Abstract
Long term survival of childhood cancers is now more than 70%. Anthracyclines, including doxorubicin, are some of the most efficacious anticancer drugs available. However, its use as a chemotherapeutic agent is severely hindered by its dose-limiting toxicities. Most notably observed is cardiotoxicity, but other organ systems are also degraded by doxorubicin use. Despite the years of its use and the amount of information written about this drug, an understanding of its cellular mechanisms is not fully appreciated. The mechanisms by which doxorubicin induces cytotoxicity in target cancer cells have given insight about how the drug damages cardiomyocytes. The major mechanisms of doxorubicin actions are thought to be as an oxidant generator and as an inhibitor of topoisomerase 2. However, other signaling pathways are also invoked with significant consequences for the cardiomyocyte. Further the interaction between oxidant generation and topoisomerase function has only recently been appreciated and the consequences of this interaction are still not fully understood. The unfortunate consequences of doxorubicin within cardiomyocytes have promoted the search for new drugs and methods that can prevent or reverse the damage caused to the heart after treatment in cancer patients. Alternative protocols have lessened the impact on newly diagnosed cancer patients. However the years of doxorubicin use have generated a need for monitoring the onset of cardiotoxicity as well as understanding its potential long-term consequences. Although a fairly clear understanding of the short-term pathologic mechanisms of doxorubicin actions has been achieved, the long-term mechanisms of doxorubicin induced heart failure remain to be carefully delineated.
Keywords: Doxorubicin, Cardiomyopathy, Topoisomerase, Heart failure, Cancer, Mitochondria, Oxidant stress, DNA damage
1. Introduction
Long term survival of childhood cancers is now more than 70% [21]. Unfortunately, adult survivors of childhood cancer are at risk for a variety of treatment-related adverse health outcomes. Using clinical criteria, survivors with a median time from diagnosis of 25 years [range 10–47 years] were assessed for the prevalence of adverse health outcomes. Among them were abnormal pulmonary function (65.2%), auditory (62.1%), endocrine conditions (62.0%), cardiac dysfunction (56.4%), and neurocognitive impairment (48.0%), whereas abnormalities involving hepatic dysfunction (13.0%), osteoporosis (9.6%), kidney dysfunction (5.0%) were less common [37], [91]. Anthracyclines, including doxorubicin, are some of the most efficacious anticancer drugs available. Their use has extended over 3 decades despite numerous side effects. The studies of childhood survivors 4 to 20 years after doxorubicin treatment observed significant decreases in fractional shortening and ejection fractions, and that was dependent upon the cumulative dose [2], [30], [31], [33], [42], [54], [55], [89], [90]. Analysis of heart transplantation patients found doxorubicin as the underlying cause in 2–3% of all cases [7]. Several reviews have been written that focus on the pathophysiology of doxorubicin cardiotoxicity for the patient [15], [47], [57], [80], [100]. This review will focus more on the cellular and molecular impacts of doxorubicin on the heart with purpose of more fully delineating the underlying molecular mechanisms that promote cardiotoxicity.
2. Chemical structure
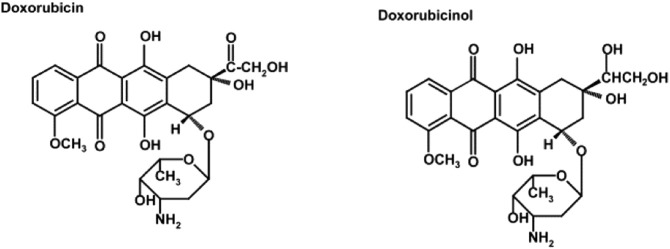
Doxorubicin, also known as Adriamycin® or Rubex®, is an anthracycline antibiotic that was discovered from a mutated strain of Streptomyces peucetius. Doxorubicin operates on several levels by different molecular mechanisms including an interaction with iron, upsetting calcium homeostasis, altering the activity of intracellular or intra-mitochondrial oxidant enzymes, and binding to topoisomerases promoting their dysfunction (Fig. 1).
Fig. 1.
Chemical structure of doxorubicin and its metabolite doxorubicinol. From Wang et.al. [103].
Doxorubicin consists of a naphthacenequinone nucleus and daunosamine, an amino sugar. Doxorubicin has both hydrophilic and hydrophobic regions, allowing it to bind to plasma proteins as well as cell membranes. Doxorubicin is also amphoteric; in having both acidic and basic functions. It is these features that make doxorubicin a versatile compound, allowing it to enter various cellular compartments. Doxorubicin can be reduced intracellularly into doxorubicinol and this metabolite also has biological activity [71]. Doxorubicin can also be reduced to a semiquinone radical by many of the intracellular oxidoreductases. Reoxidation of this radical resulted in the production of reactive oxygen species (ROS). The generation of ROS underlies one mechanism of its antineoplastic and antibiotic capabilities. Excretion is through the liver and kidney with a biphasic half life of 5 min and 30–40 h [43], [78]. Alternative forms of doxorubicin including liposomal-encapsulated forms have been developed in an attempt to decreased cardiotoxicity. These forms do not readily exit the vascular system but only in regions where the structures may be disrupted by tumor growth and are likely to have less access to the heart [54]. Various products including Doxil™, Myocet™ and DaunoXome™ are all commercially available. Meta-analysis found that although cardiotoxicity remained a function of cumulative dose, use of liposomal-encapsulated doxothracyclines lowered the incidence of cardiotoxicity [75], [94]. More recently, biodegradable microparticles or nanoparticles containing doxorubicin have been explored as a slow release mechanism of doxorubicin therapy [59], [63], [76].
3. Detection and cardiotoxicity
The increased risk of cardiac dysfunction from doxorubicin can manifest acutely during treatment or chronically weeks to years after treatment has ceased. Cardiac dysfunction may present across a board spectrum of symptoms that may range from arrhythmias to overt heart failure. Several standard approaches of cardiology have been applied towards the detection of cardiotoxicity, including electrocardiography, echocardiography, biopsy, scintigraphy, serum analysis, and genomic markers. However, not all protocols have proven to be helpful.
Standard echocardiography provides visualization of heart structure and can detect cardiac dysfunction via measures. Radionuclide based tests, such as radionuclide angiocardiography and ventriculography, when performed serially before, during, and after treatment are more sensitive to changes in cardiac function [32]. More recently, the use of radial strain has been studied. Strain is a measure of the deformation of the myocardium that occurs during the cardiac cycle and is determined by the change in length of the tissue relative to its original length [28], [51]. Doxorubicin induced damage causes myocyte death and the injured regions do not function as well as normal tissue resulting in dyssynchrony and deformation. In both human and animal studies two-dimensional strain echocardiography appeared more sensitive than standard echocardiography protocols providing additional insight into doxorubicin induced cardiac injury [36], [65].
Serum biomarkers for cardiac injury more commonly used to detect ischemia may also reflect acute doxorubicin-induced cardiomyopathy. Although elevated circulating levels of cardiac troponin T have been reported, the increases are not quantitatively consistent with the degree of doxorubicin-induced cardiac injury [72], [85]. Stronger correlations of circulating brain-type natriuretic peptide to doxorubicin-induced cardiac dysfunction have been demonstrated than with the atrial natriuretic peptide [85]. Distinct from cardiac troponin T that indicate cardiomyocyte damage, the natriuretic peptides reflect an early response to cardiac insufficiency. More recently, the use of circulating genomic biomarkers in monitoring and predicting doxorubicin-induced heart failure is also currently being investigated. These animal studies have examined changes in expression of microRNAs. Of the potential markers, both miR-208 and miR-216B were responsive to doxorubicin treatment and that miR-216B did appear dose-dependent responsive to doxorubicin [70], [98]. However, similar to radiolabeled antibodies elevated circulating levels are only detectable as a following indicator to myocyte damage.
Although used but not clinically practical, histopathological results from endomyocardial biopsies are very sensitive in detection of late-onset cardiotoxicity [72]. Alternatively, combination radiolabeled antibodies with scintigraphy is also useful. Antimyosin antibodies bind intracellularly to the heavy chain of myosin but can only do so when cell membrane integrity is compromised, allowing for localization exclusively to damaged cardiomyocytes. Carrió et al. demonstrated that increased uptake of 111In labeled antimyosin in patients with sarcomas being treated with doxorubicin was predictive of future progression of cardiotoxicity [17]. Patients with higher uptake levels of the antibody at intermediate cumulative doses of doxorubicin initially showed no simultaneous decline in left ventricular ejection fraction. However, as treatment continued, these patients with previous high uptake levels were more likely to develop cardiac dysfunction or mild congestive heart failure as a result of increased cumulative exposure than those patients with lower uptake levels at the intermediate cumulative dose. Valdés Olmos et al. investigated the sensitivity of 111In-antimyosin and scintigraphy in detecting early cardiac injury in breast cancer patients receiving low doses of doxorubicin [99]. After comparing measurements of myocardial uptake of the antibody to other assessments of cardiac dysfunction, such as left ventricular ejection fraction, it was concluded that localization of 111In-antimyosin was representative of myocyte injury, even at low cumulative doses of doxorubicin (120–150 mg/m2). These findings suggest that progressive myocyte injury precedes clinically significant cardiac dysfunction, and use of this technique may help determine and individual patient's risk of developing heart failure in response to increasing doses of doxorubicin. However, there is little information about the usefulness of this marker in predicting late-onset cardiotoxicity, which if accurate, could have a major impact on the prevention of doxorubicin-induced congestive heart failure, especially in survivors of childhood cancer.
Acute cardiotoxicity that may appear during treatment or shortly thereafter presents with abnormal changes in heartbeat, which may or may not be seen with non-specific ST-T wave changes in electrocardiograms [82], [88], [101]. Acute episodes have been reported to occur in up to 40% of patients given doxorubicin [50]. The occurrence of an acute episode has not been predictive of late onset cardiac dysfunction [74].
Beyond the early effects, sub-acute cardiotoxicity may present within a few weeks to a few months. These effects may be attributed to altered Ca+ 2 dynamics, increased oxidative stress, or altered myocardial energetics [23], [26], [68], [71]. Delayed effects have been reported in studies of childhood survivors 4 to 20 years after doxorubicin treatment where significant decreases in fractional shortening and ejection fractions were observed [2], [30], [31], [33], [42], [54], [55], [89], [90]. There appears to be a relationship between delayed development of congestive heart failure and the cumulative dose of doxorubicin received. At a cumulative dose of 500 mg/m2, there was a 4% risk of congestive heart failure. However, there is a rapid escalation in risk of congestive heart failure to 36% as cumulative doses exceeded 600 mg/m2 [61].
While cumulative dose is the greatest risk factor for developing doxorubicin- induced cardiotoxicity, several other factors may also contribute to the likelihood of developing heart failure. Receipt of other cardiotoxic chemotherapeutic drugs, such as trastuzumab or cyclophosphamide, significantly increases the chance of cardiotoxicity. Patients who received mediastinal radiation, a treatment for Hodgkin's lymphoma, had their own increased risk for cardiac dysfunction; this was increased when received in combination doxorubicin [64]. One study has suggested that gender may be significant in that females treated with doxorubicin are more likely to develop cardiac dysfunction [18]. Additionally, the younger a patient is when receiving treatment, the greater the risk of late onset cardiotoxicity. Up to 65% of children exposed to doxorubicin experience some type cardiac dysfunction at least one year after treatment [56].
4. Cardiac remodeling
In humans, depression of fractional shortening and ejection fraction are the hallmarks for doxorubicin induced degradation of cardiac function. Similar results in animal studies indicate their value as suitable surrogates for the human condition [67], [68]. The early effects of doxorubicin-induced cardiotoxicity may also be attributed to altered Ca+ 2 dynamics, increased oxidative stress, and altered myocardial energetics [23], [26], [67], [68].
Acutely, a single dose of doxorubicin has been shown to impair myocardial contractility. Doxorubicin-induced cardiomyopathy produces cytoplasmic vacuolation, swelling of the sarcoplasmic reticulum, and myofibrillar disarray [11], [12], [39], [40], [71]. Using endomyocardial biopsies from patients receiving doxorubicin, Billingham et al. observed focal lesions but without inflammatory infiltrate being present [9]. All patients receiving more than 240 mg/mm2 had evidence of degeneration, and presented initially with a loss of myofibrillar elements or vacuolar degeneration. In a separate study using daunorubicin, although morphologic disarrangements were observed in both chambers, the RV had a milder presentation [52]. Consistent with this, expression of sarcomeric proteins was impaired in the LV but not the RV. Following a single dose of 6.0 mg/kg of doxorubicin in rats, the maximal contractile force was 12% lower than controls after one week [67]. Parallel findings were observed in another study; cardiac actomyosin ATPase activity was decreased and this decrease was dose dependent [9]. The heart does attempt to compensate and maximal contractility recovered transiently to normal values three weeks after treatment, but then declined to 40% after eight weeks [67].
As a cytotoxic agent, doxorubicin diminishes the ability of tumor cells to breakdown the extracellular matrix, impairing cell mobility [71]. This would suggest that altered extracellular matrix within the heart should also be altered leading to compromised function and such an outcome might be reflected as altered diastolic dysfunction. Several cohort studies have reported diastolic and diastolic plus systolic dysfunction as an outcome of doxorubicin treatments in different patient population including children [16], [81], [111]. Ivanová et al. and others have demonstrated that mRNA and protein levels of several matrix metalloproteinases were significantly increased in cardiomyocytes by doxorubicin [3], [38]. These enzymes are significant in that they disrupt the integrity of the fibrillar collagen network resulting in left ventricular pump dysfunction. Consistent with this, decreases in the inhibitor of metalloproteinase-1 were observed following doxorubicin treatment [34]. Rescue experiments that inhibited matrix metalloproteinase activity have been used to show improved left ventricular pump function following doxorubicin treatment [20], [86]. These findings suggest the participation of the matrix metalloproteinases in the progression of doxorubicin induced heart failure by promoting myocardial extracellular remodeling.
5. Cardiomyopathy: molecular mechanisms
Doxorubicin operates on many levels by different mechanisms including an interaction with iron, altering the activity of intracellular or intra-mitochondrial oxidant enzymes, and binding to topoisomerases. Problematic in their study is the myriad of secondary pathways activated by these primary effects.
6. Free radicals and iron
The second major pathway of cell toxicity is doxorubicin induced increases in intracellular radial oxygen species. The early-onset cardiomyopathy seen after treatment with doxorubicin is thought in part to be induced by oxidative stress and mitochondrial dysfunction in cardiomyocytes. Different pathways are thought responsible for doxorubicin induced oxidant stress. Doxorubicin may cause oxidative stress by interacting directly with iron after being reduced by NADPH-cytochrome P450 reductase to yield a doxorubicin semiquinone. This reduced form of doxorubicin can complex with Fe+ 2. The free radical complex can spontaneously reduce oxygen to superoxide, resulting in the regeneration of doxorubicin and the cycle can begin again. Free oxygen radicals are also produced when doxorubicin gets reduced at Complex I of the electron transport chain. This semiquinone can reduce oxygen to generate increased levels of superoxide and other reactive oxygen species in mitochondria, leading to dysfunction. Semiquinones of doxorubicin aglycones at Complex I have also been shown to yield increased production of hydroxyl radicals, contributing to mitochondrial oxidative stress. Given the high dependence of cardiomyocytes on aerobic metabolism, cardiotoxicity may not be surprising outcome.
Doxorubicin has been shown to bind to nitric oxide synthase through a direct interaction with the enzyme [45]. This promotes monomerization of the enzyme which shifts the reaction mechanism to favor the production of superoxide rather than nitric oxide. And with greater concentrations of superoxide, interaction of the reactive oxygen species with nitric oxide becomes more likely. This reaction yields an extremely potent free radical, peroxynitrite. This free radical can cross lipid membranes, enabling it to enter the nucleus and mitochondria, potentiating the deleterious effects of oxidative stress on cardiomyocytes.
A third pathway for doxorubicin is to be metabolized to doxorubicinol, a metabolite thought to interfere with a key iron regulator protein, aconitase-1. This interaction with aconitase-1 releases free iron concentrations to the cell preventing the translation of ferritin, an iron-sequestering protein, and inhibiting the breakdown of the transferrin receptor, which allows more iron to enter the cell [8]. Transition metals, such as iron, are known to catalyze the production of free radicals, thus doxorubicinol secondarily contributes to the oxidative stress seen in cells. This contribution is supported by observations that doxorubicin-induced cardiotoxicity was increased in iron-loaded rats [73].
Studies using iron chelators have demonstrated variable effects on doxorubicin mediated cardiotoxicity. Deferoxamine appeared to offer no protective effects to doxorubicin induced cardiotoxicity [83]. In contrast, dexrazoxane (Zinecard) is an FDA approved drug approved for cardioprotection. Dexrazoxane can enter the cell, where it is metabolized into its open-ring form, ADR-925. The open-ring structure resembles the iron chelator EDTA, and can bind free iron in the cell, decreasing intracellular concentrations or can disrupt the association of iron with doxorubicin, preventing the doxorubicin-iron complex from forming, thereby decreasing superoxide production. Dexrazoxane's protective effects appear in part to be that of protecting the cardiac mitochondria. Lebrecht et al. demonstrated that cardiomyocytes from rats treated with doxorubicin and dexrazoxane for seven weeks had greater mitochondrial function than rats treated with doxorubicin alone [50]. These data suggest that the cardioprotective effects of dexrazoxane may be due to other mechanisms in addition to sequestering iron within the cell.
Other evidence also questions how critical the role of iron and reactive oxygen species are in doxorubicin-induced cardiotoxicity. Administration of antioxidants, such as N-acetylcysteine and polyphenols attenuated apoptosis in vitro or short-term animal experiments. However no significant improvement in doxorubicin-induced cardiotoxicity was observed when studied in long-term animal experiments or in clinical trials [83]. The inconclusive data on iron chelators and antioxidants creates doubt about the classic definition of oxidant stress in cardiotoxicity [83]. It does point towards experimentation the use of mitochondrial-directed antioxidants to differentiate the respective roles of cytoplasmic versus mitochondrial derive ROS.
7. Topoisomerase II
Although the oxidant role of doxorubicin was originally thought to be singular, it has become evident that doxorubicin's interaction with topoisomerases is of primary importance. Topoisomerases are highly conserved proteins present in all organisms. Humans have seven topoisomerases that may be divided into three classes; type IA, IB, and II. Topoisomerases resolve the topological difficulties of DNA replication by allowing the double helix to pass through itself. This entails a complex reaction order where 1) DNA is bound, 2) double DNA strand breaks are induced, 3) the DNA structure is rotated to relieve torsion-induced stress, and 4) religation of DNA strands. At least six isoforms of topoisomerase are present in the nucleus and mitochondria of the cardiomyocyte [58], [104], [108]. Other isoforms are present in other cell populations within the heart. Topoisomerase type IIs differ from topoisomerase type I which induce only single DNA strand breaks to relieve torsional stress. Topoisomerases II form double strand DNA breaks are the most lethal form of DNA damage [41]. Although topoisomerase activity is essential to the cell and generally protective, they may also be genotoxic [25], [30].
Topoisomerases are enzymes and several post-translational modifications exist that modulate function including myristoylation, palmitoylation, sumoylation and phosphorylation [60], [62]. Both protein kinase C and casein kinase I δ/ε activities have shown to modulate DNA cleavage activity of topoisomerase IIα [35], [107]. Dephosphorylation depresses topoisomerase relaxation of supercoiled (SC) DNA [87]. Further it has been suggested that oxidative stress alters nuclear topoisomerase function to stabilize the topoisomerase/DNA complex while inhibiting the religation process leading to an increase in DNA strand breakage [53]. Whether this is in fact the underlying mechanism of doxorubicin-induced oxidant stress remains to be determined.
Within the heart topoisomerase II proteins are found as two nuclear localized isoforms IIα and IIβ and one mitochondrial IIβ isoform. Doxorubicin is a topoisomerase inhibitor that intercalates with DNA to block progression of topoisomerase II and it binds equally to topoisomerase IIα and IIβ [95]. Doxorubicin interacts with topoisomerase and DNA to form a DNA cleavage complex that increases double strand breakage; this serving as its underlying use as a cytotoxic reagent. Zhang et al. hypothesized that acute doxorubicin cardiomyocyte toxicity may be due to the capability of doxorubicin to interact with topoisomerase IIβ. Their ideas were supported from studies using a cardiomyocyte knockout of topoisomerase IIβ in mice. While doxorubicin treatment decreased ejection fraction 10% in normal animals, it had no effect on cardiovascular function in the knockout animals [109]. This work has refocused drug development towards drugs which are protective. The FDA approved drug dexrazoxane (Zinecard) is foremost used for cardioprotection from doxorubicin. Although originally thought to be only an iron chelator (see below) it may compete with topoisomerase II as well as act to deplete topoisomerase II expression [24], [106].
Alternative approaches towards cardioprotection have been to design drugs that only target topoisomerase IIα that is not normally present within the cardiomyocytes [19]. Surprisingly, salicylate an active metabolite of acetylsalicylic acid (aspirin) has been reported as an inhibitor of topoisomerase IIα and should in theory be protective of the myocardium [6]. While as anti-cancer drugs this approach holds a great deal of promise, it is not without some drawbacks. It has become apparent over the last decade that the heart is not a static organ and that continual cell replacement from cardiac progenitor cells serves to renew the cardiomyocytes of the heart. Malignant cells and undifferentiated cells (including cardiac progenitor cells) uniquely express topoisomerase IIα, while cardiomyocytes and other differentiated cells express topoisomerase IIβ. Thus while deletion of topoisomerase IIβ may be protective of the cardiomyocytes, the long term consequence of doxorubicin treatment on cardiac progenitor cells remains unclear.
8. Calcium dysregulation
Loss of calcium homeostasis is another effect of doxorubicin. Doxorubicinol has been demonstrated to interfere with calcium sequestration by the sarcoplasmic reticulum by altering the calcium pump, SERCA, found on its membrane [69]. The sodium/potassium pump of the sarcolemma is also affected by doxorubicinol, which disrupts the sodium gradient needed for calcium to flow into the sarcolemma of a cardiomyocyte. Doxorubicinol may also interact with the ryanodine receptor, allowing an uncontrolled flow of calcium out of the sarcoplasmic reticulum. Further, doxorubicin has been shown to decrease the calcium storage capacity of mitochondria by specifically activating the selective CsA-sensitive calcium channel, exacerbating the calcium overload [110]. Disruption of these pumps and channels alters calcium homeostasis, which also leads to mitochondrial dysfunction and apoptosis. Zhou et al. demonstrated that mitochondria from doxorubicin treated hearts had a significantly lower calcium-loading capacity. Five weeks after cessation of treatment, the ability of the mitochondria to store calcium still did not improve, suggesting that these effects cannot be reversed after treatment, suggesting that these effects are both cumulative and irreversible [110]. Verapamil blocks L-type calcium channels and may effectively decreases the calcium overload caused by doxorubicin [77]. However, some studies have demonstrated that administering doxorubicin and verapamil in conjunction actually further potentiates cardiotoxicity. The mechanism behind these results remains controversial, but the hypothesis that verapamil overload itself has cardiotoxic effects, separate from doxorubicin [1], [79]. Muscle contraction is calcium-dependent and lowering intracellular calcium within cardiomyocytes would result in decreased contractility, suggesting a fine balance between usefulness and detriment.
9. Mitochondrial dysfunction
Doxorubicin accumulates in the nucleus and the mitochondria [69]. Similar to the nucleus, the mitochondria contains topoisomerase 2β, a specific target of doxorubicin. The functional role of mitochondrial topoisomerase II® remains unclear, however it has been suggested to participate in decatenating newly synthesized mtDNA circles [58]. Within the mitochondria, single strand DNA nicks also act to localize topoisomerases to the mtDNA and induce cleavage on the opposite strand [25], [46]. This would not only enhance DNA cleavage by topoisomerases in the mitochondria but would also hold mtDNA “in an open conformation” longer and expose it to further ROS attack. All of these avenues serve to interfere with mitochondrial replication and transcription causing significant imbalances in the stoichiometry of the Electron Transport Chain and promoting dysfunction. Doxorubicin may also promote apoptosis by localizing to the mitochondria and stimulating the release of cytochrome C [97]. Secondarily, the drug inducted oxidative stress and abnormally high levels of calcium within the cell, both of which are known to stimulate the release of cytochrome C and initiate apoptotic pathways through caspase activation [71]. Derivatives of doxorubicin also promote cytochrome C release by accumulating in the inner mitochondrial membrane to disrupt the electron transport chain [97]. Contrary to these ideas is evidence for an increase in the Bcl2:Bax ratio as a function of a decline in Bax, a shift which is antiapoptotic. This is possibility of the induction of a compensatory protective response against mitochondrial-mediated apoptosis, providing a mechanism for how cardiomyocytes are able to survive exposure to the drug. Overexpression of various antioxidant enzymes, such as manganese superoxide dismutase and catalase, along with increased mitochondrial efficiency were also seen after treatment with doxorubicin, supporting the existence of a compensatory response [20].
10. Endothelin-1
Alteration of endothelin-1 expression in cardiomyocytes seen after treatment with doxorubicin provides an additional mechanism of cardiotoxicity. Endothelin-1 modulates the IP3 pathway in cardiomyocytes, causing increased release of calcium from the sarcoplasmic reticulum and is thus stimulates contraction. After treatment with doxorubicin, mRNA and plasma levels of endothelin-1 in mice are significantly increased [10]. This increase in endothelin-1 caused by doxorubicin potentiates the increased calcium load in cardiomyocytes, thereby facilitating apoptosis and cardiac dysfunction. Clinically, patients with doxorubicin-induced congestive heart failure also have increased levels of endothelin-1. Although up regulation of endothelin-1 normally promotes cell survival by affecting cell signaling in the heart, the vasoconstrictive properties of this molecule in the vasculature are deleterious in doxorubicin treated patients. Normally, vasodilatation induced by nitric oxide can counteract the potent constriction caused by endothelin-1, however, as explained earlier, doxorubicin modulates the nitric oxide system. This disables endothelial cells from responding appropriately to the vasoconstriction, which facilitates the progression of hypertension and can lead to heart failure. The role of doxorubicin-induced changes on endothelin-1 and subsequent cardiomyopathy is supported by the improvement in cardiac function observed after administration with antagonists of the endothelin-1 receptor or inhibition of endothelin-converting enzyme-1.
After demonstrating doxorubicin-induced up regulation of endothelin-1 and cardiac dysfunction, Bien et al., showed that pretreatment with bosentan, an antagonist of the endothelin B receptor, resulted in marked increased systolic, diastolic, and left ventricular function as compared to mice treated only with doxorubicin [10]. Mice pretreated with the antagonist had increased stroke volume, ejection fraction, and cardiac output in addition to decreased myocardial stiffness, suggesting that endothelin-1 signaling plays a critical role doxorubicin-induced cardiac dysfunction. Further, mice treated with bosentan had decreased levels of tumor necrosis factor-(TNF), a factor known to stimulate transcription of proapoptotic proteins such as Bax. Mice that received pretreatment also had decreased Bax levels, providing evidence that blocking endothelin-1 may also participate in blocking apoptosis in cardiomyocytes, which could contribute to the improvement in cardiac function seen in these animals.
Additionally, similar results were also seen in endothelin-converting enzyme-1 heterozygous knockouts treated with doxorubicin, especially in regard to lipid peroxidation and mitochondrial damage [66]. Not only did endothelin-converting enzyme-1 heterozygotes have improved cardiac function compared to wild type after treatment with doxorubicin, but there was also preservation of mitochondrial activity. Heterozygotes treated with doxorubicin had higher ATP levels than wild types receiving treatment, suggesting maintenance of mitochondrial function was associated with decreased levels of endothelin-1. Increased expression of superoxide dismutase along with decreased cardiac lipid peroxidation was also seen in these animals, providing evidence that endothelin-1 may participate in the oxidative stress caused by doxorubicin. Further, mitochondrial biogenesis also seemed to be preserved in the heterozygote mice, demonstrated by the normal levels of a key transcription factor involved in mitochondrial biogenesis, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1 α). Transcription of this factor is inhibited by doxorubicin; however decreased levels of PGC-1 α were not seen in the knockout animals, supporting the critical role of endothelin-1 in doxorubicin-induced mitochondrial dysfunction. Collectively, these data suggest that endothelin-1 facilitates this type of cardiomyopathy and may provide insight about potential preventative treatments for this toxicity.
11. Treatment and prevention of cardiotoxicity
Doxorubicin is an effective chemotherapeutic agent and treatments that would protect patients from acute and chronic cardiotoxicity without altering cytotoxicity of abnormal cells would be the ideal goal for the management of cancer patients. Several cardioprotective agents have been explored in the treatment and prevention of doxorubicin-induced cardiomyopathy, including drugs that directly interfere with the cellular mechanisms of doxorubicin or are used in the traditional management of heart failure.
Dexrazoxane appears to be the most efficacious drug tested to date. Aside from directly competing with topoisomerase II, it can diminish oxidative stress in cardiomyocytes caused by the interaction of doxorubicin and iron, and may also stimulate expression of mitochondrial antioxidant enzymes. Based on these properties, dexrazoxane, also known as Zinecard ☐ (Pfizer Pharmaceuticals, New York, NY), has been recognized as a cardioprotective agent when administered in conjunction with doxorubicin and is now the mostly commonly used protocol.
Not perfect but good, it has been suggested in some studies that dexrazoxane may diminish the antineoplastic actions of doxorubicin when administered in conjunction. Others have depicted lower response rates and even occurrences of secondary malignancies after treatment with both drugs [96]. Barry et al. did not observe an association of dexrazoxane treatment with appearance of secondary malignant neoplasms [4]. In instances where a patient's cumulative dose of doxorubicin exceeded 300 mg/m2, such as in treatment of advanced metastatic breast cancer, dexrazoxane treatment did significantly lower incidence of congestive heart failure with little effect on time to cancer progression [92], [93].
Some traditional treatments for heart failure may also be cardioprotective in patients treated with doxorubicin. Carvedilol, a nonspecific inhibitor of adrenergic receptors, has demonstrated a protective role. Carvedilol has also been shown to have antioxidant properties as well as protect against calcium dysregulation by blocking the doxorubicin-induced down-regulation of sarcoplasmic reticular calcium pumps [44]. From preclinical studies, carvedilol was shown to reduce the histological evidence of cardiomyopathy and reduce changes in left ventricular ejection fraction when administered in combination with doxorubicin [61]. Subsequent clinical trials yielded similar outcomes, patients given carvedilol in conjunction with doxorubicin had no significant changes in left ventricular ejection fraction, whereas patients treated with doxorubicin alone had a significant decrease of about 24% [44]. In children with acute lymphoblastic leukemia that were treated with carvedilol in combination with doxorubicin found a similar efficacy of carvedilol in preventing cardiac dysfunction in children compared to studies conducted on adults [27].
Other compounds that show some promise include antioxidants such as flavonoids or probucol. The flavonoid, monohydroxyethylrutoside (monoHER), is currently being investigated in clinical trials. In preclinical studies, monoHER was administered to nude mice with human tumor xenografts in conjunction with doxorubicin [5]. They found that near term effects of doxorubicin on cardiovascular function were depressed while monoHER did not appear to alter antitumor activity. In a phase 1 study, in healthy volunteers received monoHER doses up to 1500 mg/m2 was found to be feasible [105]. Unfortunately when moved to a phase II trial with patients with metastatic cancer, monoHER did not appear to be cardioprotective and the authors suggested that the high dose chosen may have been at fault [14]. Probucol, a powerful antioxidant previously used to treat various cardiovascular diseases remains in preclinical studies, where it has been shown to offer protection against doxorubicin induced cardiotoxicity [49], [84]. More recently, phosphodiesterase-5 inhibitors, such as sildenafil, have demonstrated cardioprotective effects when administered prophylactically [22], [48]. Fisher et al. (2005) administered doxorubicin or doxorubicin and sildenafil to mice for two weeks and subsequent measures of cardiac function and levels of apoptosis were measured over the course of ten weeks [29]. Whereas mice treated with only doxorubicin had a significantly increased apoptotic index, those treated with doxorubicin and sildenafil did not.
Another example of application of a pre-existing drug to the doxorubicin induced cardiotoxicity model is rimonabant. The cannabinoid-1 receptor antagonist is approved in Europe as a weight loss drug for obese patients at high risk for other diseases such as type II diabetes and dyslipidemia [102]. Its potential is unclear since stimulation of the cannabinoid-1 receptor in the heart is cardiodepressive and it is unknown if rimonabant interacts with doxorubicin or having a positive influence by blocking the cannabinoid-1 receptor [13]. The results from one preclinical study suggested that rimonabant may counter some cardiotoxic pathways of doxorubicin. Mice treated with doxorubicin alone had significantly decreased cardiac function, shown by decreased stroke work, ejection fraction, cardiac output, and left ventricular systolic pressure, as compared to control mice that received a vehicle or rimonabant alone. In contrast, animals that received both doxorubicin and rimonabant demonstrated significantly increased cardiac function compared to those that received doxorubicin alone. However, the measurements of cardiac function in the mice treated with both drugs were still significantly lower than the control animals suggesting a need for further work [68]. Although promising, to date these preclinical studies have only examined short term outcomes.
12. Conclusions
Doxorubicin is a potent chemotherapeutic agent used to treat a variety of cancers. The chemical structure of the drug facilitates its cytotoxicity, allowing it to enter many intracellular compartments, including the nucleus and mitochondria. It is administered intravenously as a hydrochloride salt by various dose schedules in cancer patients. The efficacy of doxorubicin as a cancer treatment is attributed to the various cytotoxic mechanisms by which it acts, most notably by inducing DNA damage through interaction with topoisomerase II and production of free radicals through interaction with intracellular oxidoreductases. However, these actions of the drug also result in various hematological and gastrointestinal toxicities.
Several factors are associated with increased risk of developing cardiotoxicity after doxorubicin treatment, with increased cumulative dose being the greatest risk. Additionally, treatments with other cardiotoxic agents, radiation therapy, or preexisting cardiac conditions exacerbate this risk. Pediatric cancer patients exposed to the drug at a young age also have an increased risk of developing late-onset cardiotoxicity, although the under causes remain unclear.
Because doxorubicin is an effective cancer treatment, methods to prevent the cardiotoxicity have long been sought. Monitoring cardiotoxicity through various markers helps prevent progression to fatal congestive heart failure. Standard tests of cardiac function are often used to assess doxorubicin-induced toxicity, however certain methods have proven to better detect the early signs of cardiac injury. Although electrocardiography and echocardiography can provide useful information, radionuclide tests are currently the most reliable and accurate method for detection of cardiac dysfunction in patients treated with doxorubicin. Two-dimensional radial strain echocardiography is a newer technique that has been demonstrated to detect cardiac injury at an even earlier stage, making it an extremely valuable tool. Histological tests and scintigraphy are both sensitive to early cardiomyopathy; however these invasive techniques are less practical because of the increased risks they pose to the patient.
Drugs that can treat or prevent doxorubicin-induced cardiotoxicity would be extremely helpful for patients that have been treated with doxorubicin in the past or who have cancer that is effectively treated by doxorubicin. Several drugs are being investigated, with dexrazoxane (Zinecard™) being the most effective preventative treatment at this time. Other drugs under current investigation are flavonoids such as monoHER and carvedilol, a non-specific adrenergic receptor inhibitor. Many drugs approved for treatment of other diseases are also being evaluated for their efficacy in preventing doxorubicin-induced cardiomyopathy. These drugs include sildenafil, a phosphodiesterase-5 inhibitor, probucol, an antioxidant, and rimonabant, a cannabinoid-1 receptor antagonist. Animal studies provide evidence for the cardioprotective properties of these agents, but these studies are limited in only examining the short term consequences and little is known about their potential protective role for late onset cardiac dysfunction.
Doxorubicin is a drug that effectively treats one fatal disease, but its severe side effects can also be fatal. Identification of methods to successfully ameliorate these toxicities through preventative or alternative treatments would have a great impact on clinical oncology.
Sources of funding
This work was supported in part by National Institute of Health (HD065551) and the New York Medical College Research Endowment Fund (B494561).
Conflict of interest
The authors declared that they have no conflicts.
References
- 1.Akimoto H., Bruno N.A., Slate D.L., Billingham M.E., Torti S.V., Torti F.M. Effect of verapamil on doxorubicin cardiotoxicity: altered muscle gene expression in cultured neonatal rat cardiomyocytes. Cancer Res. 1993;53:4658–4664. [PubMed] [Google Scholar]
- 2.Armstrong G.T., Plana J.C., Zhang N., Srivastava D., Green D.M., Ness K.K., Daniel Donovan F., Metzger M.L., Arevalo A., Durand J.B., Joshi V., Hudson M.M., Robison L.L., Flamm S.D. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J. Clin. Oncol. 2006;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai P., Mabley J.G., Liaudet L., Virag L., Szabo C., Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol. Rep. 2004;11:505–508. [PubMed] [Google Scholar]
- 4.Barry E.V., Vrooman L.M., Dahlberg S.E., Neuberg D.S., Asselin B.L., Athale U.H., Clavell L.A., Larsen E.C., Moghrabi A., Samson Y., Schorin M.A., Cohen H.J., Lipshultz S.E., Sallan S.E., Silverman L.B. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J. Clin. Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 5.Bast A., Haenen G.R., Bruynzeel A.M., Van der Vijgh W.J. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc. Toxicol. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- 6.Bau J.T., Kang Z., Austin C.A., Kurz E.U. Salicylate, a catalytic inhibitor of topoisomerase II, inhibits DNA cleavage and is selective for the alpha isoform. Mol. Pharmacol. 2014;85:198–207. doi: 10.1124/mol.113.088963. [DOI] [PubMed] [Google Scholar]
- 7.Baum D., Bernstein D., Starnes V.A., Oyer P., Pitlick P., Stinson E., Shumway N. Pediatric heart transplantation at Stanford: results of a 15-year experience. Pediatrics. 1991;88:203–214. [PubMed] [Google Scholar]
- 8.Beinert H., Kennedy M.C., Stout C.D. Aconitase as iron-sulfur protein, enzyme, and iron-regulatory protein. Chem. Rev. 1996;96:2335–2374. doi: 10.1021/cr950040z. [DOI] [PubMed] [Google Scholar]
- 9.Bergson A., Inchiosa M.A., Jr. Cardiac actomyosin ATPase activity after chronic doxorubicin treatment. Res. Commun. Chem. Pathol. Pharmacol. 1985;48:57–75. [PubMed] [Google Scholar]
- 10.Bien S., Riad A., Ritter C.A., Gratz M., Olshausen F., Westermann D., Grube M., Krieg T., Ciecholewski S., Felix S.B., Staudt A., Schultheiss H.P., Ewert R., Volker U., Tschope C., Kroemer H.K. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67:10428–10435. doi: 10.1158/0008-5472.CAN-07-1344. [DOI] [PubMed] [Google Scholar]
- 11.Bier C.C., Jaenke R.S. Function of myocardial mitochondria in the adriamycin-induced cardiomyopathy of rabbits. J. Natl. Cancer Inst. 1976;57:1091–1094. doi: 10.1093/jnci/57.5.1091. [DOI] [PubMed] [Google Scholar]
- 12.Billingham M.E., Mason J.W., Bristow M.R., Daniels J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978;62:865–872. [PubMed] [Google Scholar]
- 13.Bonz A., Laser M., Kullmer S., Kniesch S., Babin-Ebell J., Popp V., Ertl G., Wagner J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Bruynzeel A.M., Niessen H.W., Bronzwaer J.G., van der Hoeven J.J., Berkhof J., Bast A., van der Vijgh W.J., van Groeningen C.J. The effect of monohydroxyethylrutoside on doxorubicin-induced cardiotoxicity in patients treated for metastatic cancer in a phase II study. Br. J. Cancer. 2007;97:1084–1089. doi: 10.1038/sj.bjc.6603994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant J., Picot J., Baxter L., Levitt G., Sullivan I., Clegg A. Clinical and cost-effectiveness of cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review. Br. J. Cancer. 2007;96:226–230. doi: 10.1038/sj.bjc.6603562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu'Lock F.A., Mott M.G., Oakhill A., Martin R.P. Left ventricular diastolic function after anthracycline chemotherapy in childhood: relation with systolic function, symptoms, and pathophysiology. Br. Heart J. 1995;73:340–350. doi: 10.1136/hrt.73.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrio I., Lopez-Pousa A., Estorch M., Duncker D., Berna L., Torres G., de Andres L. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J. Nucl. Med. 1993;34:1503–1507. [PubMed] [Google Scholar]
- 18.Chatterjee K., Zhang J., Honbo N., Karliner J.S. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W., Qiu J., Shen Y.M. Topoisomerase IIalpha, rather than IIbeta, is a promising target in development of anti-cancer drugs. Drug Discov. Ther. 2012;6:230–237. [PubMed] [Google Scholar]
- 20.Childs A.C., Phaneuf S.L., Dirks A.J., Phillips T., Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 21.Curry H.L., Parkes S.E., Powell J.E., Mann J.R. Caring for survivors of childhood cancers: the size of the problem. Eur. J. Cancer. 2006;42:501–508. doi: 10.1016/j.ejca.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Das A., Durrant D., Salloum F.N., Xi L., Kukreja R.C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 2015;147:12–21. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies K.J., Doroshow J.H. Redox cycling of anthracyclines by cardiac mitochondria I. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 24.Deng S., Yan T., Jendrny C., Nemecek A., Vincetic M., Godtel-Armbrust U., Wojnowski L. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both topoisomerase II isoforms. BMC Cancer. 2014;14:842. doi: 10.1186/1471-2407-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deweese J.E., Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2008;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doroshow J.H., Davies K.J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J. Biol. Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 27.El-Shitany N.A., Tolba O.A., El-Shanshory M.R., El-Hawary E.E. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J. Card. Fail. 2012;18:607–613. doi: 10.1016/j.cardfail.2012.06.416. [DOI] [PubMed] [Google Scholar]
- 28.Fallah-Rad N., Walker J.R., Wassef A., Lytwyn M., Bohonis S., Fang T., Tian G., Kirkpatrick I.D., Singal P.K., Krahn M., Grenier D., Jassal D.S. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J. Am. Coll. Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 29.Fisher P.W., Salloum F., Das A., Hyder H., Kukreja R.C. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 30.Floyd J.D., Nguyen D.T., Lobins R.L., Bashir Q., Doll D.C., Perry M.C. Cardiotoxicity of cancer therapy. J. Clin. Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 31.Ganz P.A., Hussey M.A., Moinpour C.M., Unger J.M., Hutchins L.F., Dakhil S.R., Giguere J.K., Goodwin J.W., Martino S., Albain K.S. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897. J. Clin. Oncol. 2008;26:1223–1230. doi: 10.1200/JCO.2007.11.8877. [DOI] [PubMed] [Google Scholar]
- 32.Ganz W.I., Sridhar K.S., Ganz S.S., Gonzalez R., Chakko S., Serafini A. Review of tests for monitoring doxorubicin-induced cardiomyopathy. Oncology. 1996;53:461–470. doi: 10.1159/000227621. [DOI] [PubMed] [Google Scholar]
- 33.Geenen M.M., Cardous-Ubbink M.C., Kremer L.C., van den Bos C., van der Pal H.J., Heinen R.C., Jaspers M.W., Koning C.C., Oldenburger F., Langeveld N.E., Hart A.A., Bakker P.J., Caron H.N., van Leeuwen F.E. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 34.Goetzenich A., Hatam N., Zernecke A., Weber C., Czarnotta T., Autschbach R., Christiansen S. Alteration of matrix metalloproteinases in selective left ventricular adriamycin-induced cardiomyopathy in the pig. J. Heart Lung Transplant. 2009;28:1087–1093. doi: 10.1016/j.healun.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Grozav A.G., Chikamori K., Kozuki T., Grabowski D.R., Bukowski R.M., Willard B., Kinter M., Andersen A.H., Ganapathi R., Ganapathi M.K. Casein kinase I delta/epsilon phosphorylates topoisomerase IIalpha at serine-1106 and modulates DNA cleavage activity. Nucleic Acids Res. 2009;37:382–392. doi: 10.1093/nar/gkn934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho E., Brown A., Barrett P., Morgan R.B., King G., Kennedy M.J., Murphy R.T. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96:701–707. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 37.Hudson M.M., Ness K.K., Gurney J.G., Mulrooney D.A., Chemaitilly W., Krull K.R., Green D.M., Armstrong G.T., Nottage K.A., Jones K.E., Sklar C.A., Srivastava D.K., Robison L.L. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova M., Dovinova I., Okruhlicova L., Tribulova N., Simoncikova P., Barte-kova M., Vlkovicova J., Barancik M. Chronic cardiotoxicity of doxorubicin involves activation of myocardial and circulating matrix metalloproteinases in rats. Acta Pharmacol. Sin. 2012;33:459–469. doi: 10.1038/aps.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaenke R.S. An anthracycline antibiotic-induced cardiomyopathy in rabbits. Lab. Investig. 1974;30:292–304. [PubMed] [Google Scholar]
- 40.Jaenke R.S. Delayed and progressive myocardial lesions after adriamycin administration in the rabbit. Cancer Res. 1976;36:2958–2966. [PubMed] [Google Scholar]
- 41.Javle M., Curtin N.J. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther. Adv. Med. Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen B.V. Cardiotoxic consequences of anthracycline-containing therapy in patients with breast cancer. Semin. Oncol. 2006;33:S15–S21. doi: 10.1053/j.seminoncol.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Joerger M., Huitema A.D., Meenhorst P.L., Schellens J.H., Beijnen J.H. Pharmacokinetics of low-dose doxorubicin and metabolites in patients with AIDS-related Kaposi sarcoma. Cancer Chemother. Pharmacol. 2005;55:488–496. doi: 10.1007/s00280-004-0900-4. [DOI] [PubMed] [Google Scholar]
- 44.Kalay N., Basar E., Ozdogru I., Er O., Cetinkaya Y., Dogan A., Inanc T., Oguzhan A., Eryol N.K., Topsakal R., Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 45.Kalivendi S.V., Kotamraju S., Zhao H., Joseph J., Kalyanaraman B. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. Effect of antiapoptotic antioxidants and calcium. J. Biol. Chem. 2001;276:47266–47276. doi: 10.1074/jbc.M106829200. [DOI] [PubMed] [Google Scholar]
- 46.Kingma P.S., Osheroff N. Spontaneous DNA damage stimulates topoisomerase II-mediated DNA cleavage. J. Biol. Chem. 1997;272:7488–7493. doi: 10.1074/jbc.272.11.7488. [DOI] [PubMed] [Google Scholar]
- 47.Kremer L.C., van Dalen E.C., Offringa M., Voute P.A. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann. Oncol. 2002;13:503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 48.Kukreja R.C. Sildenafil and cardioprotection. Curr. Pharm. Des. 2013;19:6842–6847. doi: 10.2174/138161281939131127110156. [DOI] [PubMed] [Google Scholar]
- 49.Kumar D., Kirshenbaum L.A., Li T., Danelisen I., Singal P.K. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid. Redox Signal. 2001;3:135–145. doi: 10.1089/152308601750100641. [DOI] [PubMed] [Google Scholar]
- 50.Lebrecht D., Geist A., Ketelsen U.P., Haberstroh J., Setzer B., Walker U.A. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br. J. Pharmacol. 2007;151:771–778. doi: 10.1038/sj.bjp.0707294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leitman M., Lysyansky P., Sidenko S., Shir V., Peleg E., Binenbaum M., Kaluski E., Krakover R., Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Lencova-Popelova O., Jirkovsky E., Mazurova Y., Lenco J., Adamcova M., Simunek T., Gersl V., Sterba M. Molecular remodeling of left and right ventricular myocardium in chronic anthracycline cardiotoxicity and post-treatment follow up. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li T.K., Chen A.Y., Yu C., Mao Y., Wang H., Liu L.F. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipshultz S.E., Colan S.D., Gelber R.D., Perez-Atayde A.R., Sallan S.E., Sanders S.P. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 55.Lipshultz S.E., Giantris A.L., Lipsitz S.R., Kimball Dalton V., Asselin B.L., Barr R.D., Clavell L.A., Hurwitz C.A., Moghrabi A., Samson Y., Schorin M.A., Gelber R.D., Sallan S.E., Colan S.D. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J. Clin. Oncol. 2002;20:1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 56.Lipshultz S.E., Miller T.L., Lipsitz S.R., Neuberg D.S., Dahlberg S.E., Colan S.D., Silverman L.B., Henkel J.M., Franco V.I., Cushman L.L., Asselin B.L., Clavell L.A., Athale U., Michon B., Laverdiere C., Schorin M.A., Larsen E., Usmani N., Sallan S.E. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics. 2012;130:1003–1011. doi: 10.1542/peds.2012-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lotrionte M., Biondi-Zoccai G., Abbate A., Lanzetta G., D'Ascenzo F., Malavasi V., Peruzzi M., Frati G., Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 58.Low R.L., Orton S., Friedman D.B. A truncated form of DNA topoisomerase IIbeta associates with the mtDNA genome in mammalian mitochondria. Eur. J. Biochem. 2003;270:4173–4186. doi: 10.1046/j.1432-1033.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Zhao W, Huang Y, Liu H, Marquez R, Gibbs RB, Li J, Venkataramanan R, Xu L, and Li S. Targeted delivery of doxorubicin by folic acid-decorated dual functional nanocarrier. Mol. Pharm. [DOI] [PMC free article] [PubMed]
- 60.Lynn R.M., Bjornsti M.A., Caron P.R., Wang J.C. Peptide sequencing and site-directed mutagenesis identify tyrosine-727 as the active site tyrosine of Saccharomyces cerevisiae DNA topoisomerase I. Proc. Natl. Acad. Sci. U. S. A. 1989;86:3559–3563. doi: 10.1073/pnas.86.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado V., Cabral A., Monteiro P., Goncalves L., Providencia L.A. Carvedilol as a protector against the cardiotoxicity induced by anthracyclines (doxorubicin) Rev. Port. Cardiol. 2008;27:1277–1296. [PubMed] [Google Scholar]
- 62.Madden K.R., Stewart L., Champoux J.J. Preferential binding of human topoisomerase I to superhelical DNA. EMBO J. 1995;14:5399–5409. doi: 10.1002/j.1460-2075.1995.tb00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makkouk A., Joshi V.B., Wongrakpanich A., Lemke C.D., Gross B.P., Salem A.K., Weiner G.J. Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer. AAPS J. 2015;17:184–193. doi: 10.1208/s12248-014-9676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mancuso L., Mancuso A., Scordato F., Pieri M., Valerio M.C. Malignancy and radiation-induced cardiotoxicity. Cardiovasc. Hematol. Disord. Drug Targets. 2011 doi: 10.2174/187152911798347052. [DOI] [PubMed] [Google Scholar]
- 65.Migrino R.Q., Aggarwal D., Konorev E., Brahmbhatt T., Bright M., Kalyanaraman B. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med. Biol. 2008;34:208–214. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyagawa K., Emoto N., Widyantoro B., Nakayama K., Yagi K., Rikitake Y., Suzuki T., Hirata K. Attenuation of doxorubicin-induced cardiomyopathy by endothelin-converting enzyme-1 ablation through prevention of mitochondrial biogenesis impairment. Hypertension. 2010;55:738–746. doi: 10.1161/HYPERTENSIONAHA.109.141903. [DOI] [PubMed] [Google Scholar]
- 67.Monti E., Piccinini F., Villani F., Favalli L. Myocardial contractility and heart pharmacokinetics of adriamycin following a single administration in rat. Cancer Chemother. Pharmacol. 1986;18:289–291. doi: 10.1007/BF00273406. [DOI] [PubMed] [Google Scholar]
- 68.Mukhopadhyay P., Batkai S., Rajesh M., Czifra N., Harvey-White J., Hasko G., Zsengeller Z., Gerard N.P., Liaudet L., Kunos G., Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicolay K., Fok J.J., Voorhout W., Post J.A., de Kruijff B. Cytofluorescence detection of adriamycin-mitochondria interactions in isolated, perfused rat heart. Biochim. Biophys. Acta. 1986;887:35–41. doi: 10.1016/0167-4889(86)90119-9. [DOI] [PubMed] [Google Scholar]
- 70.Nishimura Y., Kondo C., Morikawa Y., Tonomura Y., Torii M., Yamate J., Uehara T. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J. Appl. Toxicol. 2012;35:173–180. doi: 10.1002/jat.3044. [DOI] [PubMed] [Google Scholar]
- 71.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Panjrath G.S., Jain D. Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging. J. Nucl. Cardiol. 2006;13:415–426. doi: 10.1016/j.nuclcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Panjrath G.S., Patel V., Valdiviezo C.I., Narula N., Narula J., Jain D. Potentiation of doxorubicin cardiotoxicity by iron loading in a rodent model. J. Am. Coll. Cardiol. 2007;49:2457–2464. doi: 10.1016/j.jacc.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 74.Pfizer Doxorubicin Hydrochloride 2014. http://www.labeling.pfizer.com/showlabeling.aspx?id=530
- 75.Rafiyath S.M., Rasul M., Lee B., Wei G. Lamba G, and Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol. Oncol. 2012;1:10. doi: 10.1186/2162-3619-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reddy L.H., Murthy R.S. Pharmacokinetics and biodistribution studies of doxorubicin loaded poly(butyl cyanoacrylate) nanoparticles synthesized by two different techniques. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2004;148:161–166. doi: 10.5507/bp.2004.029. [DOI] [PubMed] [Google Scholar]
- 77.Rossi F., Filippelli W., Russo S., Filippelli A., Berrino L. Cardiotoxicity of doxorubicin: effects of drugs inhibiting the release of vasoactive substances. Pharmacol. Toxicol. 1994;75:99–107. doi: 10.1111/j.1600-0773.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 78.Ryu R.J. Eyal S, Kaplan HG, Akbarzadeh A, Hays K, Puhl K, Easterling TR, Berg SL, Scorsone KA,<?thyc=5?> Feldman EM, Umans JG, Miodovnik M, and Hebert MF. Pharmacokinetics of doxorubicin in pregnant women. Cancer Chemother. Pharmacol. 2014;73:789–797. doi: 10.1007/s00280-014-2406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santostasi G., Kutty R.K., Krishna G. Increased toxicity of anthracycline antibiotics induced by calcium entry blockers in cultured cardiomyocytes. Toxicol. Appl. Pharmacol. 1991;108:140–149. doi: 10.1016/0041-008x(91)90277-l. [DOI] [PubMed] [Google Scholar]
- 80.Schlitt A., Jordan K., Vordermark D., Schwamborn J., Langer T., Thomssen C. Cardiotoxicity and oncological treatments. Dtsch. Arztebl. Int. 2014;111:161–168. doi: 10.3238/arztebl.2014.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaikh A.S., Saleem A.F., Mohsin S.S., Alam M.M., Ahmed M.A. Anthracycline-induced cardiotoxicity: prospective cohort study from Pakistan. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shan K., Lincoff A.M., Young J.B. Anthracycline-induced cardiotoxicity. Ann. Intern. Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 83.Simunek T., Sterba M., Popelova O., Adamcova M., Hrdina R., Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 84.Singal P.K., Siveski-Iliskovic N., Hill M., Thomas T.P., Li T. Combination therapy with probucol prevents adriamycin-induced cardiomyopathy. J. Mol. Cell. Cardiol. 1995;27:1055–1063. doi: 10.1016/0022-2828(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 85.Sparano J.A., Brown D.L., Wolff A.C. Predicting cancer therapy-induced cardiotoxicity: the role of troponins and other markers. Drug Saf. 2002;25:301–311. doi: 10.2165/00002018-200225050-00001. [DOI] [PubMed] [Google Scholar]
- 86.Spinale F.G., Coker M.L., Bond B.R., Zellner J.L. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc. Res. 2000;46:225–238. doi: 10.1016/s0008-6363(99)00431-9. [DOI] [PubMed] [Google Scholar]
- 87.St-Amant C., Lussier S., Lehoux J., Laberge R.M., Boissonneault G. Altered phosphorylation of topoisomerase I following overexpression in an ovarian cancer cell line. Biochem. Cell Biol. 2006;84:55–66. doi: 10.1139/o05-157. [DOI] [PubMed] [Google Scholar]
- 88.Steinberg J.S., Cohen A.J., Wasserman A.G., Cohen P., Ross A.M. Acute arrhythmogenicity of doxorubicin administration. Cancer. 1987;60:1213–1218. doi: 10.1002/1097-0142(19870915)60:6<1213::aid-cncr2820600609>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 89.Steinherz L.J., Steinherz P.G., Tan C. Cardiac failure and dysrhythmias 6–19 years after anthracycline therapy: a series of 15 patients. Med. Pediatr. Oncol. 1995;24:352–361. doi: 10.1002/mpo.2950240604. [DOI] [PubMed] [Google Scholar]
- 90.Steinherz L.J., Steinherz P.G., Tan C.T., Heller G., Murphy M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 91.Strumberg D., Brugge S., Korn M.W., Koeppen S., Ranft J., Scheiber G., Reiners C., Mockel C., Seeber S., Scheulen M.E. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann. Oncol. 2002;13:229–236. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- 92.Swain S.M., Whaley F.S., Gerber M.C., Ewer M.S., Bianchine J.R., Gams R.A. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J. Clin. Oncol. 1997;15:1333–1340. doi: 10.1200/JCO.1997.15.4.1333. [DOI] [PubMed] [Google Scholar]
- 93.Swain S.M., Whaley F.S., Gerber M.C., Weisberg S., York M., Spicer D., Jones S.E., Wadler S., Desai A., Vogel C., Speyer J., Mittelman A., Reddy S., Pendergrass K., Velez-Garcia E., Ewer M.S., Bianchine J.R., Gams R.A. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 94.Swenson C.E., Bolcsak L.E., Batist G., Guthrie T.H., Jr., Tkaczuk K.H., Boxenbaum H., Welles L., Chow S.C., Bhamra R., Chaikin P. Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D-99; liposome-encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anti-Cancer Drugs. 2003;14:239–246. doi: 10.1097/00001813-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 95.Swift L.P., Cutts S.M., Nudelman A., Levovich I., Rephaeli A., Phillips D.R. The cardio-protecting agent and topoisomerase II catalytic inhibitor sobuzoxane enhances doxorubicin-DNA adduct mediated cytotoxicity. Cancer Chemother. Pharmacol. 2008;61:739–749. doi: 10.1007/s00280-007-0528-2. [DOI] [PubMed] [Google Scholar]
- 96.Tebbi C.K., London W.B., Friedman D., Villaluna D., De Alarcon P.A., Constine L.S., Mendenhall N.P., Sposto R., Chauvenet A., Schwartz C.L. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J. Clin. Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 97.Thorn C.F., Oshiro C., Marsh S., Hernandez-Boussard T., McLeod H., Klein T.E., Altman R.B. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet. Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vacchi-Suzzi C., Bauer Y., Berridge B.R., Bongiovanni S., Gerrish K., Hamadeh H.K., Letzkus M., Lyon J., Moggs J., Paules R.S., Pognan F., Staedtler F., Vidgeon-Hart M.P., Grenet O., Couttet P. Perturbation of microRNAs in rat heart during chronic doxorubicin treatment. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valdes Olmos R.A., Carrio I., Hoefnagel C.A., Estorch M., ten Bokkel Huinink W.W., Lopez-Pousa J., Dalesio O. High sensitivity of radiolabelled antimyosin scintigraphy in assessing anthracycline related early myocyte damage preceding cardiac dysfunction. Nucl. Med. Commun. 2002;23:871–877. doi: 10.1097/00006231-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 100.van Boxtel W., Bulten B.F., Mavinkurve-Groothuis A.M., Bellersen L., Mandigers C.M., Joosten L.A., Kapusta L., de Geus-Oei L.F., van Laarhoven H.W. New biomarkers for early detection of cardiotoxicity after treatment with docetaxel, doxorubicin and cyclophosphamide. Biomarkers. 2015;20:143–148. doi: 10.3109/1354750X.2015.1040839. [DOI] [PubMed] [Google Scholar]
- 101.Villani F., Monti E., Piccinini F., Favalli L., Lanza E., Rozza Dionigi A., Poggi P. Relationship between doxorubicin-induced ECG changes and myocardial alterations in rats. Tumori. 1986;72:323–329. doi: 10.1177/030089168607200315. [DOI] [PubMed] [Google Scholar]
- 102.Viner R.M., Hsia Y., Tomsic T., Wong I.C. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes. Rev. 2010;11:593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang G.X., Wang Y.X., Zhou X.B., Korth M. Effects of doxorubicinol on excitation–contraction coupling in guinea pig ventricular myocytes. Eur. J. Pharmacol. 2001;423:99–107. doi: 10.1016/s0014-2999(01)01096-2. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y., Lyu Y.L., Wang J.C. Dual localization of human DNA topoisomerase IIIalpha to mitochondria and nucleus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12114–12119. doi: 10.1073/pnas.192449499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willems A.M., Bruynzeel A.M., Kedde M.A., van Groeningen C.J., Bast A., van der Vijgh W.J. A phase I study of monohydroxyethylrutoside in healthy volunteers. Cancer Chemother. Pharmacol. 2006;57:678–684. doi: 10.1007/s00280-005-0083-7. [DOI] [PubMed] [Google Scholar]
- 106.Yan T., Deng S., Metzger A., Godtel-Armbrust U., Porter A.C., Wojnowski L. Topoisomerase II{alpha}-dependent and -independent apoptotic effects of dexrazoxane and doxorubicin. Mol. Cancer Ther. 2009;8:1075–1085. doi: 10.1158/1535-7163.MCT-09-0139. [DOI] [PubMed] [Google Scholar]
- 107.Yoshida K., Yamaguchi T., Shinagawa H., Taira N., Nakayama K.I., Miki Y. Protein kinase C delta activates topoisomerase IIalpha to induce apoptotic cell death in response to DNA damage. Mol. Cell. Biol. 2006;26:3414–3431. doi: 10.1128/MCB.26.9.3414-3431.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang H., Meng L.H., Pommier Y. Mitochondrial topoisomerases and alternative splicing of the human TOP1mt gene. Biochimie. 2007;89:474–481. doi: 10.1016/j.biochi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S., Liu X., Bawa-Khalfe T., Lu L.S., Lyu Y.L., Liu L.F., Yeh E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 110.Zhou S., Starkov A., Froberg M.K., Leino R.L., Wallace K.B. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777. [PubMed] [Google Scholar]
- 111.Zuppinger C., Timolati F., Suter T.M. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovasc. Toxicol. 2007;7:61–66. doi: 10.1007/s12012-007-0016-2. [DOI] [PubMed] [Google Scholar]