Abstract
Background
Some European countries have introduced standardised cancer patient pathways (CPPs), including urgent referrals, with the aim of diagnosing cancer at an earlier stage. This is despite a lack of evidence, particularly in patients with symptomatic cancer diagnosed via general practice.
Aim
To compare tumour stages in patients with incident cancer diagnosed via general practice before, during, and after CPP implementation in Denmark in 2008–2009.
Design and setting
A comparative cohort study of data from GPs and registries on patients with incident cancer listed with a GP before (n = 1420), during (n = 5272), and after (n = 2988) CPP implementation.
Method
χ2 test was used to compare stage distributions and logistic regression to estimate odds ratios (OR) of having local cancer after versus before CPP implementation.
Results
Distribution of tumour stages did not differ statistically significantly across time (P = 0.494) or between CPP use (P = 0.202). For all cancers combined, the OR of having local cancer after CPP implementation was 0.88 (95% confidence interval [CI] = 0.73 to 1.06) compared with before. For CPP-referred patients, the OR of having local cancer was 0.77 (95% CI = 0.62 to 0.94) compared with all patients before CPP implementation; the corresponding OR for non-CPP-referred patients was 0.96 (95% CI = 0.80 to 1.14).
Conclusion
No clear tendencies were observed confirming earlier detection of cancer after rather than before CPP implementation. CPP-referred patients had lower odds of having local cancer after CPP implementation than all patients before CPP implementation; this could be because the GPs refer patients who are ‘more ill’ as urgent referrals.
Keywords: cancer, Denmark, early diagnosis, general practice, tumour stage, urgent referral
INTRODUCTION
In recent years standardised cancer patient pathways (CPPs), including urgent referral, have been introduced in some European countries to accelerate diagnostic work.1–9 Denmark implemented CPPs in 2008 and 2009.2 The Danish CPPs consist of guidelines, including descriptions of selected alarm symptoms that may raise cancer suspicion, descriptions of medical procedures (mainly in the secondary healthcare sector), and specific timeframes for all phases (for example, 9 days from GP referral to first appointment at hospital when colorectal cancer is suspected).2,10
CPP strategies differ by country, but they tend generally to rest on the common assumption that improved prognosis (that is, better survival) can be ensured by shorter time to diagnosis and hence earlier detection of cancer. As survival is highly dependent on the tumour stage at diagnosis,11–13 tumour stage is a fair proxy for prognosis.
Although >80% of patients with cancer are diagnosed via a general practice route,14,15 the effect of CPP implementation on tumour stage has been evidenced in only three studies of patients diagnosed via a primary care route, and with conflicting results.5,16,17 In cross-sectional studies, tumour stages seem to differ according to referral routes (CPP or not) for some cancer sites: more advanced stages for lung and ovarian cancer among those urgently referred, no difference for prostate cancer, and diverging results for colorectal cancer.7,9,18–20 This could indicate that a selection is performed by the GP to comply with CPP guidelines.
The present study aimed to examine the effect of CPP implementation to identify tumours at earlier stages for seven different cancer types among symptomatic patients diagnosed via a general practice route. Furthermore, the study aimed to evaluate whether identified associations between CPP implementation and tumour stage may be interpreted as reflecting a causal relationship or whether results are biased by clinical decision making through a GP’s use of CPP referral.
METHOD
Data from GPs and registries from the Danish Cancer in Primary Care (CaP) cohort21 were used to compare tumour stage among patients with incident cancer diagnosed via a general practice route before, during, and after CPP implementation.
How this fits in
The effect of implementation of urgent referral schemes on tumour stage at diagnosis is unknown. This study found that implementation of urgent referrals (named cancer patient pathways [CPPs] in Danish) in Denmark was not associated with lower tumour stage and that urgently referred patients tended to have worse tumour stage than non-urgently referred patients. These findings can be explained by selection of a certain patient group for CPP referral and indicate that a narrow focus on a predefined checklist of specific symptoms and corresponding guidelines may lead to overlooking the double-sided nature of the clinical triage in general practice.
Setting
The study took place in Denmark, where the publicly funded healthcare system ensures free access to diagnostic procedures and treatment for all citizens. Almost all citizens (>98%) are registered with a specific general practice, which acts as gatekeeper to the rest of the healthcare system (except for otorhinolaryngologists and ophthalmologists who can be accessed directly).22 A national screening programme for breast cancer was implemented in Denmark in 2007–2009, making patients with breast cancer ineligible for the present study. Patients with prostate cancer also were ineligible because of the increased use of prostate specific antigen (PSA) tests in general practice,23 which, unrelated to CPP implementation, increased the proportion of localised tumours detected.24,25
Identification of patients and data collection
Patients were identified in hospital registers and in the Danish National Patient Registry before (1 September 2004 to 31 August 2005), during (1 October 2007 to 30 September 2008), and after (1 May 2010 to 31 August 2010) CPP implementation. Patients were eligible if they were aged ≥18 years, were listed with a GP, attended general practice as part of their diagnostic route, and were registered with a verified first-time diagnosis of colorectal cancer (ICD-10: C18–C20), lung cancer (ICD-10: C34), malignant melanoma (ICD-10: C43), head and neck cancer (ICD-10: C01–14, C30–C32, C462, and C73), upper gastrointestinal (upper GI) cancer (ICD-10: C15–C17 and C22–C26), gynaecological cancer (ICD-10: C51–C58), or urinary system cancer (ICD-10: C64–C68). The GP’s involvement was defined on the basis of the response (yes/no) to the following question in the GP questionnaire: ‘Were you/your general practice involved in diagnosing the cancer?’21
Variables used in this study
Clinical tumour stage was obtained from the Danish Cancer Registry and was based on the TNM classification of malignant tumours (T = size of Tumour, N = involved lymph Nodes, M = distant Metastasis) staging system. Tumour stage was categorised for colorectal, lung, malignant melanoma, and bladder cancers using established cancer-specific algorithms to categorise tumours with missing TNM components as: local, regional, distant, unknown (partly missing TNM components), or missing (all TNM components missing).26–29 TNM staging information for the remaining patients was categorised as local (no positive lymph nodes or metastasis), regional (positive lymph nodes), distant (metastatic cancer), missing (no T, N, and M information), or unknown (all remaining cancers).21
Exposure was the sampling time for the three sub-cohorts according to the CPP implementation: 2004/2005 = before CPP implementation (before), 2007/2008 = during CPP implementation (during), and 2010 = after CPP implementation (after). The ‘after’ group was subsequently categorised as ‘CPP-referred patients’ and ‘non-CPP-referred patients’ based on GP-reported information on referral route.21
Confounding effects were controlled for in categories of sex, comorbidity, educational level, and disposable income, and also age centred at 45 years; details are published elsewhere.21
Statistical analyses
Complete case analyses were performed. Differences in tumour stage distribution were compared using Pearson χ2 test.
Tumour stage was dichotomised into local and regional/distant combined (unknown and missing tumour stage excluded). Logistic regression was used to estimate the odds ratios (OR) of having a local tumour stage after versus before CPP implementation. Two adjusted models were considered: one with no regard of referral route (overall trend) and another with CPP-exposed patients divided into referral routes (trend by referral route). Model fit was assessed by Pearson goodness-of-fit test.
The impact of selection bias and missing data of tumour stage was investigated by sub-analyses of all complete cases, regardless of whether general practice was involved or not, including patients with a non-participating GP (n = 9736) and by multiple imputation (n = 12 346). Multiple imputations were done by a multivariate model with 1-year survival, sex, age, comorbidity, income, educational background, and cancer site as predictors of missing and unknown tumour stage, missing educational level, and income.
Analyses were done using Stata statistical software (version 13), and a value of P ≤0.05 was considered significant in all analyses.
RESULTS
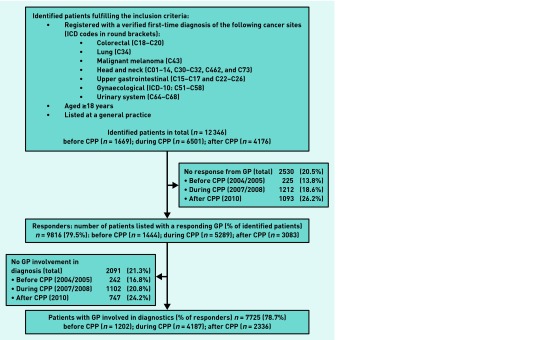
Of 12 346 patients with incident cancer identified, GP responses were received for 9816 cases (79.5%) (Figure 1). Patients with participating GPs were less likely to be male and had fewer missing data on tumour stage than other patients (data not shown). The GPs reported being involved in diagnosing cancer for 7725 (78.7%) of the included cases. The study population is described in Table 1.
Figure 1.
Flowchart showing patient identification, drop-out, and GP involvement. The box on the left indicates exclusion of patients because of no GP involvement, whereas the box on the right indicates drop-out because of GP non-response. CPP = cancer patient pathways. ICD = International Statistical Classification of Diseases and Related Health Problems.
Table 1.
Demographic characteristics of patients diagnosed through a primary care route; before, during, and after the implementation of cancer patient pathways. The ‘after’ cohort is also shown by referral route (n = 7725)
| Before | During transition | After | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Total | CPP-referred | Non-CPP referred | ||||||||||
|
|
|
|
|
|
|
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Total | 1202 | 100 | 4187 | 100 | 2336 | 100 | 772 | 100 | 1564 | 100 | 7725 | 100 |
|
| ||||||||||||
| Sex | ||||||||||||
| Female | 624 | 51.9 | 2120 | 50.6 | 1128 | 48.3 | 346 | 44.8 | 782 | 50.0 | 3872 | 50.1 |
| Male | 578 | 48.1 | 2067 | 49.4 | 1208 | 51.7 | 426 | 55.2 | 782 | 50.0 | 3853 | 49.9 |
|
| ||||||||||||
| Age groups, years | ||||||||||||
| 18–44 | 84 | 7.0 | 258 | 6.2 | 141 | 6.0 | 39 | 5.1 | 102 | 6.5 | 483 | 6.3 |
| 45–54 | 138 | 11.5 | 469 | 11.2 | 264 | 11.3 | 73 | 9.5 | 191 | 12.2 | 871 | 11.3 |
| 55–64 | 293 | 24.4 | 1040 | 24.8 | 549 | 23.5 | 196 | 25.4 | 353 | 22.6 | 1882 | 24.4 |
| 65–74 | 337 | 28.0 | 1235 | 29.5 | 724 | 31.0 | 252 | 32.6 | 472 | 30.2 | 2296 | 29.7 |
| ≥75 | 350 | 29.1 | 1185 | 28.3 | 658 | 28.2 | 212 | 27.5 | 446 | 28.5 | 2193 | 28.4 |
|
| ||||||||||||
| Diagnosis | ||||||||||||
| Colorectal | 283 | 23.5 | 1073 | 25.6 | 629 | 26.9 | 224 | 29.0 | 405 | 25.9 | 1985 | 25.7 |
| Lung | 280 | 23.3 | 1018 | 24.3 | 501 | 21.4 | 202 | 26.2 | 299 | 19.1 | 1799 | 23.3 |
| Melanoma | 125 | 10.4 | 403 | 9.6 | 236 | 10.1 | 82 | 10.6 | 154 | 9.8 | 764 | 9.9 |
| Head and neck | 74 | 6.2 | 260 | 6.2 | 180 | 7.7 | 39 | 5.1 | 141 | 9.0 | 514 | 6.7 |
| Upper gastrointestinal | 185 | 15.4 | 570 | 13.6 | 336 | 14.4 | 84 | 10.9 | 252 | 16.1 | 1091 | 14.1 |
| Gynaecological | 141 | 11.7 | 484 | 11.6 | 250 | 10.7 | 64 | 8.3 | 186 | 11.9 | 875 | 11.3 |
| Urinary system | 114 | 9.5 | 379 | 9.1 | 204 | 8.7 | 77 | 10.0 | 127 | 8.1 | 697 | 9.0 |
|
| ||||||||||||
| Comorbidity, CCI (index date = date of diagnosis) | ||||||||||||
| None | 793 | 66.0 | 2913 | 69.6 | 1636 | 70.0 | 572 | 74.1 | 1064 | 68.0 | 5342 | 69.2 |
| Moderate | 319 | 26.5 | 1051 | 25.1 | 563 | 24.1 | 169 | 21.9 | 394 | 25.2 | 1933 | 25.0 |
| High | 90 | 7.5 | 223 | 5.3 | 137 | 5.9 | 31 | 4.0 | 106 | 6.8 | 450 | 5.8 |
|
| ||||||||||||
| Educational level, ISCED | ||||||||||||
| Low (1 + 2) | 473 | 39.4 | 1874 | 44.8 | 897 | 38.4 | 310 | 40.2 | 587 | 37.5 | 3244 | 42.0 |
| Medium (3 + 4) | 421 | 35.0 | 1450 | 34.6 | 883 | 37.8 | 282 | 36.5 | 601 | 38.4 | 2754 | 35.7 |
| High (5 + 6) | 202 | 16.8 | 641 | 15.3 | 456 | 19.5 | 149 | 19.3 | 307 | 19.6 | 1299 | 16.8 |
| Missing | 106 | 8.8 | 222 | 5.3 | 100 | 4.3 | 31 | 4.0 | 69 | 4.4 | 428 | 5.5 |
|
| ||||||||||||
| Annual disposable income, OECD tertiles | ||||||||||||
| Lower | 378 | 31.4 | 1323 | 31.6 | 778 | 33.3 | 273 | 35.4 | 505 | 32.3 | 2479 | 32.1 |
| Medium | 363 | 30.2 | 1364 | 32.6 | 802 | 34.3 | 260 | 33.7 | 542 | 34.7 | 2529 | 32.7 |
| Higher | 395 | 32.9 | 1360 | 32.5 | 753 | 32.2 | 239 | 31.0 | 514 | 32.9 | 2508 | 32.5 |
| Missing | 66 | 5.5 | 140 | 3.3 | 3 | 0.1 | 0 | 0 | 3 | 0.2 | 209 | 2.7 |
CCI = Charlson’s Comorbidity Index. ISCED = International Standard Classification of Education. OECD = Organisation for Economic Co-operation and Development.
Overall tumour stage across time
The tumour stage distribution did not differ across time for all cancers combined (P = 0.494), nor for the individual cancer types (Table 2). Proportions of missing and unknown tumour stages differed statistically significantly, however, across time for all cancers combined (P <0.001) and for colorectal, lung, head and neck cancers, and gynaecological cancers (Table 2).
Table 2.
Tumour stage distribution for patients diagnosed via a primary care route shown for seven cancer types and total
| Before | During | After | P-valuea | CPP-referred | Non-CPP-referred | P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Colorectal cancer (n= 1985) | ||||||||||||
| Local | 86 | 30.4 | 340 | 29.0 | 201 | 32.0 | 71 | 31.7 | 130 | 32.1 | ||
| Regional | 67 | 23.7 | 279 | 23.8 | 176 | 28.0 | 67 | 29.9 | 109 | 26.9 | ||
| Distant | 63 | 22.3 | 245 | 29.4 | 166 | 26.4 | 0.874c | 62 | 27.7 | 104 | 25.7 | 0.847c |
| Unknown | 60 | 21.2 | 202 | 17.2 | 82 | 13.0 | 23 | 10.3 | 59 | 14.6 | ||
| Missing | 7 | 2.5 | 7 | 0.6 | 4 | 0.6 | 0.003d | 1 | 0.4 | 3 | 0.7 | 0.569d |
|
| ||||||||||||
| Lung cancer (n= 1799) | ||||||||||||
| Local | 51 | 18.2 | 184 | 18.1 | 68 | 13.6 | 27 | 13.4 | 41 | 13.7 | ||
| Regional | 53 | 18.9 | 167 | 16.4 | 97 | 19.4 | 41 | 20.3 | 56 | 18.7 | ||
| Distant | 149 | 53.2 | 630 | 61.9 | 322 | 64.3 | 0.055c | 132 | 65.3 | 190 | 63.5 | 0.946c |
| Unknown | 23 | 8.2 | 36 | 3.5 | 14 | 2.8 | 2 | 1.0 | 12 | 4.0 | ||
| Missing | 4 | 1.4 | 1 | 0.1 | 0 | 0.0 | <0.001d | 0 | 0.0 | 0 | 0.0 | 0.244d |
|
| ||||||||||||
| Malignant melanoma (n= 764) | ||||||||||||
| Local | 84 | 67.2 | 277 | 68.7 | 175 | 74.2 | 60 | 73.2 | 115 | 74.7 | ||
| Regional | 10 | 8.0 | 39 | 9.7 | 21 | 8.9 | 8 | 9.8 | 13 | 8.4 | ||
| Distant | 9 | 7.2 | 15 | 3.7 | 7 | 3.0 | 0.319c | 3 | 3.7 | 4 | 2.6 | 0.853c |
| Unknown | 18 | 14.4 | 65 | 16.1 | 33 | 14.0 | 11 | 13.4 | 22 | 14.3 | ||
| Missing | 4 | 3.2 | 7 | 1.7 | 0 | 0.0 | 0.159d | 0 | 0.0 | 0 | 0.0 | 0.950d |
|
| ||||||||||||
| Head and neck cancer (n= 514) | ||||||||||||
| Local | 25 | 33.8 | 96 | 36.9 | 71 | 39.4 | 16 | 41.0 | 55 | 39.0 | ||
| Regional | 38 | 51.4 | 130 | 50.0 | 65 | 36.1 | 18 | 46.2 | 47 | 33.3 | ||
| Distant | 2 | 2.7 | 11 | 4.2 | 6 | 3.3 | 0.347c | 1 | 2.6 | 5 | 3.5 | 0.704c |
| Unknown | 2 | 2.7 | 18 | 6.9 | 37 | 20.6 | 4 | 10.3 | 33 | 23.4 | ||
| Missing | 7 | 9.5 | 5 | 1.9 | 1 | 0.6 | <0.001d | 0 | 0.0 | 1 | 0.7 | 0.360d |
|
| ||||||||||||
| Upper gastrointestinal cancer (n= 1091) | ||||||||||||
| Local | 32 | 17.3 | 81 | 14.2 | 52 | 15.5 | 13 | 15.5 | 39 | 15.5 | ||
| Regional | 32 | 17.3 | 124 | 21.8 | 63 | 18.8 | 19 | 22.6 | 44 | 17.5 | ||
| Distant | 70 | 37.8 | 248 | 43.5 | 147 | 43.8 | 0.536c | 35 | 41.7 | 112 | 44.4 | 0.623c |
| Unknown | 43 | 23.2 | 108 | 18.9 | 72 | 21.4 | 17 | 20.2 | 55 | 21.8 | ||
| Missing | 8 | 4.3 | 9 | 1.6 | 2 | 0.6 | 0.057d | 0 | 0.0 | 2 | 0.8 | 0.782d |
|
| ||||||||||||
| Gynaecological cancer (n= 875) | ||||||||||||
| Local | 95 | 67.4 | 308 | 63.6 | 165 | 66.0 | 38 | 59.4 | 127 | 68.3 | ||
| Regional | 17 | 12.1 | 48 | 9.9 | 24 | 9.6 | 10 | 15.6 | 14 | 7.5 | ||
| Distant | 15 | 10.6 | 61 | 12.6 | 38 | 15.2 | 0.742c | 11 | 17.2 | 27 | 14.5 | 0.136c |
| Unknown | 8 | 5.7 | 59 | 12.2 | 22 | 8.8 | 4 | 6.3 | 18 | 9.7 | ||
| Missing | 6 | 4.3 | 8 | 1.7 | 1 | 0.4 | 0.049d | 1 | 1.6 | 0 | 0.0 | 0.108d |
|
| ||||||||||||
| Urinary system cancer (n= 697) | ||||||||||||
| Local | 79 | 69.3 | 244 | 64.4 | 136 | 66.7 | 47 | 61.0 | 89 | 70.1 | ||
| Regional | 2 | 1.8 | 20 | 5.3 | 12 | 5.9 | 6 | 7.8 | 6 | 4.7 | ||
| Distant | 22 | 19.3 | 84 | 22.2 | 33 | 16.2 | 0.241c | 13 | 16.9 | 20 | 15.7 | 0.526c |
| Unknown | 8 | 7.0 | 21 | 5.5 | 22 | 10.8 | 10 | 13.0 | 12 | 9.4 | ||
| Missing | 3 | 2.6 | 10 | 2.6 | 1 | 0.5 | 0.084d | 1 | 1.3 | 0 | 0.0 | 0.463d |
|
| ||||||||||||
| All cancers (n= 7725) | ||||||||||||
| Local | 452 | 37.6 | 1530 | 36.5 | 868 | 37.2 | 272 | 35.3 | 596 | 38.1 | ||
| Regional | 219 | 18.2 | 807 | 19.3 | 458 | 19.6 | 168 | 21.8 | 289 | 18.5 | ||
| Distant | 330 | 27.5 | 1294 | 30.9 | 719 | 30.8 | 0.494c | 257 | 33.3 | 462 | 29.5 | 0.067c |
| Unknown | 162 | 13.5 | 509 | 12.2 | 282 | 12.1 | 71 | 9.2 | 211 | 13.5 | ||
| Missing | 39 | 3.2 | 47 | 1.1 | 9 | 0.4 | <0.001d | 3 | 0.4 | 6 | 0.4 | 0.006d |
Test for differences in tumour stage distribution across time.
Test for differences in tumour stage distribution between CPP-referred and non-CPP-referred patients.
Missing and unknown stage excluded.
Missing and unknown stage included.
CPP = cancer patient pathway.
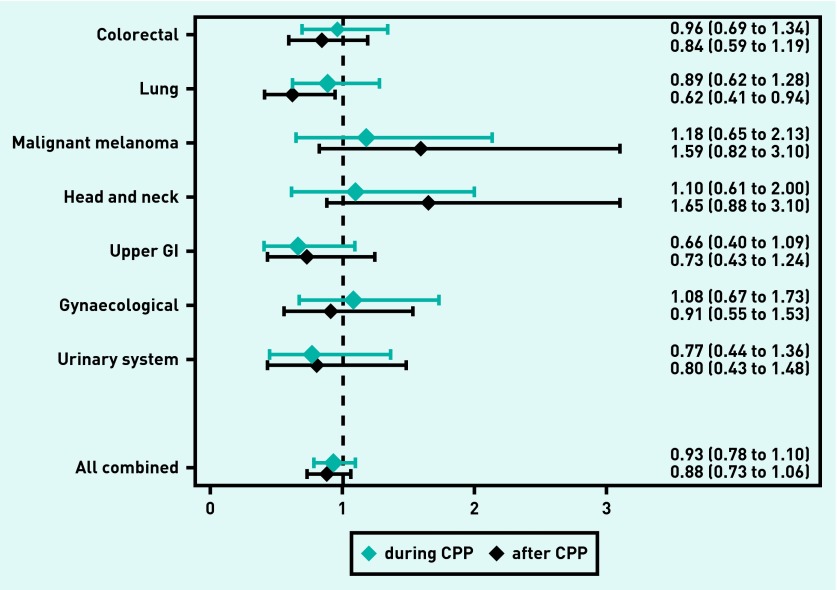
For all cancers combined, the OR of having local cancer was 0.88 (95% CI = 0.73 to 1.06) after CPP implementation compared with before (Figure 2). Patients with lung cancer had an OR of 0.62 (95% CI = 0.41 to 0.94) of having local cancer after CPP implementation compared with before (Figure 2). The ORs of having local cancer during CPP implementation compared with before were similar for all cancer types and for all cancers combined (Figure 2). The sensitivity analyses showed no changes in estimates for all patients (regardless of involvement of general practice), nor after multiple imputations (Appendices 1–3).
Figure 2.
Odds ratios and 95% CIs for patients with incident cancer with non-missing tumour stage diagnosed through a primary care route having local cancer during (blue) and after (black) CPP implementation compared with before CPP implementation. Values <1 indicate less likelihood of having local cancer compared with before CPP implementation. All values are adjusted for sex, age, cancer site, comorbidity, educational level, and household income. GI = gastrointestinal.
Tumour stage by referral route
The tumour stage distributions did not differ between CPP-referred and non-CPP-referred patients (Table 2). Yet, for all cancers combined, non-CPP-referred patients had higher proportions of unknown and missing tumour stages than CPP-referred patients (P = 0.006).
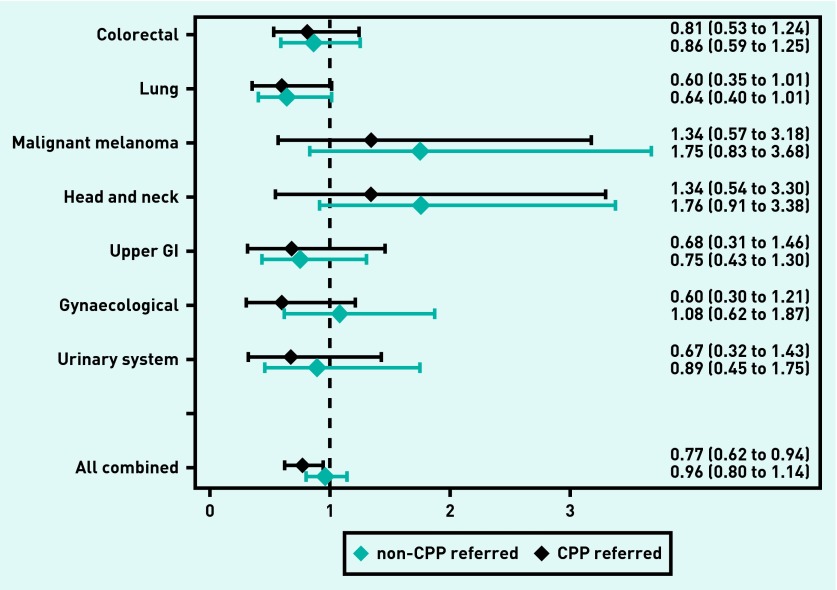
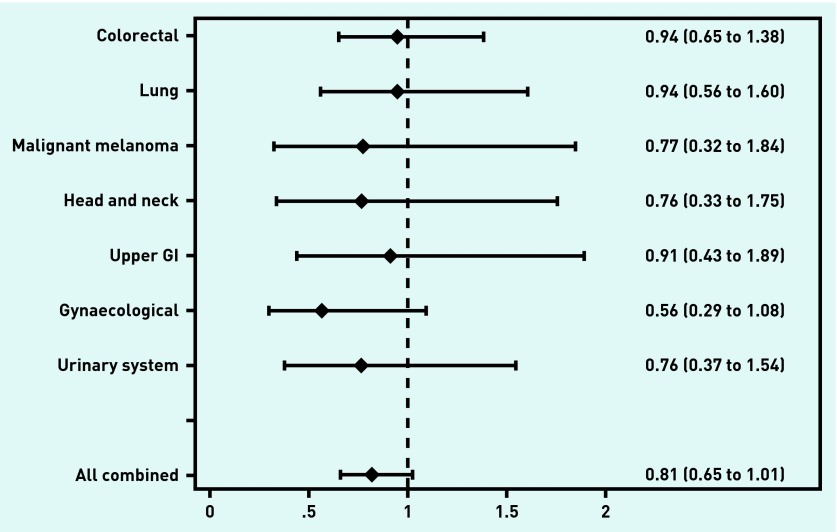
For all cancers combined, an OR of 0.77 (95% CI = 0.62 to 0.94) of having local cancer was identified for CPP-referred patients compared with the total group of patients before CPP implementation (Figure 3). Similar patterns were observed across different cancer types, but all 95% CIs of the ORs included 1 (Figure 3). For all cancers combined, CPP-referred patients tended to be less likely than non-CPP-referred patients to be diagnosed with localised cancer (OR 0.81, 95% CI = 0.65 to 1.01, P = 0.066) (Figure 4).
Figure 3.
Odds ratios and 95% CIs for patients with incident cancer, with non-missing tumour stage diagnosed through a primary care route of having local cancer shown for CPP-referred patients (black) and non-CPP-referred patients (blue), both compared with all patients before CPP implementation. Values <1 indicate less likelihood of having local cancer compared with before CPP implementation. All values are adjusted for sex, age, cancer site, comorbidity, educational level, and household income. GI = gastrointestinal.
Figure 4.
Odds ratios and 95% CIs of local cancer among CPP-referred patients compared with non-CPP-referred patients. Values <1 indicate that CPP-referred patients are less likely to have localised cancer than non-CPP-referred patients. All values are adjusted for sex, age, cancer site, comorbidity, educational level, and household income. GI = gastrointestinal.
DISCUSSION
Summary
As the purpose of CPPs is to improve the prognosis of patients with cancer, a more favourable distribution of tumour stage after CPP implementation is anticipated. Yet, the present study found no evidence of a higher likelihood of having local cancer across time for all cancers combined for all patients with cancer, nor for patients diagnosed through general practice. Yet, the proportion of missing recordings of tumour stage decreased over time; this could indicate more complete staging and more complete records of staging information.
CPP-referred patients generally had a lower likelihood of having local cancer after CPP implementation compared with all patients before CPP implementation. CPP-referred patients also tended to be less likely to have local cancer than non-CPP-referred patients.
The results are likely to reflect more complete registration of tumour stage, stage migration, lack of statistical precision, and confounding by severity.
Strengths and limitations
The collective impact of CPP implementation was analysed, with emphasis on the case-mix of diagnoses, that is, variations across administrative time and space. Even though this study focused on patients diagnosed through general practice, the discussed methodological issues also refer to the analyses comparing all patients (Appendices 1–3).
The study design does not permit inference of causality, but it carries a risk that the association found between CPP implementation and localised tumour stage is coincidental. Furthermore, the study design cautions that the findings of more advanced tumours among non-CPP-referred patients cannot be rigorously interpreted as a causal effect of CPPs with the potential to disadvantage this group.
Selection bias was reduced during the identification of patients as all patients with cancer were included, regardless of symptom and cancer site, even late-registered patients.9,20 Furthermore, the high response rate of 79% reduced the risk of selection bias. Although selection bias cannot be ruled out entirely, its role in this study seems to be minor as the sensitivity analyses displayed similar results.
The quality of available information may have changed over the study period because of clinical practice, increased intensity of investments, and modernisation of the Danish Cancer Registry (DCR) (from paper to electronic registration).30,31 These changes may have biased the observed tumour stage across time. Incomplete staging information decreased over time, which may indicate improvements in staging and data-recording practices. Yet, missing data of tumour stages in the DCR is a well-known concern.26–29,32 The present sensitivity analyses displayed identical results, however, which suggest that this potential bias alone cannot explain the findings.
Misclassification of tumour stages may have occurred in the present study for three reasons: data entry errors in the DCR, misclassification of tumours (local, regional, or distant), and registration time of tumour stage (at diagnosis or at treatment start). It is believed that the potential misclassification of stages is non-differential and thus would tend to underestimate the associations found across time. Although bias related to registration time and data entry errors may be differentiated across time, it is believed that it may have led to underestimation of the ORs presented.
The confidence intervals were comparatively large (0.73 to 1.06), indicating that the non-significant findings may be a result of low statistical precision, although the observed OR of having local cancer was 0.88.
Slightly more distant/metastatic cancers were found in this study than those reported by the clinical databases of the Danish Multidisciplinary Cancer Groups (DMCGs) for colorectal, lung, and ovarian cancers during 2000–2012,33 which calls into question the quality of TNM staging in the DCR.
Comparison with existing literature
Three former studies have compared tumour stage across time among patients diagnosed through a general practice route during CPP implementation, but these were restricted to include only highly selected groups of patients with two cancer types.5,16,17 The results of no difference in tumour stages among patients with colorectal cancer are in line with the study by Zafar and colleagues.17 The present findings contrast, however, the findings of more stage IV head and neck cancers reported by Lyhne and colleagues5 and of more Dukes’ stage A colorectal cancers reported by Cerdán-Santacruz and colleagues.16
No previous studies were found of patients with lung cancer diagnosed through a primary care route that compared tumour stages before and after CPP implementation. As the Danish CPP guidelines recommended intensified and better quality in the diagnostic work-up,2 this may have directed a shift towards more thorough pre-therapeutic assessment, which could lead to more severely staged tumours at the time of diagnosis after CPP implementation.
Recent evidence has shown that malignant melanoma was more likely to be diagnosed as stage I cancer during 2009–2011 compared with 2004–2008.34 This could be because of greater awareness mediated by a large national skin cancer campaign launched in 2007.34
It is believed that the finding that CPP-referred patients tend to be less likely to have local cancer than non-CPP-referred patients mainly reflects confounding by severity, that is, bias stemming from the inherent differences in prognosis given the severity of the patient’s disease.35 Clearly, the chance of being referred to CPP increases as the underlying disease evolves and produces more severe symptoms.36 This may also be why tumour stages tend to differ according to referral routes (CPP or not) in other studies,7,9,18–20 may explain why GPs select the most severely ill cases for CPP referral,37 and may explain why the patients referred and diagnosed within the 2-week wait framework had higher tumour stage than other patients.38 Therefore, the level of disease may in itself be a confounder and thus may constitute an unacknowledged methodological problem, which challenges testing the effect of CPP implementation on earlier detection of cancer in symptomatic patients.
Implications for research and practice
The possibility that part of the findings can be explained by confounding by severity, which originates from the selection of certain patient groups who are referred to the CPP route, indicates that a narrow focus on a predefined checklist of specific symptoms and corresponding guidelines may lead to disregarding the double-sided nature of the clinical triage in general practice: the GP is expected to spot (and refer) the seriously ill patients, but the GP is also expected to prevent healthy people from getting unnecessary examinations at hospitals.39
The double-sided nature of the clinical triage stresses the need for faster diagnostic work-up for patients who are not eligible for CPP referral. The introduction of CPPs allows the GPs to refer the most ill patients, but these patients may not profit the most in terms of earlier cancer stage from faster diagnosis. Furthermore, the present findings that the GPs refer the most ill patients to the CPPs (in line with the guidelines) and stage migration over time may indicate that tumour stage is an insufficient measure to evaluate the effectiveness of CPPs in an observational study design that spans many years. Even though no improvement in tumour stage was observed in this study, patients with symptomatic cancer may still benefit from more timely diagnosis in terms of less morbidity, better performance scores, and increased patient satisfaction. This needs to be tested in future studies.
Acknowledgments
We thank data manager Kaare Rud Flarup at the Research Centre for Cancer Diagnosis in Primary Care, Research Unit for General Practice, for his outstanding and meticulous help with setting up and maintaining the database and enabling register linkage at Statistics Denmark. We also thank the employees at Statistics Denmark for providing the data platform and the secure data environment.
Appendix 1. Tumour stages before, during, and after CPP implementation for all identified patients
| Before | During | After | Total | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| n | % | n | % | n | % | n | % | ||
| Colorectal cancer (n= 3067) | |||||||||
| Local | 109 | 28.8 | 520 | 31.7 | 339 | 32.3 | 968 | 31.6 | |
| Regional | 84 | 22.2 | 402 | 24.5 | 266 | 25.4 | 752 | 24.5 | |
| Distant | 80 | 21.1 | 381 | 23.2 | 265 | 25.3 | 726 | 23.7 | 0.981a |
| Unknown | 87 | 23.0 | 315 | 19.2 | 171 | 16.3 | 573 | 18.7 | |
| Missing | 19 | 5.0 | 22 | 1.3 | 7 | 0.7 | 48 | 1.6 | <0.001b |
|
| |||||||||
| Lung cancer (n= 3038) | |||||||||
| Local | 84 | 20.5 | 298 | 18.5 | 171 | 16.8 | 553 | 18.2 | |
| Regional | 76 | 18.5 | 277 | 17.2 | 198 | 19.5 | 551 | 18.1 | |
| Distant | 203 | 49.5 | 958 | 59.4 | 607 | 59.7 | 1768 | 58.2 | 0.068a |
| Unknown | 36 | 8.8 | 76 | 4.7 | 40 | 3.9 | 152 | 5.0 | |
| Missing | 11 | 2.7 | 3 | 0.2 | 0 | 0.0 | 14 | 0.5 | <0.001b |
|
| |||||||||
| Malignant melanoma (n= 1137) | |||||||||
| Local | 99 | 61.5 | 408 | 68.7 | 287 | 75.1 | 794 | 69.8 | |
| Regional | 12 | 7.5 | 56 | 9.4 | 30 | 7.9 | 98 | 8.6 | |
| Distant | 13 | 8.1 | 24 | 4.0 | 15 | 3.9 | 52 | 4.6 | 0.085a |
| Unknown | 21 | 13.0 | 90 | 15.2 | 50 | 13.1 | 161 | 14.2 | |
| Missing | 16 | 9.9 | 16 | 2.7 | 0 | 0.0 | 32 | 2.8 | <0.001b |
|
| |||||||||
| Head and neck cancer (n= 951) | |||||||||
| Local | 49 | 37.1 | 217 | 46.8 | 144 | 40.6 | 410 | 43.1 | |
| Regional | 53 | 40.2 | 187 | 40.3 | 120 | 33.8 | 360 | 37.9 | |
| Distant | 5 | 3.8 | 23 | 5.0 | 13 | 3.7 | 41 | 4.3 | 0.820a |
| Unknown | 5 | 3.8 | 28 | 6.0 | 75 | 21.1 | 108 | 11.4 | |
| Missing | 20 | 15.2 | 9 | 1.9 | 3 | 0.8 | 32 | 3.4 | <0.001b |
|
| |||||||||
| Upper gastrointestinal cancer (n= 1767) | |||||||||
| Local | 43 | 17.0 | 124 | 13.7 | 106 | 17.3 | 273 | 15.4 | |
| Regional | 43 | 17.0 | 192 | 21.3 | 105 | 17.2 | 340 | 19.2 | |
| Distant | 82 | 32.4 | 363 | 40.2 | 253 | 41.4 | 698 | 39.5 | 0.060a |
| Unknown | 69 | 27.3 | 209 | 23.1 | 140 | 22.9 | 418 | 23.7 | |
| Missing | 16 | 6.3 | 15 | 1.7 | 7 | 1.1 | 38 | 2.2 | <0.001b |
|
| |||||||||
| Gynaecological cancer (n= 1279) | |||||||||
| Local | 117 | 64.3 | 423 | 60.6 | 250 | 62.7 | 790 | 61.8 | |
| Regional | 17 | 9.3 | 69 | 9.9 | 36 | 9.0 | 122 | 9.5 | |
| Distant | 24 | 13.2 | 104 | 14.9 | 70 | 17.5 | 198 | 15.5 | 0.733a |
| Unknown | 11 | 6.0 | 89 | 12.8 | 40 | 10.0 | 140 | 10.9 | |
| Missing | 13 | 7.1 | 13 | 1.9 | 3 | 0.8 | 29 | 2.3 | <0.001b |
|
| |||||||||
| Urinary system cancer (n= 1107) | |||||||||
| Local | 99 | 65.1 | 387 | 65.6 | 243 | 66.6 | 729 | 65.9 | |
| Regional | 3 | 2.0 | 25 | 4.2 | 20 | 5.5 | 48 | 4.3 | |
| Distant | 27 | 17.8 | 121 | 20.5 | 59 | 16.2 | 207 | 18.7 | 0.274a |
| Unknown | 11 | 7.2 | 41 | 6.9 | 40 | 11.0 | 92 | 8.3 | |
| Missing | 12 | 7.9 | 16 | 2.7 | 3 | 0.8 | 31 | 2.8 | <0.001b |
|
| |||||||||
| Total (n= 12 346) | |||||||||
| Local | 600 | 35.6 | 2377 | 36.6 | 1540 | 36.9 | 4517 | 36.6 | |
| Regional | 288 | 17.3 | 1208 | 18.6 | 775 | 18.6 | 2271 | 18.4 | |
| Distant | 434 | 26.0 | 1974 | 30.4 | 1282 | 30.7 | 3690 | 29.9 | 0.364a |
| Unknown | 240 | 14.4 | 848 | 13.0 | 556 | 13.3 | 1644 | 13.3 | |
| Missing | 107 | 6.4 | 94 | 1.5 | 23 | 0.6 | 224 | 1.8 | <0.001b |
Missing and unknown data excluded.
Missing and unknown data included.
CPP = cancer patient pathway.
Appendix 2. Odds ratios and 95% CIs for patients with incident cancer, regardless of GP questionnaire response, of having local cancer during and after CPP implementation compared with before CPP implementation. Values <1 indicate less likelihood of having local cancer compared with before CPP implementation
| During | After | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Raw | Adjusted | Raw | Adjusted | |||||
|
|
|
|
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Colorectal | 1.00 | 0.77 to 1.30 | 0.98 | 0.73 to 1.31 | 0.96 | 0.73 to 1.27 | 0.92 | 0.68 to 1.24 |
| Lung | 0.80 | 0.61 to 1.05 | 0.80 | 0.60 to 1.08 | 0.71 | 0.53 to 0.95 | 0.69 | 0.51 to 0.94 |
| Malignant melanoma | 1.29 | 0.78 to 2.12 | 1.19 | 0.70 to 2.03 | 1.61 | 0.94 to 2.76 | 1.55 | 0.87 to 2.77 |
| Head and neck | 1.22 | 0.80 to 1.87 | 1.25 | 0.80 to 1.97 | 1.28 | 0.82 to 2.00 | 1.41 | 0.88 to 2.26 |
| Upper gastrointestinal | 0.65 | 0.44 to 0.97 | 0.62 | 0.41 to 0.95 | 0.86 | 0.57 to 1.30 | 0.81 | 0.53 to 1.25 |
| Gynaecological | 0.86 | 0.58 to 1.27 | 0.89 | 0.59 to 1.36 | 0.83 | 0.54 to 1.26 | 0.85 | 0.54 to 1.32 |
| Urinary system | 0.80 | 0.51 to 1.26 | 0.80 | 0.48 to 1.33 | 0.93 | 0.58 to 1.51 | 0.80 | 0.47 to 1.37 |
|
| ||||||||
| Total | 0.90 | 0.80 to 1.01 | 0.90 | 0.77 to 1.04 | 0.90 | 0.79 to 1.02 | 0.90 | 0.77 to 1.05 |
Adjusted for sex, age, cancer site, comorbidity, educational level, and household income.
CPP = cancer patient pathway. OR = odds ratio. Estimates marked by bold indicate statistically significant at P<0.05 level.
Appendix 3. Odds ratios and 95% CIs after multiple imputation for identified patients with incident cancer of having local cancer during and after CPP implementation compared with before CPP implementation. Values <1 indicate less likelihood of having local cancer compared with before CPP implementation
| During | After | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Raw | Adjusted | Raw | Adjusted | |||||
|
|
|
|
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Colorectal | 1.02 | 0.78 to 1.33 | 1.02 | 0.78 to 1.34 | 0.96 | 0.72 to 1.29 | 0.96 | 0.72 to 1.29 |
| Lung | 0.79 | 0.61 to 1.03 | 0.81 | 0.62 to 1.06 | 0.70 | 0.52 to 0.93 | 0.70 | 0.53 to 0.94 |
| Malignant melanoma | 1.25 | 0.77 to 2.04 | 1.25 | 0.76 to 2.04 | 1.57 | 0.92 to 2.68 | 1.55 | 0.90 to 2.67 |
| Head and neck | 1.21 | 0.78 to 1.88 | 1.25 | 0.80 to 1.95 | 1.26 | 0.80 to 2.68 | 1.29 | 0.81 to 2.05 |
| Upper GI | 0.58 | 0.39 to 0.87 | 0.59 | 0.39 to 0.89 | 0.79 | 0.53 to 1.18 | 0.81 | 0.53 to 1.22 |
| Gynaecological | 0.84 | 0.57 to 1.23 | 0.84 | 0.57 to 1.23 | 0.83 | 0.55 to 1.25 | 0.83 | 0.55 to 1.25 |
| Urinary system | 0.80 | 0.52 to 1.24 | 0.82 | 0.53 to 1.29 | 0.90 | 0.56 to 1.44 | 0.88 | 0.54 to 1.42 |
|
| ||||||||
| Total | 0.90 | 0.80 to 1.02 | 0.88 | 0.77 to 1.02 | 0.91 | 0.79 to 1.03 | 0.91 | 0.78 to 1.06 |
Adjusted for sex, age, cancer site, comorbidity, educational level, and household income.
CPP = cancer patient pathway. OR = odds ratio. Estimates marked by bold indicate statistically significant at P<0.05 level.
Funding
This study was funded by the Novo Nordisk Foundation, the Danish Cancer Society, the Health Foundation (Helsefonden), the Danish foundation TrygFonden, and the Foundation for Primary Health Care Research of the Central Denmark Region (Praksisforskningsfonden). The funders did not have any influence on the study.
Ethical approval
The study was approved by the Danish Data Protection Agency (rec. no. 2009–41–3471). The Danish National Board of Health (now: the Danish Patient Safety Authority) gave permission to obtain information from the GPs’ medical records without patient consent. According to the committee on health research ethics in the Central Denmark Region, no ethical approval was needed for the study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Prades J, Espinas JA, Font R, et al. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): a mixed methods study. Br J Cancer. 2011;105(6):753–759. doi: 10.1038/bjc.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians — a national Danish project. Health Policy. 2012;105(1):65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer. 2009;101(Suppl 2):S5–S8. doi: 10.1038/sj.bjc.6605383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toustrup K, Lambertsen K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer — a fast track study. Acta Oncol. 2011;50(5):636–641. doi: 10.3109/0284186X.2010.551139. [DOI] [PubMed] [Google Scholar]
- 5.Lyhne NM, Christensen A, Alanin MC, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49(7):1627–1633. doi: 10.1016/j.ejca.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Vallverdu-Cartie H, Comajuncosas-Camp J, Orbeal-Saenz RA, et al. Results of implementation of a fast track pathway for diagnosis of colorectal cancer. Rev Esp Enferm Dig. 2011;103(8):402–407. doi: 10.4321/s1130-01082011000800003. [DOI] [PubMed] [Google Scholar]
- 7.Valentin-Lopez B, Ferrandiz-Santos J, Blasco-Amaro JA, et al. Assessment of a rapid referral pathway for suspected colorectal cancer in Madrid. Fam Pract. 2012;29(2):182–188. doi: 10.1093/fampra/cmr080. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health . The NHS cancer plan. London: DH; 2000. [Google Scholar]
- 9.Guzmán Laura KP, Bolíbar R, I, Alepuz MT, et al. Impact on patient care time and tumor stage of a program for fast diagnostic and treatment of colorectal cancer. Rev Esp Enferm Dig. 2011;103(1):13–19. doi: 10.4321/s1130-01082011000100003. [DOI] [PubMed] [Google Scholar]
- 10.The National Board of Health . Pakkeforløb for kræft i tyk-og endetarm. Copenhagen: Danish National Board of Health; 2012. [Standardised Cancer Patient Pathway for colorectal cancer]. Report No: 3.0. [Google Scholar]
- 11.Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000–2007. Acta Oncol. 2013;52(5):919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 12.Walters S, Maringe C, Butler J, et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000–2007: a population-based study. Br J Cancer. 2013;108(5):1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68(6):551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 14.Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen MB, Hansen RP, Hansen DG, et al. Secondary care intervals before and after the introduction of urgent referral guidelines for suspected cancer in Denmark: a comparative before-after study. BMC Health Serv Res. 2013;13(1):348. doi: 10.1186/1472-6963-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerdán-Santacruz C, Cano-Valderrama O, Cárdenas-Crespo S, et al. Colorectal cancer and its delayed diagnosis: have we improved in the past 25 years? Rev Esp Enferm Dig. 2011;103(9):458–463. doi: 10.4321/s1130-01082011000900004. [DOI] [PubMed] [Google Scholar]
- 17.Zafar A, Mak T, Whinnie S, Chapman MA. The 2-week wait referral system does not improve 5-year colorectal cancer survival. Colorectal Dis. 2012;14(4):e177–e180. doi: 10.1111/j.1463-1318.2011.02826.x. [DOI] [PubMed] [Google Scholar]
- 18.Neal RD, Allgar VL, Ali N, et al. Stage, survival and delays in lung, colorectal, prostate and ovarian cancer: comparison between diagnostic routes. Br J Gen Pract. 2007;57(536):212–219. [PMC free article] [PubMed] [Google Scholar]
- 19.Chohan DP, Goodwin K, Wilkinson S, et al. How has the ‘two-week wait’ rule affected the presentation of colorectal cancer? Colorectal Dis. 2005;7(5):450–453. doi: 10.1111/j.1463-1318.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 20.Bevis PM, Donaldson OW, Card M, et al. The association between referral source and stage of disease in patients with colorectal cancer. Colorectal Dis. 2008;10(1):58–62. doi: 10.1111/j.1463-1318.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 21.Jensen H, Torring ML, Larsen MB, Vedsted P. Existing data sources for clinical epidemiology: Danish Cancer in Primary Care (CaP) cohort. Clin Epidemiol. 2014;6:237–246. doi: 10.2147/CLEP.S62855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen KM, Andersen JS, Sondergaard J. General practice and primary health care in Denmark. J Am Board Fam Med. 2012;25(Suppl 1):S34–S38. doi: 10.3122/jabfm.2012.02.110216. [DOI] [PubMed] [Google Scholar]
- 23.Mukai TO, Bro F, Pedersen KV, Vedsted P. Brug af undersøgelse for prostataspecifikt antigen. [Use of prostate-specific antigen testing] Ugeskr Laeger. 2010;172(9):696–700. [PubMed] [Google Scholar]
- 24.Outzen M, Brasso K, Martinussen N, et al. Prostate cancer in Denmark 1978–2009 — trends in incidence and mortality. Acta Oncol. 2013;52(4):831–836. doi: 10.3109/0284186X.2012.702922. [DOI] [PubMed] [Google Scholar]
- 25.Hjertholm P, Fenger-Gron M, Vestergaard M, et al. Variation in general practice prostate-specific antigen testing and prostate cancer outcomes: an ecological study. Int J Cancer. 2015;136(2):435–442. doi: 10.1002/ijc.29008. [DOI] [PubMed] [Google Scholar]
- 26.Deleuran T, Sogaard M, Froslev T, et al. Completeness of TNM staging of small-cell and non-small-cell lung cancer in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4(Suppl 2):39–44. doi: 10.2147/CLEP.S33315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostenfeld EB, Froslev T, Friis S, et al. Completeness of colon and rectal cancer staging in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4(Suppl 2):33–38. doi: 10.2147/CLEP.S32362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froslev T, Grann AF, Olsen M, et al. Completeness of TNM cancer staging for melanoma in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4(Suppl 2):5–10. doi: 10.2147/CLEP.S32064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland-Bill L, Froslev T, Friis S, et al. Completeness of bladder cancer staging in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4(Suppl 2):25–31. doi: 10.2147/CLEP.S31542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danish National Board of Health . National Cancer Plan II Danish National Board of Health recommendations for improving cancer healthcare services. Copenhagen: Danish National Board of Health; 2005. [Google Scholar]
- 31.Danish National Board of Health . Det moderniserede Cancerregister — metode og kvalitet. Copenhagen: Danish National Board of Health; 2009. [The modernised Danish Cancer Registry — method and quality] [Google Scholar]
- 32.Nguyen-Nielsen M, Froslev T, Friis S, et al. Completeness of prostate cancer staging in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4(Suppl 2):17–23. doi: 10.2147/CLEP.S32004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danske Multidisciplinære Cancer Grupper (DMCG.dk) Danish Breast Cancer Cooperative Group (DBCG) Dansk Lunge Cancer Gruppe (DLCG) Danish Colorectal Cancer Group (DCCG) Dansk Gynækologisk Cancer Gruppe (DGCG) DMCG.dk Benchmarking Consortium — rapport om canceroverlevelse i Danmark 1995–2012. 2014. [Benchmarking Consortium — report on cancer survival in Denmark 1995–2012] http://dmcg.dk/dmcgdk-benchmarking-consortium/ (accessed 10 Mar 2016)
- 34.Bay C, Kejs AM, Storm HH, Engholm G. Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-based Register Study 1989–2011. Cancer Epidemiol. 2015;39(1):1–7. doi: 10.1016/j.canep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Salas M, Hofman A, Stricker BHC. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149(11):981–983. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Chen H, Xiang J, et al. Relationship between tumor size and disease stage in non-small cell lung cancer. BMC Cancer. 2010;10:474. doi: 10.1186/1471-2407-10-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen H, Torring ML, Olesen F, et al. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14(1):636. doi: 10.1186/1471-2407-14-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forrest LF, Adams J, White M, Rubin G. Factors associated with timeliness of post-primary care referral, diagnosis and treatment for lung cancer: population-based, data-linkage study. Br J Cancer. 2014;111(9):1843–1851. doi: 10.1038/bjc.2014.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X483175. [DOI] [PMC free article] [PubMed] [Google Scholar]