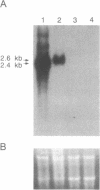
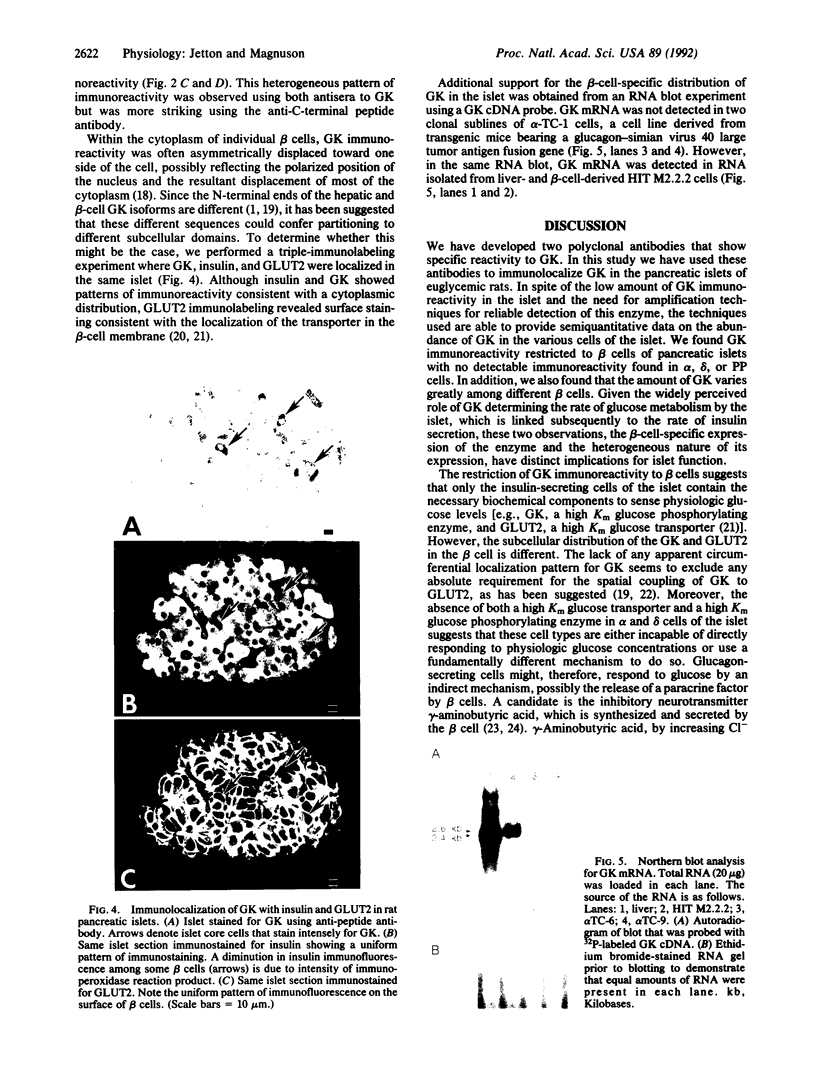
Abstract
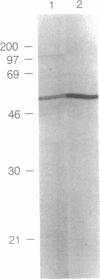
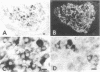
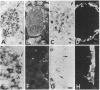
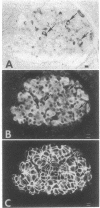
The cellular location of glucokinase (GK), a key component of the glucose-sensing mechanism of the pancreatic islet, was determined using immunocytochemical techniques. In rat islets, GK immunoreactivity was detected only in beta cells with no immunoreactivity detected in alpha, delta, or pancreatic polypeptide-containing (PP) cells. However, within various beta cells, GK immunoreactivity varied considerably. Most beta cells displayed relatively low levels of cytoplasmic immunoreactivity whereas other beta cells stained intensely for this enzyme. Colocalization studies of GK and GLUT2, the high Km glucose transporter of beta cells, confirmed that these proteins are located in different subcellular domains of beta cells. The lack of GK immunoreactivity in glucagon- and somatostatin-secreting cells in islets suggests that these cells are not directly responsive to glucose or utilize a fundamentally different mechanism for sensing glucose fluctuations. Moreover, the differential expression of GK among pancreatic beta cells suggests that glucose phosphorylation is the probable enzymatic control point for the functional diversity of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert S., Hanahan D., Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988 Apr 22;53(2):295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Bedoya F. J., Oberholtzer J. C., Matschinsky F. M. Glucokinase in B-cell-depleted islets of Langerhans. J Histochem Cytochem. 1987 Oct;35(10):1089–1093. doi: 10.1177/35.10.3305701. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988 May;37(5):616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- Boyd A. E., 3rd, Hill R. S., Oberwetter J. M., Berg M. Calcium dependency and free calcium concentrations during insulin secretion in a hamster beta cell line. J Clin Invest. 1986 Mar;77(3):774–781. doi: 10.1172/JCI112374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelje T. C., Scharp D. W., Sorenson R. L. Three-dimensional imaging of intact isolated islets of Langerhans with confocal microscopy. Diabetes. 1989 Jun;38(6):808–814. doi: 10.2337/diab.38.6.808. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry D. J., Appel N. M., Garry M. G., Sorenson R. L. Cellular and subcellular immunolocalization of L-glutamate decarboxylase in rat pancreatic islets. J Histochem Cytochem. 1988 Jun;36(6):573–580. doi: 10.1177/36.6.2896676. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Pilot P. R., Nouspikel T., Milburn J. L., Quaade C., Hughes S., Ucla C., Newgard C. B. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G. M., Jepson M. A., Trayer I. P., Walker D. G. The compartmentation of glycolytic and gluconeogenic enzymes in rat kidney and liver and its significance to renal and hepatic metabolism. Histochem J. 1986 Jan;18(1):45–53. doi: 10.1007/BF01676198. [DOI] [PubMed] [Google Scholar]
- Liang Y., Jetton T. L., Zimmerman E. C., Najafi H., Matschinsky F. M., Magnuson M. A. Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and pituitary. J Biol Chem. 1991 Apr 15;266(11):6999–7007. [PubMed] [Google Scholar]
- Liang Y., Najafi H., Matschinsky F. M. Glucose regulates glucokinase activity in cultured islets from rat pancreas. J Biol Chem. 1990 Oct 5;265(28):16863–16866. [PubMed] [Google Scholar]
- Magnuson M. A., Shelton K. D. An alternate promoter in the glucokinase gene is active in the pancreatic beta cell. J Biol Chem. 1989 Sep 25;264(27):15936–15942. [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Quaade C., Hughes S. D., Milburn J. L. Glucokinase and glucose transporter expression in liver and islets: implications for control of glucose homoeostasis. Biochem Soc Trans. 1990 Oct;18(5):851–853. doi: 10.1042/bst0180851. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L. The insulin cell: its cellular environment and how it processes (pro)insulin. Diabetes Metab Rev. 1986;2(1-2):71–106. doi: 10.1002/dmr.5610020106. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Berggren P. O., Bokvist K., Ericson H., Möhler H., Ostenson C. G., Smith P. A. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989 Sep 21;341(6239):233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986 Feb;162(2):507–520. doi: 10.1016/0014-4827(86)90354-x. [DOI] [PubMed] [Google Scholar]
- Schauder P., McIntosh C., Arends J., Arnold R., Frerichs H., Creutzfeldt W. Somatostatin and insulin release from isolated rat pancreatic islets stimulated by glucose. FEBS Lett. 1976 Oct 1;68(2):225–227. doi: 10.1016/0014-5793(76)80441-3. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., In't Veld P. A., Pipeleers D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan Y., Meda P., Neufeld M., Orci L. Stimulation of insulin secretion reveals heterogeneity of pancreatic B cells in vivo. J Clin Invest. 1987 Jul;80(1):175–183. doi: 10.1172/JCI113045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelman G., Alpert S., Hanahan D. Proliferation, senescence, and neoplastic progression of beta cells in hyperplasic pancreatic islets. Cell. 1988 Jan 15;52(1):97–105. doi: 10.1016/0092-8674(88)90534-x. [DOI] [PubMed] [Google Scholar]
- Teitelman G. Insulin cells of pancreas extend neurites but do not arise from the neuroectoderm. Dev Biol. 1990 Dec;142(2):368–379. doi: 10.1016/0012-1606(90)90357-o. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Trus M., Zawalich K., Gaynor D., Matschinsky F. Hexokinase and glucokinase distribution in the liver lobule. J Histochem Cytochem. 1980 Jun;28(6):579–581. doi: 10.1177/28.6.7391551. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Bonner-Weir S. Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest. 1990 Apr;85(4):983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesslén N., Pipeleers D., Van de Winkel M., Rorsman P., Hellman B. Glucose stimulates the entry of Ca2+ into the insulin-producing beta cells but not into the glucagon-producing alpha 2 cells. Acta Physiol Scand. 1987 Oct;131(2):230–234. doi: 10.1111/j.1748-1716.1987.tb08231.x. [DOI] [PubMed] [Google Scholar]