Abstract
Background
Hepatitis B (HBV), hepatitis C (HCV), and HIV blood-borne viruses (BBV) are associated with chronic ill health and mortality. Early diagnosis reduces disease transmission, delays progression, and improves outcomes. Routine opt-out testing for BBV in primary care may be viable in identifying unknown disease.
Aim
To assess the viability and yield of routine opt-out testing for BBV.
Design and setting
A multicentre, prospective, routine opt-out testing study of BBV in primary care between September 2014 and February 2015 across four sites in Dublin, Ireland.
Method
All adult patients attending for routine blood tests were offered an additional BBV test during a 6-month period. All individuals were given an information leaflet before phlebotomy and were given the choice to opt out of BBV testing.
Results
In total, 1188 patients were invited to participate in the study and 1063 (89.5%) opted to be tested (95% confidence interval [CI] = 87.7% to 91.2%). A total of 125 patients (10.5%) opted out. There were 10 positive results, four new diagnoses, and six previously known. There were two new HBV and two new HCV diagnoses, a yield of four per 1000 (95% CI = 0.9 to 7.5 cases per 1000). No new HIV cases were diagnosed.
Conclusion
This study indicates that testing for BBV in patients presenting for routine blood tests in primary care is viable. The yield of HBV and HCV suggests that opt-out testing should be considered in primary care to increase detection rates of BBV.
Keywords: general practice, hepatitis B, hepatitis C, HIV, primary health care, screening
INTRODUCTION
Blood-borne viruses (BBV) are associated with chronic ill health and considerable mortality. Hepatitis B virus (HBV) infection causes a vaccine-preventable disease transmitted through contact with blood or body fluids from an infected person. The risk of developing chronic HBV infection reduces with age, but once developed there is a 40% risk of progression to hepatic cirrhosis or hepatocellular carcinoma.1 The Irish Health Protection and Surveillance Centre (HPSC) reported 445 cases of newly identified chronic HBV infection in 2014 and a general population prevalence rate of <1%.1 Screening for HBV in Ireland is currently targeted at particular high-risk groups including people born in HBV-endemic countries, injecting drug users, contacts of known cases, and people with multiple sexual partners. An opt-out testing strategy operates at sexual health clinics and antenatal clinics across the country.2 Most patients screened for chronic HBV infection are asymptomatic and unaware of their status. Early detection of chronic HBV infection will allow treatment with antiviral drugs, which can stop viral replication and minimise liver damage.
In 2014 the number of people newly diagnosed with hepatitis C virus (HCV) infection in Ireland was 710.1 It is estimated that a possible 20 000–50 000 people living in the country are unaware of their HCV infection.2 The predominant risk factors for HCV infection in Ireland are drug use (80%) and receipt of blood products.2 In primary care the current standard practice is opt-in testing for HCV in people with known risk factors or symptomatic liver disease. Three-quarters of all people infected with HCV will develop chronic infection, and after 20 years 5–20% develop liver cirrhosis with 1.5–2.5% of patients with cirrhosis progressing to hepatocellular carcinoma.3 Sustained virological response (SVR) synonymous with HCV cure can now be achieved using antiviral treatment.4 It is important that cases of HCV infection are identified early and treated to prevent disease complications and reduce the spread of infection.5
The HPSC reported an 11% increase in 2014 in the number of people newly diagnosed with HIV, compared with 2013 with 49% of those newly diagnosed presenting at either late or advanced stage.1 Prompt diagnosis of HIV infection and appropriate early treatment can reduce HIV-related illness for affected individuals, prevent disease transmission, and reduce the economic burden associated with late diagnosis.6 Current standard practice in Irish primary care is to offer patients a risk-based opt-in blood test for HIV. The 2008 UK British HIV Association guidelines state that an HIV test should be considered in settings in which diagnosed HIV prevalence in the local population exceeds two per 1000.7 In 2012, the six specialist HIV centres in Ireland collaborated to report an HIV-diagnosed prevalence rate of over two per 1000 among 15–59-year-olds in the Dublin area.8
How this fits in
Standard practice in primary care is to offer targeted opt-in tests for blood-borne viruses (BBV) based on patient risk profiles. This study supports research suggesting that routine opt-out testing is viable compared with targeted tests among the general population. Such testing may help to identify previously unknown cases of BBVs.
It is suggested that a review of testing strategy for BBVs should be considered in Ireland. This study supports research suggesting that routine opt-out testing is viable compared with targeted tests among the general population.9
METHOD
Setting and participants
This is a multicentre prospective opt-out study of BBV across four primary care sites in Dublin, Ireland, conducted between September 2014 and February 2015. In total, the combined practice population is approximately 15 000 patients. The four primary care sites are all general practices located in areas of relative deprivation in Dublin City. All individuals aged >18 years who presented for routine blood tests during the study period were offered an additional blood test to screen for BBV. The tests performed were HBV surface antigens, antibody tests for HCV, and an antigen–antibody combination assay for HIV. Further confirmatory testing was done when a positive result was found. The cost of the initial screen for the three pathogens is approximately €25 (∼£20).
Sample collection
A meeting was held in each practice before commencing the study to educate all relevant members of the practice team. Posters were placed in the waiting room areas detailing the study. Before blood testing, patients were given an information leaflet about the study. The leaflet was modified for primary care from a leaflet used in an opt-out BBV screening study in the emergency department of a nearby hospital. The patients were given the choice to opt out of having the additional blood sample drawn.
All patients who were offered blood tests had their decision documented and coded in their electronic file. Coding in the electronic chart prevented the same patient being offered testing on more than one occasion. All information retrieved from individual patients was anonymised and entered into an electronic spreadsheet.
Informed verbal consent was obtained from each patient who opted in and were documented in the patient file. This is now the standard of care in HIV testing as it is thought that obtaining written consent is unnecessary and may discourage HIV testing by making it an exception.10
Testing for BBV was performed on the blood sample either at St James’s Hospital or the National Virus Reference Laboratory (NVRL), Dublin, depending on where each practice routinely sent its blood samples.
RESULTS
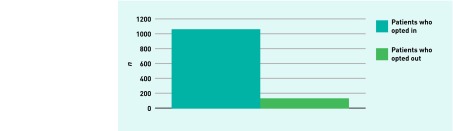
In total, 1188 patients (775 [65.2%] female, 413 [34.8%] male) were offered BBV screening during the 6-month study period. This represents approximately 8% of the total practice populations. Of those, 1063 patients (89.5%) opted in (95% CI = 87.7% to 91.2%), and 125 patients (10.5%) opted out (Figure 1). The median age of those tested was 54 years (Table 1) and this is reflective of the median age of the total patient population.
Figure 1.
Number of patients offered blood-borne virus testing and opting decision.
Table 1.
Sex and age characteristics of patients offered blood-borne virus testing and opting decision
| Opted in | Opted out | Total | |
|---|---|---|---|
| Male, n (%) | 406 (89.6) | 47 (10.3) | 453 |
| Female, n (%) | 657 (89.4) | 78 (10.6) | 735 |
| Total, n (%) | 1063 (89.2) | 125 (10.5) | 1188 |
| Median age, years | 54 | 57 | 54 |
| Age range, years | 18–96 | 18–93 | 18–96 |
Of the 1063 patients who opted in, 657 (61.8%) were female and 406 (38.2%) were male. The median age of this group was 54 years. Of the 125 patients who opted out, 78 (62.4%) were female and 47 (37.6%) were male. The median age was 57 years. There was no significant difference between age or sex in those patients who opted in or out of testing.
In total, 10 of the 1063 patients who were tested had positive results. There were two new diagnoses of HBV and two new diagnoses of HCV, a yield of four per 1000 (95% CI = 0.9 to 7.5 cases per 1000). All eight cases of HCV were confirmed as active. No new HIV cases were diagnosed.
All new cases were immediately referred to specialist care in tertiary referral centres, as agreed with the services before initiating the study. Of the six previously known cases of HCV identified during the study, two patients were not routinely attending a hospital specialist and have now been referred back to specialist care.
DISCUSSION
Summary
The aim of this study was to assess the viability and yield of routine opt-out testing for BBV in primary care. Of the patients invited to participate in the study, 89.5% opted to have testing for BBV. In total, 10 of the 1063 patients who were tested had positive results. There were two new diagnoses of HBV. Eight individuals tested positive for HCV, of which two cases were new diagnoses. No cases of HIV were identified.
The median age of those who opted out of testing was 57 years, which was marginally higher than that of the total sample population of 54 years. There was no significant difference between age or sex in those patients who opted in or out of testing.
The yield of four per 1000 is reflective of a larger study conducted in the emergency department of a Dublin inner-city teaching hospital.11 In this study 8839 individual patient samples were taken. There were 447 (5.1%) positive results, of which 85 (1.0%) were new. Of those, 70 (0.8%), 20 (0.2%), and 58 (0.7%) were new diagnoses of HIV, HBV, and HCV, respectively. The present study conducted in the primary care setting mirrored the prevalence of HBV and HCV in that hospital study, being two (0.2%) and two (0.2%), respectively.11
The current study concludes that offering routine opt-out BBV tests in primary care is viable. The yields of HBV and HCV from this study suggest that opt-out testing in primary care may be an option in diagnosing unknown disease.
Strengths and limitations
This is the first routine opt-out testing study for BBVs to be conducted in primary care. The high uptake rate of 89.5% confirms that it is viable to conduct routine opt-out testing for BBV in primary care.
The study yield of 10 positive results including two new cases of HBV, two new cases of HCV, and two cases of HCV lost to follow-up is reflective of a larger study of 10 000 blood tests conducted in the emergency department of a nearby tertiary referral centre in St James’s Hospital, Dublin, during a similar time period.11
This present study is limited in that it was a relatively small study (with four primary care sites) and was conducted over a short time period.
Comparison with existing literature
No literature was found on any other studies that screen for BBV in Irish primary care; however, a few studies in primary care have been cited in international journals. In the UK between 2009 and 2010, eight pilot projects were set up looking at HIV testing in different healthcare settings, which included three pilots in primary care.12 Two of the primary care projects aimed to offer testing to newly registering patients within the surgeries, and one aimed to offer testing to all patients attending the practice. One of the primary care pilots included 10 GP surgeries in the Brighton area, with a 59% uptake of tests. Another primary care pilot in the London area had 18 surgeries and this had a 62% uptake. In the London primary care pilot, 19 people were discovered to be newly diagnosed out of 2713 who took the test, giving a prevalence of 7 per 1000.12 In a separate pilot study involving patients from the emergency department, outpatients department, acute medical unit, and general practice, 92% agreed with the statement ‘It is acceptable for me to be offered an HIV test’.
In the US, routine opt-out screening for HIV has been recommended for individuals aged 13–64 years in all healthcare settings by the Centers for Disease Control and Prevention (CDC) since 2006.13 A study conducted between January and April 2010 in a primary care centre in Dayton, Ohio, offered adult patients aged <65 years a routine opt-out HIV test. In total, 272 patients were offered HIV testing, of whom 46 patients (17%) agreed. No new positive diagnoses were made.14 The authors suspect that the uptake was low because patients who had no medical insurance had to pay $115 (∼£80) upfront for the test and 43% of the study practice population were not insured. In the present study, the additional BBV blood test was provided at no additional cost to the patient.
In 2013, between February and June, a study was conducted in southern Spain testing patients for HIV in a primary care setting. It was an opt-out testing strategy for patients aged 20–55 years. HIV testing was offered to 508 patients, with 368 (72%) choosing to be tested. No positive results were identified during the study.15
The present study appears to be the first of its kind conducted in primary care that combines testing for HBV, HCV, and HIV screening. When compared with the aforementioned studies in the US, UK, and Spain, it achieved a significantly higher uptake rate. The higher uptake rate may have been achieved through use of practice nurses, who performed most of the phlebotomy, in the execution of the study protocol.
Implications for research and practice
The yield of four new cases of BBV in over 1000 individuals tested, which correlates with rates obtained in a larger emergency department study in a nearby tertiary referral centre, supports the concept of routine opt-out testing for BBV in primary care. Opt-out testing in primary care may be an option in diagnosing BBV.
Given the success of these studies in confirming the viability of opt-out BBV testing in both primary and secondary healthcare settings, there is a clear opportunity to develop current testing strategies across both primary and secondary care. Qualitative studies are needed in primary care to assess the feasibility and acceptability of such strategies.
Furthermore, the low levels of patients who opted out of testing in this study suggest that opt-out primary care testing may help reduce the stigma previously associated with such blood tests.
Acknowledgments
We would like to thank the staff of the general practice sites involved in the study, our colleagues in the Trinity College Dublin/ HSE General Practice Training Programme, and Department of Public Health and Primary Care. We wish to also thank Dr Sarah O’Connell in the Department of GU Medicine and Infectious Diseases at St James’s Hospital, Dublin, and Dr Cillian De Gascun of the National Virus Reference Laboratory (NVRL), Dublin.
Funding
No funding was obtained for this study. The costs of processing blood samples were accommodated by the laboratory sites.
Ethical approval
Ethical approval for the study protocol was granted by the Trinity College Dublin/HSE General Practice Training Programme ethics committee.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Health Protection Surveillance Centre HPSC annual epidemiological report 2014. http://www.hpsc.ie/AboutHPSC/AnnualReports/File,15505,en.pdf (accessed 15 Apr 2016)
- 2.Thornton L, Murphy N, Jones L, et al. Determination of the burden of hepatitis C virus infection in Ireland. Epidemiol Infect. 2012;140(8):1461–1468. doi: 10.1017/S0950268811001920. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Hepatitis C Working Group Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44(1):20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 4.Innes H, Hutchison S, Allen S, et al. Excess liver related morbidity of chronic hepatitis C patients who achieve a sustained viral response and are discharged from care. Hepatology. 2011;54(5):1547–1558. doi: 10.1002/hep.24561. [DOI] [PubMed] [Google Scholar]
- 5.Conjeeveram H. Continued progress against hepatitis C infection. JAMA. 2015;313(17):1716–1717. doi: 10.1001/jama.2015.4368. [DOI] [PubMed] [Google Scholar]
- 6.May M, Gomples M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ. 2011;343:d6016. doi: 10.1136/bmj.d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.British HIV Association. British Association for Sexual Health and HIV, British Infection Society UK national guidelines for HIV testing 2008. http://www.bhiva.org/documents/guidelines/testing/glineshivtest08.pdf (accessed 15 Apr 2016) [Google Scholar]
- 8.Tuite H, Horgan M, Mallon P, et al. Antiretroviral treatment and viral load responses in HIV-infected patients accessing specialist care in Ireland. European Congress of Clinical Microbiology and Infectious Disease; London. 2012. http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=145623&XNSPRACHE_ID=2&XNKONGRESS_ID=161&XNMASKEN_ID=900 (accessed 15 Apr 2016) [Google Scholar]
- 9.Thornton AC, Rayment M, Elam G, et al. Exploring staff attitudes to routine HIV testing in non-traditional settings: a qualitative study in four healthcare facilities. Sex Transm Infec. 2012;88(8):601–606. doi: 10.1136/sextrans-2012-050584. [DOI] [PubMed] [Google Scholar]
- 10.Royal College of Physicians Testing for HIV: concise guideline. 2009. https://www.rcplondon.ac.uk/guidelines-policy/testing-hiv (accessed 15 Apr 2016)
- 11.Connell SO, Lillis D, O Dea S, et al. Results of a universal testing programme for blood borne viruses in an urban emergency department, including rates of diagnosis and linkage to care. AASLD, the Liver Meeting; 7–11 Nov 2014; Boston, MA. 2014. http://hdl.handle.net/2262/74982 (accessed 15 Apr 2016) [Google Scholar]
- 12.Health Protection Agency Time to test for HIV: expanding healthcare and community HIV testing in England. 2011 http://www.nhivna.org/documents/Publications/Guidelines/TTT-InterimReport.pdf (accessed 15 Apr 2016) [Google Scholar]
- 13.Branson B, Handsfield H, Lampe M, et al. Revised recommendations for HIV testing of adults, adolescents and pregnant women in health-care settings. 2006 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm (accessed 15 Apr 2016) [PubMed] [Google Scholar]
- 14.Anim M, Markert RJ, Okoye NE, Sabbagh W. HIV screening of patients presenting for routine medical care in a primary care setting. J Prim Care Community Health. 2013;4(1):28–30. doi: 10.1177/2150131912448071. [DOI] [PubMed] [Google Scholar]
- 15.Diaz F, Ortega P, Antequera P, et al. HIV infection early diagnosis experience in primary care. J Int AIDS Soc. 2014;17(suppl 3):19597. doi: 10.7448/IAS.17.4.19597. [DOI] [PMC free article] [PubMed] [Google Scholar]