Abstract
Background
Screening for serum cryptococcal antigen (CrAg) may identify those at risk for disseminated cryptococcal disease (DCD), and pre-emptive fluconazole treatment may prevent progression to DCD. In August 2012, the Western Cape Province (WC), South Africa, adopted provider-initiated CrAg screening. We evaluated the implementation and effectiveness of this large-scale public-sector program during its first year, September 1, 2012—August 31, 2013.
Methods
We used data from the South African National Health Laboratory Service, WC provincial HIV program, and nationwide surveillance data for DCD. We assessed the proportion of eligible patients screened for CrAg (CrAg test done within 30 days of CD4 date) and the prevalence of CrAg positivity. Incidence of DCD among those screened was compared with those not screened.
Results
Of 4,395 eligible patients, 26.6% (n=1170) were screened. The proportion of patients screened increased from 15.9% in September 2012 to 36.6% in August 2013. The prevalence of positive serum CrAg was 2.1%. Treatment data were available for 13 of 24 CrAg-positive patients; nine of 13 were treated with fluconazole. Nine (0.8%) incident cases of DCD occurred among the 1170 patients who were screened for CrAg vs. 49 (1.5%) incident cases among the 3225 patients not screened (p=0.07).
Conclusions
Relatively few eligible patients were screened under the WC provider-initiated CrAg screening program. Unscreened patients were nearly twice as likely to develop DCD. CrAg screening can reduce the burden of DCD, but needs to be implemented well. To improve screening rates, countries should consider laboratory-based reflexive screening when possible.
Keywords: South Africa, Cryptococcal meningitis, Cryptococcal antigen, CrAg, Screening, Fluconazole prophylaxis, Prevention
INTRODUCTION
Cryptococcal disease is a leading cause of mortality among people living with HIV (PLHIV) with advanced immunosuppression in sub-Saharan Africa [1]. Given the limited availability of medications such as amphotericin B and flucytosine to treat disseminated cryptococcal disease [2, 3], and mortality rates of 30%-70% associated with this disease [4], prevention of cryptococcal disease has emerged as an important strategy to reduce AIDS-associated mortality [5].
Cryptococcal antigen (CrAg) is detectable in the serum a median of 22 days (range: 5-234) before symptoms of meningitis appear [6], suggesting that screening for antigenemia during the asymptomatic phase, before onset of disseminated disease, is possible. Pre-emptive treatment with fluconazole for those with asymptomatic antigenemia may prevent progression to meningitis and other forms of disseminated disease. In 2009, a retrospective study in South Africa showed a serum CrAg prevalence of 6% among antiretroviral therapy (ART)-naive patients with CD4+ T-lymphocyte (CD4) counts <100 cells/μl. More than a quarter (28%) of those who screened CrAg positive and did not receive pre-emptive fluconazole therapy went on to develop CM and 34% died, compared with no cases of meningitis and an 11% mortality rate among those who screened CrAg-negative [7]. In 2010, a small study from Uganda showed that 71% of CrAg-positive patients who were treated with fluconazole survived to 30 months compared to 0% among those not treated with fluconazole. Based on this and other evidence, the World Health Organization (WHO) issued rapid advice guidelines in 2011 conditionally recommending screening of all ART-naïve patients with CD4 counts less than 100 cells/μl for presence of cryptococcal antigenemia and pre-emptive fluconazole treatment of those who screened CrAg positive prior to ART initiation in high CrAg prevalence settings [8].
In April 2012 South Africa’s National Strategic Plan on HIV, sexually transmitted infections (STIs) and tuberculosis (TB) was revised to include guidance on CrAg screening and pre-emptive treatment [9]. Four months later, Western Cape became the first province in South Africa to adopt CrAg screening and treatment as part of the HIV care package in the public sector. The approach was provider-initiated screening, with the program relying on individual health care providers to order the CrAg test for eligible patients once their CD4 count was known.
We evaluated the implementation of this public sector provider-initiated CrAg screen-and-treat intervention in the Western Cape from September 1, 2012—August 31, 2013, the first year of the screening program. We also evaluated the effectiveness of CrAg screening by assessing the difference in incidence of disseminated cryptococcal disease between patients who were screened versus those eligible but not screened.
METHODS
Study setting
An estimated 260,000 adults are living with HIV infection in the Western Cape and HIV prevalence is nearly 4% [11]. ART was initiated in over 31,000 patients in 2012 in the province; slightly less than 20% of these patients presented with a CD4 count of <100 cells/μL [12]. All patients with a new HIV diagnosis are advised to undergo clinical staging and to obtain a CD4 cell count within one week of initial presentation. ART is initiated for all patients with CD4 cell count <350 cells/μl (guidelines changed in 2015 to include all patients with CD4 cell count <500 cells/μl); those with CD4 cell count <100 cells/μl are given higher priority for treatment initiation.
In August 2012, the Western Cape HIV/AIDS, STI and TB (HAST) directorate sent a circular to all (approximately 200) provincial ART facilities with ten updates to antiretroviral treatment guidelines. This circular also contained CrAg screening guidance, instructing clinicians to offer CrAg screening to HIV-infected adults not currently on ART who present with a CD4 cell count <100 cells/μl; fluconazole treatment was recommended for all CrAg-positive asymptomatic patients, and referral for LP was recommended for symptomatic patients (headache). No specific clinical training on the CrAg screen-and-treat initiative was offered at that time. Providers were responsible for ordering a separate blood draw for CrAg testing for a patient with a CD4 cell count <100/ cells/μl. Providers did not receive any feedback on how well they were doing with ordering CrAg tests on eligible patients.
Laboratory methods
Venipuncture was performed and blood samples, collected in a red top, plain non-serum separator tube, were transported to one of three clinical laboratories in the province. The laboratories used the Cryptococcal Antigen Latex Agglutination (LA) System (Meridian Bioscience, Inc., Cincinnati, OH) or the Cryptococcus Antigen Latex Agglutination Test System (ImmunoMycologics, Norman, OK) to perform CrAg testing. All patients in the evaluation were tested using LA because the CrAg lateral flow assay (LFA) was not approved for use in South Africa at the initiation of the screening program.
Study design
This was a retrospective cohort study of the CrAg screen-and-treat initiative in the Western Cape Province. The primary study outcomes were the proportion of eligible patients screened for CrAg and the prevalence of CrAg positivity among those who were screened. The secondary outcome was the incidence of laboratory-confirmed disseminated cryptococcal disease among those who were screened compared with those who were not screened. As a third component of the analysis, we examined all cases of disseminated cryptococcal disease among PLHIV in the Western Cape between September 1 2012—August 31, 2013 and assessed CD4 count and ART status at the time patients presented with cryptococcal disease to understand which interventions (e.g. earlier HIV diagnosis, ART initiation, CrAg-screen-and-treat, etc.) would best address the burden of cryptococcal disease.
Data sources
No specific monitoring and evaluation system was in place for the CrAg screening program in the Western Cape. Therefore, data for this study were obtained from several large, pre-existing provincial and national databases.
-
(1)
The National Health Laboratory Services (NHLS)’s Corporate Data Warehouse, a centralized repository for diagnostic laboratory results generated by NHLS laboratories throughout South Africa: we extracted all CD4 counts (during 2007—2013) and serum CrAg tests performed in the Western Cape during the study period (September 1, 2012—August 31, 2013) from this source.
-
(2)
TIER.Net, an electronic, province-wide database used in the majority of Western Cape provincial clinics captures patient ART status, including ART start date, demographics, safety blood results and outcomes [12]. Data from TIER.Net were used to determine ART status (naïve vs. experienced) of potentially eligible patients.
-
(3)
The National Institute for Communicable Diseases’ GERMS-SA surveillance program is a laboratory-based surveillance program which captures all cases of disseminated cryptococcal disease that have occurred in South Africa during 2005-2013 diagnosed in NHLS labs and other labs serving private hospitals, and the military and mining sector. From this database we identified patients with prior, concurrent, and incident cryptococcal disease.
-
(4)
Where available, we examined individual patient charts and pharmacy records to obtain information on fluconazole treatment for those who screened positive for CrAg.
Unique health identifiers were not available on all patient records from the datasets described above. Therefore the databases were matched using probabilistic linkage based on all available patient identifiers. Matching was facilitated by STATA 13.1 and Fine Grained Record Linkage (FRIL) software [13].
Definitions
Screening-eligible patient
A patient was defined as eligible for screening if he or she was ≥ 18 years old at the time a CD4 count was obtained, ART-naïve, had no prior or concurrent cryptococcal disease, and presented with a CD4 count <100 cells/μL for the first time to a Western Cape provincial outpatient clinic during September 1, 2012—August 31, 2013 (hereafter, referred to as a qualifying CD4 count). Patients who presented to care at a hospital were excluded since the screening guidelines were intended for outpatient settings. Additionally, patients presenting to care at a Cape Town city clinic (as opposed to a Western Cape provincial clinic) were also excluded because Cape Town city clinics did not initiate CrAg screening until August 2013.
CrAg screened
serum CrAg LA test was performed ≤7 days before to ≤30 days after the qualifying CD4 count was obtained. The seven day window prior to CD4 test was chosen to account for any time delays and discrepancies between the time a sample is obtained and the time it takes to get processed in the lab and for reporting to occur.
Prior cryptococcal disease
Disseminated cryptococcal disease (including CM and cryptococcemia) diagnosed before the date of qualifying CD4 count.
Concurrent cryptococcal disease
Disseminated cryptococcal disease diagnosed at the time of or ≤14 days from the date of qualifying CD4 count.
Incident cryptococcal disease
Disseminated cryptococcal disease diagnosed >14 days after the date of qualifying CD4 count.
Fluconazole treated
A patient who received fluconazole (any dose and any duration) after the date of the positive serum CrAg result.
Data analysis
Categorical variables were analysed using the chi-squared test and medians of continuous variables were analysed using the Wilcoxon rank sum test. The level of significance was set at α=0.05.
RESULTS
Proportion screened and prevalence of cryptococcal antigenemia
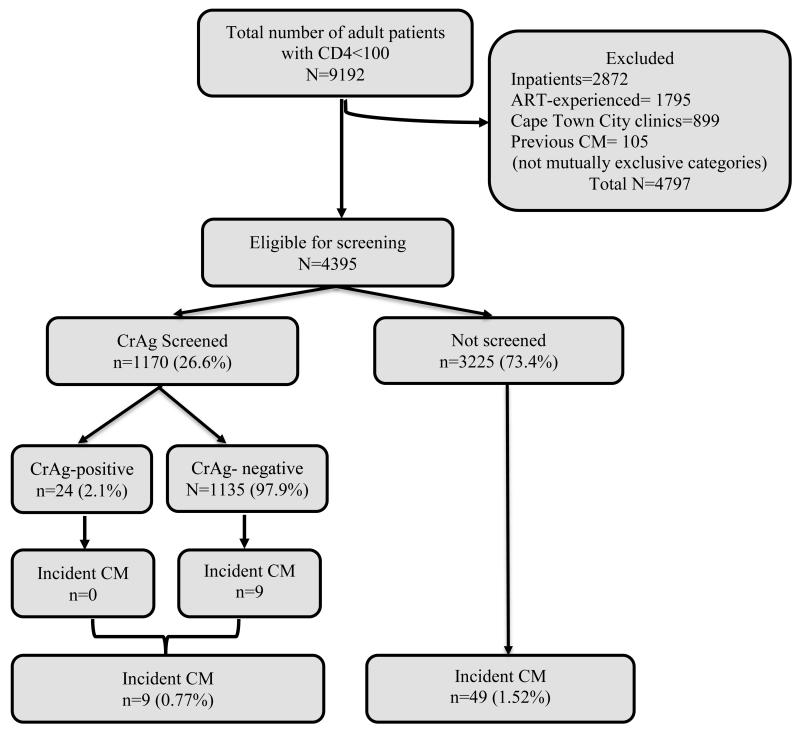
There were 9,192 HIV-positive adults who presented to care with a CD4 count <100 cells/μL for the first time during September 1, 2012—August 31, 2013 in the Western Cape. In total, 4,797 patients (52.2%) were excluded from analysis as screening-ineligible for the following reasons: 1,795 (19.5%) were ART-experienced at the time the CD4 count was obtained, 2,872 (31.5%) presented to care at a district hospital or tertiary care, 899 (9.8%) presented at a Cape Town city clinic, and 105 (1.1%) had prior or concurrent disseminated cryptococcal disease (categories not mutually exclusive). Although not the focus of this analysis, notably, the CrAg prevalence in the hospitalized patients was 3.4% and among ART-experienced patients was 2.8%.
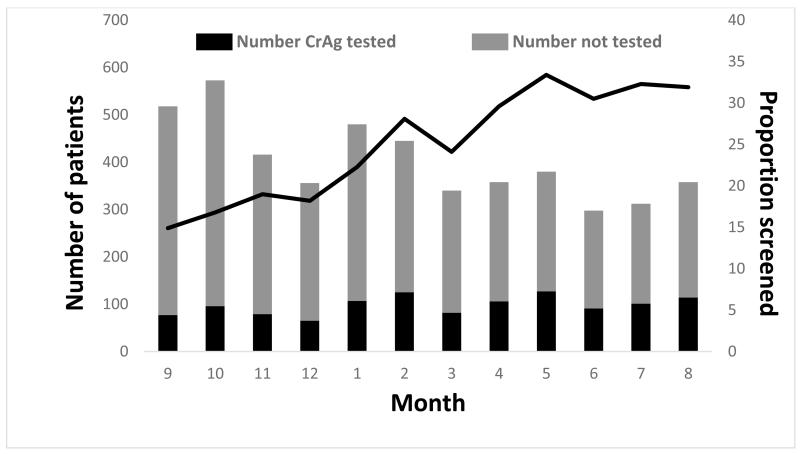
The resulting cohort eligible for screening included 4,395 patients. The median age was 34 years (interquartile range: 29-41) and 50.1% (n=2,204) were female. Twenty-seven percent (n=1,170) of those eligible had a CrAg test done 7 days before to 30 days after their CD4 count test (our pre-defined criterion for being considered CrAg screened). Prevalence of CrAg-positivity among CrAg-screened patients was 2.1% (n=24/1170). The proportion of patients screened increased during the study period (Figure 1), from 15.9% in September 2012, the first month of the screening program, to 36.6% in August 2013, the end of the first year of the screening program. If the screening window was limited to ≤7 days before to ≤14 days after the CD4 count test, 17.5% (n=767) of patients would be considered screened.
Figure 1.
Proportion of eligible patients with screening CrAg test by month in the Western Cape Province, September 2012-August 2013
Incidence of cryptococcal disease among screening-eligible patients
There were nine (0.8%) incident cases of disseminated cryptococcal disease among the 1170 patients screened for CrAg compared with 49 (1.5%) incident cases among the 3225 patients not screened (p=0.07) (Figure 2). All nine cases in the screened group occurred in patients with a negative CrAg screening result. Among these nine patients, the time from CD4 test date to date of diagnosis of cryptococcal disease ranged from 35 to 497 days (median of 103 days) (Table 1). Three of 9 patients had been prescribed ART prior to the date they developed cryptococcal disease. None of the 24 screened, CrAg-positive patients developed cryptococcal disease. Fluconazole treatment data were available for 13 of 24 CrAg-positive patients; 9 were treated with fluconazole following testing. The median time between CD4 date and date of diagnosis of cryptococcal disease among the 49 patients who were not screened and developed cryptococcal disease was 155 days (range: 17-589).
Figure 2.
Incidence of disseminated cryptococcal disease among patients screened and not screened for CrAg, Western Cape Province, September 1, 2012-August 31, 2013
Table 1. Characteristics of patients eligible for cryptococcal antigen (CrAg) screening, Western Cape, South Africa, September 1, 2012-August 31, 2013.
| CrAg screened (n=1151) | Not screened (n=3225) | P value | |
|---|---|---|---|
| Female, n (%) | 555 (48.2) | 1649 (51.3) | 0.02 |
| Median age, years (IQR) | 35.2 (29.6-41.4) | 34.3 (29.3-40.8) | 0.05 |
| Incidence of disseminated cryptococcal disease, n (%) | 9 (0.8) | 49 (1.5) | 0.07 |
| Median time to CM, days (IQR) | 103 (69-119) | 155 (70-262) | 0.48 |
| ART started, n (%) | 853 (74.1) | 1556 (48.3) | <0.001 |
| Median time to ART among those who started ART, days (IQR) | 24(16-36) | 33 (18-62) | <0.001 |
CrAg: cryptococcal antigen; CM: cryptococcal meningitis; ART: antiretroviral treatment
A higher proportion of CrAg screened patients were prescribed ART (72.9%, 853/1170) compared with those were not screened (48.3%, n=1556/3225) (p<0.001). The median time from CD4 testing to ART initiation was 24 days for those screened compared with 33 days for those not screened (p<0.001).
Disseminated Cryptococcal Disease in the Western Cape among all PLHIV
There were 421 patients with disseminated cryptococcal disease in the Western Cape during the study period; of these, 74 did not have a CD4 count available for analysis and were therefore excluded. Of the remaining 317 patients, 80.7% (n=280) had a CD4 count of <100 cells/μL, of whom 5.7% (n=16) had had prior cryptococcal disease. Another 45.4% (n=127) had concurrent cryptococcal disease (within two weeks of their CD4 count). Finally, 48.9% (n=137) presented with incident disease.
DISCUSSION
In this evaluation of a large public sector CrAg screening program in the Western Cape, South Africa, we found that only 1 in 4 eligible patients were screened during the first year of the program, though the proportion of eligible patients screened increased to 1 in 3 towards the end of the evaluation period. Among patients who were eligible for screening, the incidence of cryptococcal disease among those screened for CrAg was approximately half that of those not screened (0.8% vs. 1.5%).
The Western Cape was the first province to adopt CrAg screening province-wide in South Africa. Prior to screening program initiation, the two screening strategies were considered: laboratory-based reflex testing versus provider-initiated testing. In a laboratory-based reflex testing strategy, the CrAg screening test is not dependent on a provider’s order; instead, it is reflexively (routinely) performed in the laboratory on any blood sample with CD4 count <100 cells/μL. Since the same blood sample is used for CrAg testing, reflexive testing would also reduce time to result since an additional patient visit and a blood draw for CrAg testing would not be required. The disadvantages of laboratory-base reflexive testing include potentially “wasted” testing of ineligible patients, such as those already receiving ART, and those getting repeat CD4 testing, patients not returning for CD4 result and therefore not receiving their CrAg test result and any necessary antifungal treatment, and clinicians potentially not knowing how to act on a test they did not specifically order.
The provider-initiated screening approach was ultimately chosen for several reasons. Logistically, laboratories in the Western Cape were willing to assume the additional burden of CrAg testing by request. The LFA was unavailable at the time of program initiation, meaning that an additional tube of blood was required for CrAg testing with LA (LFA can be performed on remnant blood from CD4 test but LA cannot) and generated the additional cost associated with drawing an extra tube of blood on every patient before the CD4 count was available. Additionally, provider-initiated screening could be quickly implemented by sending out a routine circular updating clinicians on the new guidance, whereas high-level coordination between the Department of Health and the NHLS would have been required for reflexive laboratory-based testing. It should be noted that once the provider-initiated screening strategy was chosen, no formal training related to CrAg screening was held at the provincial or local levels; each clinic manager was responsible for informing clinic staff about the new screening recommendations.
Thus, despite the rapid implementation of provider-initiated screening in the Western Cape, a low proportion of eligible patients were screened in the absence of formal training of clinical providers. Even if the proportion of screened patients increased further in the second and third years of the program with more training, it is unlikely to reach optimal levels because it relies on individual providers to remember to order the test. From other medical interventions such as antenatal syphilis screening among pregnant women, it is known that screening interventions that depend on individual providers can be poorly implemented [14]. In contrast, reflex laboratory testing for CrAg conducted on the same blood sample used for the CD4 test, piloted in two other South African provinces, resulted in a >95% CrAg screening rate for patients with CD4 count <100 cells/μL[15]. It is imperative that a high proportion of the eligible population be screened for CrAg for the screening program to be effective; missed screening opportunities result in additional cases of disseminated cryptococcal disease, adding to the morbidity, mortality, and substantial healthcare and other economic and social costs.
Another notable finding from the provider-initiated approach to CrAg screening is the delay from CD4 testing to CrAg screening. We found that while nearly one quarter of patients were screened within a 30-day window from the qualifying CD4 count, only 17% were screened within two weeks. Ideally, screening should take place within two weeks to prevent CM, given that the median duration of cryptococcal antigenemia in the serum before onset of meningitis is approximately 3 weeks [6]. Patients presenting to care with CD4 counts below 100 cells/μL are severely immunosuppressed and their risk of developing opportunistic infections and dying increases with each week they are not started on ART [16]. Provider-initiated screening potentially delays ART initiation, especially for the large group of patients who screen CrAg-negative (>95%), because it may require an additional patient visit and a blood draw for screening. There is also potential risk of loss to follow up with the additional time and economic burden from having an extra clinic visit for CrAg testing.
We found a CrAg prevalence of approximately 2% in our study. This is lower than the 6% prevalence reported in a previous study that retrospectively tested stored serum samples of patients presenting to a large ART clinic in the Western Cape [7]. It is possible that certain clinics have a higher prevalence than others, and a province-wide evaluation results in lower numbers than a study at a single clinic, or because the previous study was conducted eight years earlier, when the landscape of HIV care and treatment was different in South Africa. A prevalence of 2% is comparable to that reported in other studies on the prevalence of antigenemia in southern Africa [15]. A study examining the cost-effectiveness of a cryptococcal screening program found that screening for CrAg is cost-effective when the prevalence is as low as 0.6% [17]. Additionally, we found that CrAg prevalence was substantial in other groups not included in recommendations for CrAg screening (3.4% among inpatients and 2.8% among ART-experienced patients). Including these groups in screening recommendations could have an even larger impact on cryptococcal disease prevention. All cases of disseminated cryptococcosis in the screened group occurred among those who screened CrAg-negative. This is likely due to two reasons. First, the LA test used for detecting serum CrAg in the program is less sensitive than the LFA [10]. It is possible that some patients who truly had antigenemia were not identified by screening and therefore developed cryptococcal disease. Second, only three out of the nine patients who developed cryptococcal disease were started on ART immediately after screening. Patients who screen negative for CrAg but for whom ART is not initiated remain profoundly immunosuppressed and remain at risk for cryptococcal disease. This finding highlights the importance of combining CrAg screening with prompt initiation of ART in order to reduce the burden of cryptococcal disease. A recently published randomized controlled trial conducted in Tanzania and Zambia found that among severely immunosuppressed patients, a 28% reduction in mortality occurred when CrAg screening was combined with ART adherence support [18].
Fluconazole uptake among those who screened CrAg-positive was lower than desired. We could not find any evidence of receipt of fluconazole in about 30% of patients for whom we had records available. The finding of low uptake of fluconazole may have to do with poor patient follow-up, poor provider compliance with guidelines, lack of availability of fluconazole at some clinics, or poor record keeping (fluconazole was prescribed but not documented). This finding underscores the importance of patient and provider education, supply chain management, good documentation, and the need for adherence to all elements of the screening and treatment cascade for effective prevention of disseminated cryptococcal disease. The higher proportion of ART initiation and shorter time to initiation in the screened group likely reflects better overall care that this group receives. It is also possible that screening for CrAg is a tool to retain patients in care and that patients who get screened are also more likely to return to care to get started on ART.
Finally, we found that of all evaluable cases of disseminated cryptococcal disease among PLHIV in the Western Cape during the study period, approximately half occurred in patients at least two weeks after they presented with a low CD4 count. These cases could potentially have been prevented with a combination of CrAg screening and treatment, prompt ART initiation, and retention of patients in care. An additional one-third of the burden of cryptococcal disease was seen in patients who presented with CM at the time of CD4 count testing. CrAg screening would not have prevented disease, since they already have disseminated disease. Efforts to improve earlier HIV diagnosis and linkage to care would likely address this portion of the burden of cryptococcal disease.
There are several limitations to this evaluation. First, whereas the Western Cape is the only province in South Africa to have implemented a unique patient identifier, the identifier was not available on all datasets and duplicate identifiers still occurred for some patients. Therefore, we relied on probabilistic methods for further matching based on a limited number of variables to match patients from the different data sources. Incomplete matching may have resulted in an underestimate of the proportion of the patients screened. However, we made every attempt to optimize matching by liberalizing matching criteria, such as allowing for misspelling of patient names and transposition of birth dates; we manually cross-checked matches of thousands of records that were not perfectly matched. Second, we may not have captured all incident cases of cryptococcal disease. The GERMS SA cryptococcal disease surveillance program is a laboratory-based surveillance system, which theoretically captures most cases of cryptococcal disease in the country. However, it is possible that we missed some cases of cryptococcal disease, though there is no reason to believe that the surveillance system would have preferentially captured either CrAg screened or non-CrAg screened patients. Third, because this is not a randomized controlled trial, we cannot conclude that the reduced incidence of cryptococcal disease in the screened group was specifically due to the screening program. We found that a significantly higher proportion of those screened started ART compared with those who were not screened. It is possible that the difference in incidence is also partly explained by better ART services and retention in care in those clinics or with those providers who also performed CrAg screening.
In March 2015, the National Department of Health incorporated CrAg screening into the national consolidated HIV guidelines, along with other changes, which include initiating ART with a CD4 count threshold of 500 cells/μL, an increase from the previous threshold of 350 cells/μL [19]. Inclusion of CrAg screening in the South African national guidelines may help make more clinicians aware of the guidance and may result in increased screening rates, though this is yet to be evaluated.
In conclusion, provider-initiated screening, as implemented in the Western Cape, did not reach optimal screening coverage in the first year of the program. Public sector programs implementing CrAg screening should consider reflexive lab-based CrAg screening to optimize screening if laboratory infrastructure can support this. Other provinces have successfully piloted reflexive screening recently. In addition, LFA should be considered in place of LA for CrAg testing due to its high sensitivity, lower cost, and ease of use. If provider-initiated screening is to be continued in the Western Cape or considered by other countries, it should be paired with systematic and intensive training of clinical staff to improve screening coverage and subsequent patient management. Sites initiating screening programs should consider putting a thorough monitoring and evaluation system in place before initiation to document screening program performance. In addition to CrAg screening and pre-emptive treatment, it is essential to increase HIV testing and early diagnosis and improve ART initiation rates in order to address the burden of cryptococcal disease.
Acknowledgments
Sources of Funding:
NL Was funded by the Wellcome Trust, London, UK
GM is supported by the Wellcome Trust (098316), the South African Medical Research Council and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 64787). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential Conflict of Interest:
Nelesh P. Govender has received honoraria from MSD Pty (Ltd) South Africa, Astellas and Pfizer for speaking engagements, travel grants from MSD Pty (Ltd) South Africa and has received an investigator-initiated research grant from Pfizer South Africa for an unrelated surveillance project.
REFERENCES
- 1.Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–e444. doi: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- 2.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 3.Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005647.pub2. CD005647. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58:736–745. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JN, Harrison TS, Govender N, et al. Routine cryptococcal antigen screening for HIV-infected patients with low CD4+ T-lymphocyte counts--time to implement in South Africa? S Afr Med J. 2011;101:232–234. doi: 10.7196/samj.4752. [DOI] [PubMed] [Google Scholar]
- 6.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis JN1, Lawn SD, Vogt M, et al. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Rapid advice: Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. WHO guidelines; 2011. Available at: http://www.who.int/hiv/pub/cryptococcal_disease2011/en/ [PubMed] [Google Scholar]
- 9.National Strategic Plan on HIV, STIs and TB 2012-2016. Available at: http://www.sahivsoc.org/upload/documents/National_Strategic_Plan_2012.pdf.
- 10.Lindsley MD, Mekha N, Baggett HC, et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011;53:321–325. doi: 10.1093/cid/cir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Western Cape Government HIV/AIDS Information. Available at: https://westerncape.gov.za/service/anti-retroviral-therapy.
- 12.Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17:18908. doi: 10.7448/IAS.17.1.18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurczyk P, Lu JJ, Xiong L, et al. FRIL: A tool for comparative record linkage. AMIA Annu Symp Proc. 2008:440–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Gloyd S, Chai S, Mercer MA. Antenatal syphilis in sub-Saharan Africa: missed opportunities for mortality reduction. Health Policy Plan. 2001;16:29–34. doi: 10.1093/heapol/16.1.29. [DOI] [PubMed] [Google Scholar]
- 15.National Institute of Communicable Diseases Monthly Surveillance Report. Available at: http://www.nicd.ac.za/assets/files/Monthly%20NICD%20Surveillance%20Report%20-%20August%202015.pdf.
- 16.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–342. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis JN, Harrison TS, Lawn SD, et al. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One. 2013;8:e69288. doi: 10.1371/journal.pone.0069288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mfinanga S, Chanda D, Kivuyo SL, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173–2182. doi: 10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- 19.National Department of Health, South Africa National Consolidated Guidelines for PMTCT and the Management of HIV in Children, Adolescents, and Adults. 2015 Apr; Available at: http://www.sahivsoc.org/upload/documents/ART%20Guidelines%2015052015.pdf.