. pp. 405–462. [Google Scholar]
- 16.Arasaradnam RP, et al. Insights into 'fermentonomics': evaluation of volatile organic compounds (VOCs) in human disease using an electronic'e-nose'. Journal of medical engineering & technology. 2011;35:87–91. doi: 10.3109/03091902.2010.539770. [DOI] [PubMed] [Google Scholar]
- 17.Altomare DF, et al. Exhaled volatile organic compounds identify patients with colorectal cancer. British journal of surgery. 2013;100:144–150. doi: 10.1002/bjs.8942. [DOI] [PubMed] [Google Scholar]
- 18.Walton C, et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflammatory Bowel Diseases. 2013;19:2069–2078. doi: 10.1097/MIB.0b013e31829a91f6. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, et al. Selected Ion Flow Tube-MS Analysis of Headspace Vapor from Gastric Content for the Diagnosis of Gastro-Esophageal Cancer. Analytical chemistry. 2012;84:9550–9557. doi: 10.1021/ac302409a. [DOI] [PubMed] [Google Scholar]
- 20.Garner CE, et al. Volatile organic compounds from faeces and their potential for diagnosis of gastrointestinal disease. The FASEB Journal. 2007;21:1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- 21.Khalid T, et al. A Pilot Study Combining a GC-Sensor Device with a Statistical Model for the Identification of Bladder Cancer from Urine Headspace. PloS one. 2013 doi: 10.1371/journal.pone.0069602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner M, et al. Headspace screening of fluid obtained from the gut during colonoscopy and breath analysis by proton transfer reaction-mass spectrometry: A novel approach in the diagnosis of gastro-intestinal diseases. International Journal of Mass Spectrometry. 2005;243:151–154. [Google Scholar]
- 23.Jayasena H, Khalid T, Probert CS. PTU-076 Diagnostic Potential of Volatile Organic Compounds as Faecal Biomarkers in Inflammatory Bowel Disease. Gut. 2013;62(Suppl 1):A75–A76. [Google Scholar]
- 24.de Lacy Costello B, Shepherd SF, Ratcliffe NM. The use of MS for the investigation of irritable bowel syndrome and inflammatory bowel disease. Clinincal Lab International magazine. 2013;37:18–20. [Google Scholar]
- 25.Meij TG, et al. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: proof of principle study. International Journal of Cancer. 2013 doi: 10.1002/ijc.28446. [DOI] [PubMed] [Google Scholar]
- 26.De Meij TG, et al. Su1268 Fecal Gas Analysis by Electronic Nose of Pediatric IBD Patients and Healthy Controls: A Pilot Study. Gastroenterology. 2013;144:S–443. [Google Scholar]
- 27.Bensalah k, Montorsi F, Shariat SF. Challenges of Cancer Biomarker Profiling. European Urology. 2007;52:1601–1609. doi: 10.1016/j.eururo.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 28.Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA journal. 2013 doi: 10.1186/1878-5085-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45(suppl 2):II1–II5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walmsley RS, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. The Lancet. 1980;315:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 32.de Lacy Costello B, Ewen RJ, Ratcliffe NM, Sivanand PS. Thick film organic vapour sensors based on binary mixtures of metal oxides. Sensors and Actuators B: Chemical. 2003;92:159–166. [Google Scholar]
- 33.Altman G, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caccaro R, D'Incá R, Sturniolo GC. Clinical utility of calprotectin and lactoferrin as markers of inflammation in patients with inflammatory bowel disease. Expert review of clinical immunology. 2010;6:551–558. doi: 10.1586/eci.10.26. [DOI] [PubMed] [Google Scholar]
- 35.Wilson S, et al. Prevalence of Irritable Bowel Syndrome: a community survey. British Journal of General Practice. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- 36.Swidsinski A, et al. Azathioprine and Mesalazine-induced effects on the mucosal flora in patients with IBD colitis. Inflammatory Bowel Diseases. 2007;13:51–56. doi: 10.1002/ibd.20003. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed I. Faecal volatile organic metabolites (VOMs) as novel diagnostic biomarkers in inflammatory bowel disease. doi: 10.1111/apt.13522. [in preparation] [DOI] [PubMed] [Google Scholar]
- 38.Ahmed I, et al. an investigation of Fecal Volatile Organic Metabolites in Irritable Bowel Syndrome. PloS one. 2013;8:e58204. doi: 10.1371/journal.pone.0058204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hrdlička L, et al. Analysis of volatile compounds in the breath of patients with inflammatory bowel diseases. Gastroenterologie a Hepatologie. 2012;66:125–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.