Abstract
Background
In 2012, hepatitis B virus (HBV) testing of people born in a country with a prevalence of ≥2% was recommended in the UK. Implementation of this recommendation requires an understanding of prior HBV testing practice and coverage, for which there are limited data.
Aim
To estimate the proportion of migrants tested for HBV and explore GP testing practices and barriers to testing.
Design and setting
A cross-sectional study of (a) migrants for whom testing was recommended under English national guidance, living in Bristol, and registered with a GP in 2006–2013, and (b) GPs practising in Bristol.
Method
NHS patient demographic data and HBV laboratory surveillance data were linked. A person was defined as ‘HBV-tested’ if a laboratory result was available. An online GP survey was undertaken, using a structured questionnaire.
Results
Among 82 561 migrants for whom HBV testing was recommended, 9627 (12%) were ‘HBV-tested’. The HBV testing coverage was: Eastern Africa 20%; Western Africa 15%; South Eastern Asia 9%; Eastern Asia 5%. Of 19 GPs, the majority did not use guidelines to inform HBV testing in migrants and did not believe routine testing of migrants was indicated; 12/17 GPs stated that workload and lack of human, and financial resources were the most significant barriers to increased testing.
Conclusion
The majority of migrants to a multicultural UK city from medium-/high-prevalence regions have no evidence of HBV testing. Much greater support for primary care in the UK and increased GP awareness of national guidance are required to achieve adherence to current testing guidance.
Keywords: cross-sectional studies, diagnosis, general practice, hepatitis B, transients and migrants, UK
INTRODUCTION
Worldwide, 240 million people have chronic hepatitis B (HBV) infection and 780 000 people die of the disease annually.1 Treatment is available and can slow disease progression and improve survival.2 The World Health Organization (WHO) classifies countries according to the hepatitis B surface antigen (HBsAg) into low (<2%), intermediate (2–8%), and high (>8%) prevalence.3 Chronic HBV infection disease burden in low-prevalence countries is mainly attributed to migrants from higher-prevalence countries.4
In the UK, a low-prevalence country, the majority of chronic HBV infection occurs among migrant populations who acquired their infection outside of the UK.5 The number of people with chronic HBV infection living in the UK is unknown — with estimates ranging from 86 000 to 326 000.6–8 UK testing strategies include antenatal screening,9 and testing blood donors and at-risk populations, including people who inject drugs, prisoners, patients undergoing haemodialysis, and healthcare workers.10 A 2013 UK study demonstrated that 1.1% of tested individuals were positive for HBsAg;11 in 2009, the prevalence of HBV among migrants in the UK was estimated to be 4%,12 ranging from 0.1% to 17.4% depending on ethnic group and study method.13–18
In 2012, the National Institute for Health and Care Excellence (NICE) recommended that all people born in a country with HBV prevalence of ≥2% should be offered a HBV test and that testing is offered in primary care.19 In 2011, approximately 7.5 million (13%) individuals living in England and Wales were born outside of the UK.20 The implementation of this guidance has major implications in terms of the costs and resources needed, and requires understanding of current testing coverage and GP testing practice in migrants. Such data are not routinely available in the UK, as country of birth information is not captured in HBV testing surveillance,21 and there have been no previous studies of HBV testing coverage and GP testing practices in migrants in the UK.
The aim of this service evaluation was to estimate the number of migrants for whom HBV testing is recommended under UK national guidance in a large multicultural UK city and the proportion tested and infected. Furthermore, this study aimed to explore testing practices and barriers to implementation of the national guidance among GPs in Bristol.
How this fits in
National guidance recommends HBV testing for migrants born in medium- and high-prevalence countries. GP testing practice and coverage in the UK is not presently known. The present study determines the proportion of migrants tested and explores GP testing practices, facilitators, and barriers to this. Only a small proportion of migrants were tested for HBV. Most GPs did not test routinely for HBV; the main barriers were lack of resources, workload, and guidance awareness.
METHOD
Hepatitis B testing and prevalence study
Study population
This study was undertaken in Bristol, a UK city with an estimated population of 428 234 in 2011, of whom 60 226 (14%) were born outside of the UK.22 The study population was defined as all individuals residing in Bristol, registered with a GP at any time between April 2006 and September 2013, and whose country or United Nations subregion of birth had a HBV prevalence of ≥2% as stated in the NICE guidance.
Data sources
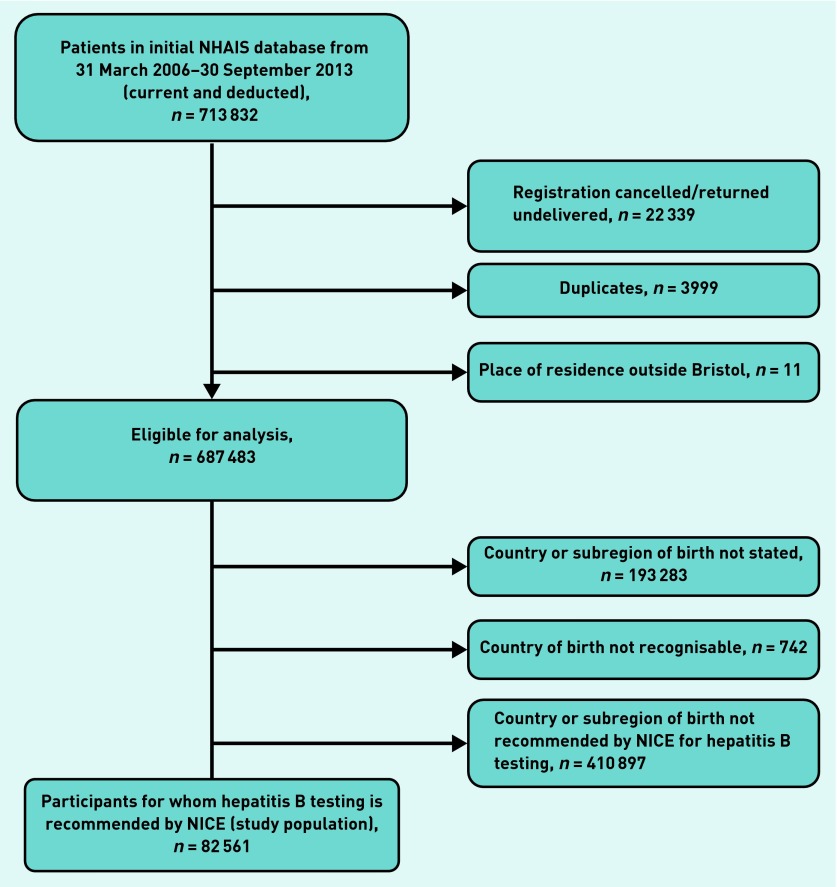
The study population was identified using the English National Health Authority Information System (NHAIS) — a database that includes sociodemographic characteristics of all patients registered at any time with a GP in England; a country of birth was assigned to each individual based on the ‘place of birth’ field. Where the place of birth was ambiguous, for example, the place recorded existed in more than one country or was unrecognisable, or missing, it was recorded as unknown. Duplicates and those not part of the defined study population were removed (Figure 1). Hepatitis B test results were provided by Bristol Public Health Laboratory (PHLB) for all patients tested during the study period. The dataset included: patient’s name or code; date of birth; NHS number; date of request; requestor and location of requestor; HBV serology (HBsAg, hepatitis B core antibody, hepatitis B e-antigen, hepatitis B e-antibody); and HBV DNA.
Figure 1.
Flowchart of selection process of the study participants. NHAIS = English National Health Authority Information System. NICE = National Institute for Health and Care Excellence.
Migrants were defined as ‘HBV tested’ if any HBV serology or HBV DNA test was performed during the study period; and as ‘HBV infected’ if HBsAg was positive or HBV DNA was detected. The requestor of the chronologically first HBV test in the study period was defined as ‘GP’ if the requestor was a GP practice, and ‘Antenatal’ (ANC) if either the ‘requestor’ or the ‘location’ field included any of the terms ‘midwives’, ‘antenatal’, ‘early pregnancy’, or ‘maternity’. The patient demographic database was linked with the laboratory dataset using the name, date of birth, and NHS number (if available).
GP survey
A survey of Bristol GPs to assess HBV testing practice in primary care and to explore barriers and facilitators to testing was undertaken. Bristol electoral wards (administrative small areas) were classified into high (≥30%), medium (10–29%), and low (<10%) population density of black and ethnic minority (BME) groups using 2011 census data,23 considering that GPs in higher BME density wards may be more aware of hepatitis B and test more. Invitations to participate were sent to one GP from 8 out of 8 GP practices located in high BME density wards and a stratified simple random sample of 6 out of 21 in medium and 10 out of 27 in low BME density wards; additionally, they were asked to encourage other GPs in their practice to participate. Data were collected from December 2013 to July 2014 using an online structured questionnaire that collected information on views and practices regarding HBV testing, barriers and facilitators to testing, awareness of NICE guidance, satisfaction with available resources, and resources needed to implement the guidance.
Data analysis
A descriptive analysis of the study population demographics was carried out and the proportion of individuals who were HBV tested and infected was estimated. The association between sociodemographic characteristics and BME density categories and HBV testing and infection was explored using Poisson regression to produce estimates of crude prevalence ratios (uPR). To explore bias from missing data, the proportion of tested and infected individuals for whom the country of birth was unknown was calculated. Statistical analysis was performed in Excel Microsoft Office 2010 and Stata 12 software.
RESULTS
Participants
Of 687 483 individuals identified, the country of birth was unknown for 194 025 (28%). Among 493 458 individuals with a known country of birth, 410 897 (83%) were born in low-prevalence countries, of which 387 569 (94%) were born in the UK; 82 561 (17%) were identified as migrants born in a country with ≥2% HBV prevalence, comprising the study population. Of these, 3987 (5%) were from high-prevalence countries.
The median age of the study population was 33 years (interquartile range 25–41); 50% were female (Table 1). A total of 39% were born in Asia; 18% in Eastern Europe, 18% in Southern Asia, and 14% in Eastern Africa. Poland (12%), Somalia (8%), and India (8%) were the commonest countries of birth.
Table 1.
Sociodemographic characteristics of study populationa
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Female | 41 255 | 50 |
| Male | 41 306 | 50 |
|
| ||
| Age group, years | ||
| <5 | 986 | 1 |
| 5–13 | 4211 | 5 |
| 14–17 | 2518 | 3 |
| 18–24 | 11 889 | 14 |
| 25–34 | 28 407 | 34 |
| 35–44 | 19 773 | 24 |
| 45–54 | 8158 | 10 |
| 55–64 | 3675 | 5 |
| >65 | 2944 | 4 |
|
| ||
| GP registration status | ||
| Current | 66 220 | 80 |
| Deducted | 16 341 | 20 |
|
| ||
| Regionb | ||
| Asia | 32 341 | 39 |
| Europe | 24 134 | 29 |
| Africa | 20 038 | 24 |
| Latin America and the Caribbean | 5958 | 7 |
| Oceania | 90 | 0.1 |
|
| ||
| Subregionb | ||
| Eastern Europe | 14 752 | 18 |
| Southern Asia | 14 446 | 18 |
| Eastern Africa | 11 926 | 14 |
| Southern Europe | 9382 | 11 |
| Eastern Asia | 7801 | 10 |
| South-Eastern Asia | 5544 | 7 |
| Western Asia | 4395 | 5 |
| Western Africa | 3854 | 5 |
| Caribbean | 3688 | 5 |
| Southern Africa | 2595 | 3 |
| South America | 1942 | 2 |
| Northern Africa | 1161 | 1 |
| Middle Africa | 502 | 0.6 |
| Central America | 328 | 0.4 |
| Central Asia | 155 | 0.2 |
| Pacific Islands | 90 | 0.1 |
n = 82 561.
Based on the United Nations geographical categorisation and composition of each region and subregion.
Compared with individuals with a known country of birth, those with an unknown country of birth were older (proportional age >54 years: 11% versus 56%, respectively, uPR 4.15, 95% CI = 4.11 to 4.19), were more likely to reside in low BME density wards (36% versus 49%, uPR 1.48, 95% CI = 1.47 to 1.50), and more likely to be registered with a GP before 20 September 2011 (= median date of registration with a GP) (45% versus 64%, respectively, uPR 1.68, 95% CI = 1.66 to 1.69).
Hepatitis B testing
Of 82 561 individuals in this study population, 9627 (12%) had evidence of a HBV test (Table 2); of whom 7201 (75%) were female. The proportion tested was greater for females (7201/41 255; 17%) than males (2426/41 306; 6%) (uPR 2.97, 95% CI = 2.84 to 3.11), and by age, compared with other age groups, greatest for the 35–44 years age group in both sexes (18% versus 10%, uPR 1.87, 95% CI = 1.79 to 1.95, 30% for females, uPR 2.16, 95% CI = 2.06 to 2.27; 8% for males, uPR 1.69, 95% CI = 1.56 to 1.83) and least for children and adolescents (0.5–2%, uPR 0.10, 95% CI = 0.08 to 0.12). The regions, subregions, and countries of birth with the highest proportion tested were: Africa (17%); Eastern Africa (20%) Middle Africa (19%), and Western Africa (15%); and Somalia (23%), Gambia (19%), Sudan (18%), and Pakistan (18%) (Table 3). Among the tested study populations, the requestor of their first chronological test in the study period was ‘GP’ for 4078 (42%) and ‘ANC’ for 3857 (40%).
Table 2.
Sociodemographic characteristics of study participants tested for hepatitis B
| Characteristic | Female | Male | All | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| n/N | % | n/N | % | n/N | % | |
| All participants tested | 7201/41 255 | 17 | 2426/41 306 | 6 | 9627/82 561 | 12 |
|
| ||||||
| Age group, years | ||||||
| <5 | 1/501 | 0.2 | 4/485 | 0.8 | 5/986 | 0.5 |
| 5–13 | 17/2092 | 0.8 | 29/2119 | 1.4 | 46/4211 | 1.1 |
| 14–17 | 26/1325 | 2 | 21/1193 | 2 | 47/2518 | 2 |
| 18–24 | 443/6758 | 7 | 115/5131 | 2 | 558/11 889 | 5 |
| 25–34 | 3346/14 961 | 22 | 635/13 446 | 5 | 3981/28 407 | 14 |
| 35–44 | 2644/8734 | 30 | 924/11 039 | 8 | 3568/19 773 | 18 |
| 45–54 | 466/3422 | 14 | 423/4736 | 9 | 889/8158 | 11 |
| 55–64 | 140/1777 | 8 | 166/1898 | 9 | 306/3675 | 8 |
| >65 | 118/1685 | 7 | 109/1259 | 9 | 227/2944 | 8 |
|
| ||||||
| BME density category, %a | ||||||
| <10% | 1713/8849 | 19 | 479/8428 | 6 | 2192/17 277 | 13 |
| 10–29% | 2172/18 589 | 12 | 682/16 810 | 4 | 2854/35 399 | 8 |
| ≥30% | 2847/10 777 | 26 | 1129/13 139 | 9 | 3976/23 916 | 17 |
| >65 | 118/1685 | 7 | 109/1259 | 9 | 227/2944 | 8 |
|
| ||||||
| GP registration status | ||||||
| Currently registered | 6384/32 558 | 20 | 2136/33 662 | 6 | 8520/66 220 | 13 |
| Deducted | 817/8697 | 9 | 290/7644 | 4 | 1107/16 341 | 7 |
|
| ||||||
| Requestor of first chronological test | ||||||
| Antenatal | 3857/7201 | 54 | – | – | – | – |
| GP/primary care | 2452/7201 | 34 | 1626/2426 | 67 | 4078/9627 | 42 |
| Other | 892/7201 | 12 | 800/2426 | 33 | 1692/9627 | 18 |
BME density category: percentage of black and ethnic minority (BME) inhabitants in the Bristol administrative ward of participants’ GP practice.
Table 3.
Proportion of study population tested for hepatitis B by region, subregion, and selected country of birtha
| Region/subregion of birthb | Study population, N | Ever tested | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Female | Male | Total | |||||
|
|
|
|
|||||
| n | % | n | % | n | % | ||
| Africa | 20 038 | 2521 | 27 | 965 | 9 | 3486 | 17 |
| Eastern Africa | 11 926 | 1739 | 30 | 624 | 10 | 2363 | 20 |
| Middle Africa | 502 | 65 | 27 | 30 | 12 | 95 | 19 |
| Northern Africa | 1161 | 99 | 24 | 46 | 6 | 145 | 12 |
| Southern Africa | 2595 | 228 | 17 | 67 | 5 | 295 | 11 |
| Western Africa | 3854 | 390 | 23 | 198 | 9 | 588 | 15 |
|
| |||||||
| Asia | 32 341 | 2451 | 15 | 806 | 5 | 3257 | 10 |
| Central Asia | 155 | 8 | 8 | 3 | 6 | 11 | 7 |
| Eastern Asia | 7801 | 330 | 7 | 97 | 3 | 427 | 5 |
| Southern Asia | 14 446 | 1420 | 23 | 475 | 6 | 1895 | 13 |
| South Eastern Asia | 5544 | 410 | 13 | 105 | 5 | 515 | 9 |
| Western Asia | 4395 | 283 | 17 | 126 | 5 | 409 | 9 |
|
| |||||||
| Latin America and the Caribbean | 5958 | 512 | 17 | 195 | 7 | 707 | 12 |
| Caribbean | 3688 | 360 | 20 | 154 | 8 | 514 | 14 |
| Central America | 328 | 23 | 12 | 7 | 5 | 30 | 9 |
| South America | 1942 | 129 | 12 | 34 | 4 | 163 | 8 |
|
| |||||||
| Europe | 24 134 | 1707 | 13 | 458 | 4 | 2165 | 9 |
| Eastern Europe | 14 752 | 1245 | 15 | 263 | 4 | 1508 | 10 |
| Southern Europe | 9382 | 462 | 10 | 195 | 4 | 657 | 7 |
|
| |||||||
| Oceania (Pacific Islands) | 90 | 10 | 19 | 2 | 5 | 12 | 13 |
|
| |||||||
| Country of birth | |||||||
| Somalia | 6885 | 1177 | 37 | 409 | 11 | 1586 | 23 |
| Gambia | 655 | 89 | 30 | 36 | 10 | 125 | 19 |
| Pakistan | 4021 | 564 | 33 | 176 | 8 | 740 | 18 |
| Sudan | 622 | 78 | 37 | 34 | 8 | 112 | 18 |
| Bangladesh | 1414 | 184 | 34 | 52 | 6 | 236 | 17 |
| Philippines | 1300 | 168 | 21 | 55 | 11 | 223 | 17 |
| Iraq | 819 | 85 | 39 | 43 | 7 | 128 | 16 |
| Zimbabwe | 1494 | 178 | 21 | 67 | 10 | 245 | 16 |
| Jamaica | 3016 | 308 | 21 | 133 | 9 | 441 | 15 |
| Kenya | 1048 | 108 | 20 | 34 | 7 | 142 | 14 |
| Ghana | 600 | 53 | 21 | 26 | 7 | 79 | 13 |
| Nigeria | 2060 | 182 | 19 | 94 | 8 | 276 | 13 |
| Czech Republic | 785 | 77 | 16 | 12 | 4 | 89 | 11 |
| India | 6466 | 527 | 18 | 173 | 5 | 700 | 11 |
| Romania | 1395 | 115 | 16 | 39 | 6 | 154 | 11 |
| South Africa | 2347 | 198 | 17 | 61 | 5 | 259 | 11 |
| Turkey | 872 | 60 | 20 | 35 | 6 | 95 | 11 |
| Bulgaria | 532 | 43 | 15 | 9 | 4 | 52 | 10 |
| Poland | 9481 | 826 | 16 | 166 | 4 | 992 | 10 |
| Russia | 706 | 60 | 13 | 14 | 6 | 74 | 10 |
| Slovakia | 940 | 77 | 15 | 16 | 4 | 93 | 10 |
| Thailand | 828 | 81 | 13 | 0 | 0 | 81 | 10 |
| Sri Lanka | 795 | 62 | 18 | 12 | 3 | 74 | 9 |
| Brazil | 911 | 59 | 12 | 15 | 4 | 74 | 8 |
| Hungary | 1405 | 87 | 12 | 21 | 3 | 108 | 8 |
| Iran | 1149 | 53 | 13 | 40 | 5 | 93 | 8 |
| Italy | 2049 | 105 | 11 | 68 | 6 | 173 | 8 |
| Japan | 570 | 41 | 11 | 2 | 1 | 43 | 8 |
| Portugal | 1058 | 41 | 9 | 30 | 5 | 71 | 7 |
| Greece | 879 | 33 | 9 | 21 | 4 | 54 | 6 |
| Hong Kong | 1830 | 80 | 8 | 32 | 4 | 112 | 6 |
| China | 4418 | 178 | 7 | 49 | 3 | 227 | 5 |
| Cyprus | 655 | 22 | 7 | 9 | 3 | 31 | 5 |
| Singapore | 576 | 18 | 5 | 8 | 3 | 26 | 5 |
| Spain | 3489 | 131 | 7 | 29 | 2 | 160 | 5 |
| Malaysia | 1892 | 64 | 7 | 20 | 2 | 84 | 4 |
| South Korea | 594 | 17 | 5 | 8 | 3 | 25 | 4 |
Data presented for countries with ≥500 migrants (86% of total population; N = 82 561.
Based on the UN geographical categorisation and composition of each region and subregion.
The proportion of the study population tested varied from 2% to 23% at practice level (excluding practices with fewer than 100 patients from medium-/high-prevalence countries). Practices in high BME density wards were more likely to test their migrant population for HBV than practices in low-/medium-density BME wards (17% versus 10%, uPR 1.74, 95% CI = 1.66 to 1.81). The lowest proportion tested was observed in a student health practice (2%, 168/7645), in which only 0.9% of 4418 Chinese students were tested.
Hepatitis B infection
Overall, among the HBV-tested subset of the study population, 5% (457/9627) were infected with hepatitis B, including 262 out 2426 (11%) of tested males and 195 out of 7201 (3%) of tested females (uPR 3.99, 95% CI = 3.31 to 4.80). Among the individuals tested in GP practices 249 out of 4078 (6%) were positive for HBV, including 158 out of 1626 (10%) males and 91 out of 2452 (4%) females (uPR 2.62, 95% CI = 2.02 to 3.39).
Of 410 897 individuals from low-prevalence countries, 36 661 (9%) were tested for HBV, and, among those tested, 106 (0.29%) were infected. Among 194 025 individuals with an unknown country of birth, 14 971 (8%) were tested for HBV and, among those tested, 0.53% were infected. Comparing individuals from medium-/high-prevalence countries, those coming from low prevalence and with an unknown country of birth were less likely to be tested for HBV (uPR 0.77, 95% CI = 0.75 to 0.78 and uPR 0.66, 95% CI = 0.64 to 0.68, respectively) and be infected (uPR 0.06, 95% CI = 0.05 to 0.08 and uPR 0.11, 95% CI = 0.09 to 0.14, respectively).
GP survey: views, practices, and barriers to HBV testing
Of 24 GP practices invited, 13 participated in the survey, with 19 GPs responding. The response was highest among practices in high BME density wards (7/8) compared with medium- (3/6) and low-density wards (3/10). GPs were predominantly male (10/17) of white ethnicity (16/17) with a mean age of 46 years (standard deviation [SD] 14); they were practising medicine for a mean of 18 years (SD 10).
Regarding guideline awareness, of 15 GPs who answered this question, 14 were not aware of the NICE guidance recommending routine HBV testing of migrants (Table 4). Regarding their views on testing, a minority of 19 GPs stated that routine HBV testing was indicated for migrants born in China (5/19), Somalia (4/19), Poland (2/19), or India (1/19) (Table 5). Most GPs indicated that testing should only be offered if the person presented with signs or symptoms of hepatitis B or after an active assessment for likelihood of infection. Regarding their practice, only three of 18 GPs indicated that it was their routine practice to offer opportunistic testing of patients born in high-prevalence countries.
Table 4.
GPs’ views and practices regarding hepatitis B testing of migrants born in intermediate- and high-prevalence countries
| Category | n | Total | |
|---|---|---|---|
| Use/awareness of HBV guidance | Use of local/national guidelines or other HBV resourcesa | 4 | 19 |
| Awareness of existence of NICE guidance PH43b | 1 | 15 | |
| Testing practice (before NICE guidance)c | Tested opportunistically migrants born in high-prevalence countries (for example, China, sub-Saharan Africa) | 3 | 19 |
| Future plans for testing (possible/very likely)d | Offer opportunistic and new registered patient testing | 17 | 19 |
| Start active case finding | 14 | 19 | |
| Barriers to testinge | Lack of resources (human, financial, logistical) and available time | 12 | 17 |
| Patients’ issues (awareness of HBV, acceptance of testing, compliance) | 10 | 17 | |
| Lack of HBV awareness of healthcare staff | 7 | 17 | |
| Language problems | 5 | 17 | |
| Usefulness of additional resources (very/a bit useful)f | Set up support for a person in the practice to perform contact tracing | 18 | 18 |
| Translated sample letters | 17 | 18 | |
| Automated flags in the GP electronic system for eligible patients | 17 | 17 | |
| Continuing professional development opportunities on hepatitis B | 17 | 17 | |
| Country of birth information available | 16 | 17 | |
| Improved access to translators | 13 | 15 |
HBV = Hepatitis B virus. The survey questions and possible answers were:
Do you routinely follow any local or national guidelines or use any resources to assist your decision making regarding testing for hepatitis B in migrants? Answers: (a) No (b) Yes.
Before receiving this questionnaire were you aware of the recent NICE public health guidance (Hepatitis B and C: ways to promote and offer testing to people at increased risk of infection, PH43) recommending that all people born in areas of intermediate or high hepatitis B prevalence should be offered a hepatitis B test? Answers: (a) No, this guidance had not been brought to my attention (b) Yes.
Prior to NICE guidance (December 2012 PH43), did you perform opportunistic testing for hepatitis B for patients born in regions of high hepatitis B prevalence (for example, China, sub-Saharan Africa)? Answers: (a) Yes this was my routine practice (b) No this was not my routine practice (c) Other.
Given that NICE public health guidance (PH43) now recommends testing in primary care for hepatitis B in all patients born in high- or intermediate-prevalence countries, how likely are you to do the following (in consultation with your partners)? Answers: (a) Already started (b) Very likely (c) Possible (d) Very unlikely (e) I don’t know.
In your opinion, what are the most significant barriers to testing eligible migrants for hepatitis B in your practice? (1 being the most important barrier). Answers: [free text].
What is your opinion on the current available resources and how useful would you find additional resources in assisting you with hepatitis B case finding? Answers: (a) Very useful (b) A bit useful (c) Not useful.
Table 5.
GPs’ views regarding hepatitis B testing practices on migrants born in intermediate- and high-prevalence countries (N = 19)
| Routine testing is indicated | Person should be actively assessed for likelihood ofhepatitis B, and offeredtesting on a case-by-case basis | Testing should be offered only if person presents with signs or symptoms of hepatitis | Other | |
|---|---|---|---|---|
| Country | ||||
| China | 5 | 8 | 6 | 0 |
| Somalia | 4 | 8 | 7 | 0 |
| Poland | 2 | 7 | 10 | 0 |
| India | 1 | 11 | 7 | 0 |
Question: Which of the statements best reflects the view that you held regarding hepatitis B testing for people born in the following countries, prior to NICE guidance (December 2012 PH43) on the topic?
When asked to state significant barriers to implementation of the NICE guidance, 12/17 GPs cited workload and lack of human and financial resources, with nine ranking workload as the most important barrier. Other barriers cited were: patient-related issues (10/17), such as awareness of HBV and acceptance of testing; lack of HBV awareness of healthcare staff (7/17); and language barrier (5/17). Overall, GPs indicated dissatisfaction with the resources and available guidance regarding HBV testing of migrant patients; the median satisfaction score among 19 GPs was 2 (range: 0–5) on a 0 to 10 scale. Almost all GPs reported that additional resources and support, such as support for contact tracing, translated sample letters, country of birth information, and automated flags for eligible patients, would be useful.
DISCUSSION
Summary
Testing for HBV among migrants born in countries with moderate/high prevalence was low; for 88% there was no evidence of testing. Proportionately more females than males were tested, notably among the 25–44-year-old age group, due to antenatal screening.24 Testing in children and adolescents was very low and may be partially attributed to higher vaccination coverage among this population. Practices serving high BME density wards had the highest proportion of tested individuals. GPs showed limited awareness of national guidance on migrant testing, did not routinely test migrants born in medium-/high-prevalence countries, and expressed dissatisfaction with the resources and support available to them.
Strengths and limitations
The study population used to determine HBV testing coverage was drawn from a regularly updated national population database (NHAIS database) expected to include the vast majority of migrant residents. Individuals missed will have included those not registered with a GP. Overall GP registration rates in the UK population are very high25 but irregular migrants make up approximately 7% of the UK migrant population26 and are under-represented in the present study. The study population was drawn from a single UK city and results cannot be generalised to the whole of the UK, as testing practice may differ.
The country of birth was unavailable for one-third of the NHAIS population. The place of birth field in the NHAIS database is filled from data collected at registration with a general practice. There is variation in the registration data requested between practices. Over time, more practices have included this field in their registration forms, and it is likely that those in areas of high BME density adopted this practice earlier. This variation in data collection is reflected in the high number of individuals for whom the country of birth is unknown, with this group being older, registered earlier, and more commonly residing in low BME areas. The low HBV testing coverage (8%) and low HBV prevalence (0.53%) in the group with an unavailable country of birth suggests that their omission from the study group is unlikely to have had a major impact on this study’s conclusion — that HBV testing coverage of migrants from target countries is very low.
The laboratory that supplied the HBV testing dataset provides all non-private HBV testing for the study area. Testing of the study population not captured will have included testing before the study period or before moving to the study area, testing through private practice, and anonymous testing at sexual health clinics. Data linkage between the population and the laboratory database may have been incomplete due to errors in identifiers used, thereby underestimating the proportion tested.
The GP survey had a high response rate among practices in high BME density wards and a medium to low response rate among practices in medium and low BME density wards; thus, the findings may not be generalisable to all GPs in these areas. However, one of the major findings from the survey — that HBV testing in primary care is done on the basis of clinical indicators and not as a routine — is corroborated by the much higher infection prevalence in tested males, where testing is elective, compared with the prevalence in females, where a proportion of tests are performed as antenatal screening, and by the high prevalence in the tested population in primary care (6%) when compared with the prevalence for the whole Bristol migrant population; estimated as 1.7%.27
Comparison with existing literature
To the authors’ knowledge, this is the first estimate of HBV testing coverage to be published in a UK migrant population. The finding of low coverage in the UK is in line with previous reports indicating that key stakeholders do not identify immigrant populations as a priority for HBV testing28 and that most GPs do not routinely screen migrants from endemic countries.29 However, individual GP practices in the UK may have HBV screening programmes targeting specific migrant populations, resulting in higher testing coverage.30 Testing coverage of migrant populations outside the UK demonstrated mixed results but was generally low.31,32
GPs’ guideline awareness and professional development were associated with increased screening in the US and Australia;33–35 language difficulties have been identified as barriers to testing and treatment.34,36 In the UK, new entrant migrants identified non-migrant-friendly services and disease-related stigma as barriers to testing.37
The overall prevalence of HBV infection in the present study is similar to previous estimates of 4%;12 3.3%;5 and 6.4%38 in selected UK migrant populations but may reflect selective testing, as it is significantly higher than the 1.7% estimated antenatal prevalence in the Bristol migrant population.27 The higher HBV prevalence in males is consistent with previous UK studies39,40 and again reflects greater selective testing in the male population.
Implications for research and practice
It is important to determine if other UK regions have similar low HBV testing coverage in migrant populations, because the application of the NICE guidance in large untested populations in primary care will require significant resources, with major cost implications that need to be carefully budgeted. The low testing coverage in migrants has clear consequences for patient access to treatment and for public health interventions in case-finding and disease prevention. The low awareness of the NICE guidance and the testing views of GPs suggest that the guidance needs to be promoted and healthcare professionals educated on HBV. The dissatisfaction of the GPs regarding the available resources (for example, patient information material), and their concerns regarding lack of time and staffing, reflected a true lack of supporting resources. Public health authorities could support the implementation of NICE guidance by providing easily accessible online resources, building the human resource capacity, and developing testing strategies, such as opportunistic, active, and new registrants’ testing. Targeted testing of children should be considered because of the very low levels of testing coverage and given the evidence of child-to-child intrafamilial spread.1,41 GP electronic systems could be adapted to allow easy identification of at-risk patients, that is, easy access to country of birth information and use of automated messages.
Acknowledgments
The authors thank all the GPs for participating in the study. Thanks also go to: Dr Andre Charlett, Head, Statistics, Modelling and Economics Department, Public Health England; and Neville Verlander, Statistician, Statistics, Modelling and Economics Department, Public Health England for their support and advice in the statistical analysis. Grateful thanks go to Dr Kostas Danis, Scientific Coordinator, European Programme for Intervention Epidemiology Training, European Centre for Disease Prevention and Control, for his valuable advice during protocol development and data analysis.
Funding
This project has been supported by an educational grant via the Gilead UK and Ireland Fellowship Programme. It was also funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at the University of Bristol, in partnership with Public Health England (PHE). The views expressed herein are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, or PHE.
Ethical approval
Approval from an Ethics Committee for the study was not required, as this was a service evaluation. This decision was accepted by the Regional Ethics Committee Centre (Bristol). Management of data with personal identifiers complied with Caldicott guidelines.42
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Alexandra Cochrane has received research funding from Gilead UK and Ireland Fellowship Programme. The Gilead UK and Ireland Fellowship Programme had no role in the design of the study, data collection, analysis, interpretation, abstract preparation, or the decision to submit. All other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.World Health Organization Hepatitis B. Fact sheet No 204. Updated July 2015. http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed 4 Mar 2016)
- 2.National Clinical Guideline Centre Hepatitis B (chronic): diagnosis and management. 2013. CG165. https://www.nice.org.uk/guidance/cg165/resources/hepatitis-b-chronic-diagnosis-and-management-35109693447109 (accessed 15 Feb 2016)
- 3.World Health Organization Hepatitis B: introduction. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index1.html (assessed 14 Mar 2016)
- 4.European Centre for Disease Prevention and Control Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. 2010. http://ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C%20_EU_neighbourhood.pdf (accessed 4 Mar 2016)
- 5.Hahne S, Ramsay M, Balogun K, et al. Incidence and routes of transmission of hepatitis B virus in England and Wales, 1995–2000: implications for immunisation policy. J Clin Virol. 2004;29(4):211–220. doi: 10.1016/j.jcv.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health Getting ahead of the curve: a strategy for combating infectious diseases (including other aspects of health protection) 2002. http://webarchive.nationalarchives.gov.uk/20130107105354/ http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4060875.pdf (accessed 4 Mar 2016)
- 7.Hope VD, Eramova I, Capurro D, Donoghoe MC. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol Infect. 2014;142(2):270–286. doi: 10.1017/S0950268813000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pendleton S, Wilson-Webb P. Rising curve: chronic hepatitis B infection in the UK. London: Hepatitis B Foundation UK; 2007. [Google Scholar]
- 9.Department of Health Screening of pregnant women for hepatitis B and immunisation of babies at risk. 1998. http://webarchive.nationalarchives.gov.uk/20130107105354/ http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4011840.pdf (accessed 4 Mar 2016)
- 10.European Centre for Disease Prevention and Control Surveillance and prevention of hepatitis B and C in Europe. 2010 http://ecdc.europa.eu/en/publications/Publications/101012_TER_HepBandC_survey.pdf (accessed 4 Mar 2016) [Google Scholar]
- 11.Public Health England Annual report from the sentinel surveillance study of blood borne virus testing in England: data for January to December 2013. Health Protection Report: weekly report. 2014;8(29):1–11. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/345716/hpr2914_senthep.pdf (accessed 15 Feb 2016) [Google Scholar]
- 12.Advisory Group on Hepatitis . Case-finding for hepatitis B and C virus infection in minority ethnic populations in the UK. Advisory Group on Hepatitis; 2009. [Google Scholar]
- 13.Newell A, Sullivan A, Halai R, Boag F. Sexually transmitted diseases, cervical cytology and contraception in immigrants and refugees from the former Yugoslavia. Venereology. 1998;11(1):25–27. [PubMed] [Google Scholar]
- 14.Aweis D, Brabin BJ, Beeching NJ, et al. Hepatitis B prevalence and risk factors for HBsAg carriage amongst Somali households in Liverpool. Commun Dis Public Health. 2001;4(4):247–252. [PubMed] [Google Scholar]
- 15.Kawsar M, Goh BT. Hepatitis B virus infection among Chinese residents in the United Kingdom. Sex Transm Infect. 2002;78(3):166–168. doi: 10.1136/sti.78.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin G, Shoeb D, Solaiman S, et al. Prevalence of chronic viral hepatitis in people of south Asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. J Viral Hepat. 2010;17(5):327–335. doi: 10.1111/j.1365-2893.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- 17.McPherson S, Valappil M, Moses SE, et al. Targeted case finding for hepatitis B using dry blood spot testing in the British-Chinese and South Asian populations of the North-East of England. J Viral Hepat. 2013;20(9):638–644. doi: 10.1111/jvh.12084. [DOI] [PubMed] [Google Scholar]
- 18.Vedio AB, Ellam H, Rayner F, et al. Hepatitis B: report of prevalence and access to healthcare among Chinese residents in Sheffield UK. J Infect Public Health. 2013;6(6):448–455. doi: 10.1016/j.jiph.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence Hepatitis B and C: ways to promote and offer testing to people at increased risk of infection. 2012. PH43. https://www.nice.org.uk/guidance/ph43/resources/hepatitis-b-and-c-testing-people-at-risk-of-infection-1996356260293 (accessed 4 Mar 2016)
- 20.Office for National Statistics Detailed country of birth and nationality analysis from the 2011 Census of England and Wales. 2013 http://www.ons.gov.uk/ons/dcp171776_310441.pdf (accessed 4 Mar 2016) [Google Scholar]
- 21.Health Protection Services Migrant health: infectious diseases in non-UK born populations in the United Kingdom. An update to the baseline report — 2011. 2011 http://webarchive.nationalarchives.gov.uk/20140714084352/ http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317131998682 (accessed 4 Mar 2016) [Google Scholar]
- 22.Office for National Statistics . 2011 Census: KS204EW country of birth, local authorities in England and Wales. UK: 2012. http://www.ons.gov.uk/ons/rel/census/2011-census/key-statistics-for-local-authorities-in-england-and-wales/rft-table-ks204ew.xls (accessed 4 Mar 2016) [Google Scholar]
- 23.Office for National Statistics 2011 Census: QS211EW Ethnic group (detailed), wards in England and Wales. UK Data Service Census Support. 2012. http://www.ons.gov.uk/ons/rel/census/2011-census/key-statistics-and-quick-statistics-for-wards-and-output-areas-in-england-and-wales/rft-qs211ew-ward.zip (accessed 4 Mar 2016)
- 24.Public Health England Antenatal screening for infectious diseases in England: summary report for 2013. Infection Reports. 2014;8(43) https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/375211/hpr4314_ntntlscrng.pdf (accessed 4 Mar 2016) [Google Scholar]
- 25.Health and Social Care Information Centre Attribution data set GP-registered populations scaled to ONS population estimates — 2011. 2012. http://www.hscic.gov.uk/searchcatalogue?productid=4710&q=title%3a%22Attribution+data+set+GP-registered+populations%22&sort=Relevance&size=10&page=1#top (accessed 4 Mar 2016)
- 26.Gordon I, Scanlon K, Travers T, Whitehead C. Economic impact on the London and UK economy of an earned regularisation of irregular migrants to the UK. 2009 http://www.lse.ac.uk/geographyAndEnvironment/research/london/pdf/irregular%20migrants%20full%20report.pdf (accessed 4 Mar 2016) [Google Scholar]
- 27.Cochrane A, Evlampidou I, Irish C, et al. Hepatitis B infection prevalence by country of birth in migrant populations in a large UK city. J Clin Virol. 2015;68:79–82. doi: 10.1016/j.jcv.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Jones L, Bates G, McCoy E, et al. A practice survey of activities and interventions that aim to raise awareness among, and/or engage with, groups who are at an increased risk of hepatitis B and C infection. 2013 https://www.nice.org.uk/guidance/ph43/evidence/hepatitis-b-and-c-ways-to-promote-and-offer-testing-mapping-review2 (accessed 4 Mar 2016) [Google Scholar]
- 29.Ahmad A, Falla A, Veldhuijzen I, et al., editors. Current hepatitis B and C screening practices for first generation migrants and barriers to screening: results from an online questionnaire survey of experts in Germany, The Netherlands, Hungary, Italy, UK and Spain. 2014. http://hiveurope.eu/Portals/0/Conference%202014/Poster%20presentations/PO3_09.pdf (accessed 4 Mar 2016)
- 30.Gregory A, Vedio A, Stone B, et al. Targeted testing in primary care demonstrates high prevalence of hepatitis B infection within the Slovak-Roma population in Sheffield, UK. J Viral Hepat. 2014;21(10):e138–e139. doi: 10.1111/jvh.12287. [DOI] [PubMed] [Google Scholar]
- 31.Niederau C. Chronic hepatitis B in 2014: great therapeutic progress, large diagnostic deficit. World J Gastroenterol. 2014;20(33):11595–11617. doi: 10.3748/wjg.v20.i33.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516–1523. doi: 10.1007/s10620-010-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallman JB, Arsalla A, Park V, et al. Screening for hepatitis B, C and nonalcoholic fatty liver disease: a survey of community-based physicians. Aliment Pharmacol Ther. 2009;29(9):1019–1024. doi: 10.1111/j.1365-2036.2009.03961.x. [DOI] [PubMed] [Google Scholar]
- 34.Lai C, Nguyen T, Hwang J, et al. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Canc Educ. 2007;22(1):37–41. doi: 10.1007/BF03174373. [DOI] [PubMed] [Google Scholar]
- 35.Wallace J, Hajarizadeh B, Richmond J, McNally S. Investigating general practice and hepatitis B. 2012. https://www.latrobe.edu.au/arcshs/downloads/arcshs-research-publications/General-Practice-and-Hepatitis-B-Final-Report.pdf (accessed 4 Mar 2016)
- 36.Guirgis M, Yan K, Bu YM, Zekry A. General practitioners’ knowledge and management of viral hepatitis in the migrant population. Intern Med J. 2012;42(5):497–504. doi: 10.1111/j.1445-5994.2011.02440.x. [DOI] [PubMed] [Google Scholar]
- 37.Seedat F, Hargreaves S, Friedland JS. Engaging new migrants in infectious disease screening: a qualitative semi-structured interview study of UK migrant community healthcare leads. PLoS One. 2014;9(10):e108261. doi: 10.1371/journal.pone.0108261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One. 2012;7(9):e44611. doi: 10.1371/journal.pone.0044611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Health Protection Agency Targeting testing in England: health protection services sentinel surveillance of blood-bourne virus testing (SBV) in England Annual review 2011. 2013 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/363846/Sentinel_Surveillance_Annual_Review_2011.pdf (accessed 4 Mar 2016) [Google Scholar]
- 40.Tedder RS, Rodger AJ, Fries L, et al. The diversity and management of chronic hepatitis B virus infections in the United Kingdom: a wake-up call. Clin Infect Dis. 2013;56(7):951–960. doi: 10.1093/cid/cis1013. [DOI] [PubMed] [Google Scholar]
- 41.Zervou EK, Gatselis NK, Xanthi E, et al. Intrafamilial spread of hepatitis B virus infection in Greece. Eur J Gastroenterol Hepatol. 2005;17(9):911–915. doi: 10.1097/00042737-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 42.The Caldicott Committee Report on the review of patient-identifiable information. 1997 http://webarchive.nationalarchives.gov.uk/20130107105354/ http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4068404.pdf (accessed 4 Mar 2016) [Google Scholar]