Clinical Question
Can we reduce delays in correctly assigning a diagnosis of monogenic diabetes?
INTRODUCTION
A GP will often be the first health professional involved in making a diagnosis of diabetes. For adults who do not need immediate insulin treatment, a default diagnosis of type 2 diabetes is the most common outcome, but a small proportion of patients will actually have maturity-onset diabetes of the young (MODY; a type of monogenic diabetes).
MODY is inherited in an autosomal dominant pattern and estimated to account for 1–2% of all diabetes.1 MODY is most commonly caused by mutations in genes encoding the transcription factors HNF1A, HNF4A, and HNF1B, and the glycolytic enzyme glucokinase (GCK).1
Making a correct molecular diagnosis is crucial, as this allows optimal treatment and therefore the best long-term outcome. It is estimated that around 80% of MODY patients are misdiagnosed as type 1 (T1DM) or type 2 diabetes (T2DM),1 and a delay of >10 years from presentation of diabetes to molecular diagnosis is frequently reported.2 Two cases where genetic referral for MODY was arranged at presentation of diabetes are described.
CASE PRESENTATIONS
Case 1
An 18-year-old female presented to her GP with 2 kg weight loss, mild polydipsia, and polyuria but otherwise well. Her fasting blood glucose of 12 mmol/L was diagnostic of diabetes (normal range 3.9–5.5 mmol/L). Her mother had been diagnosed with T1DM at age 15 years and treated with insulin. Her paternal grandfather had diabetes diagnosed in his forties and was treated successfully with an oral agent for four decades.
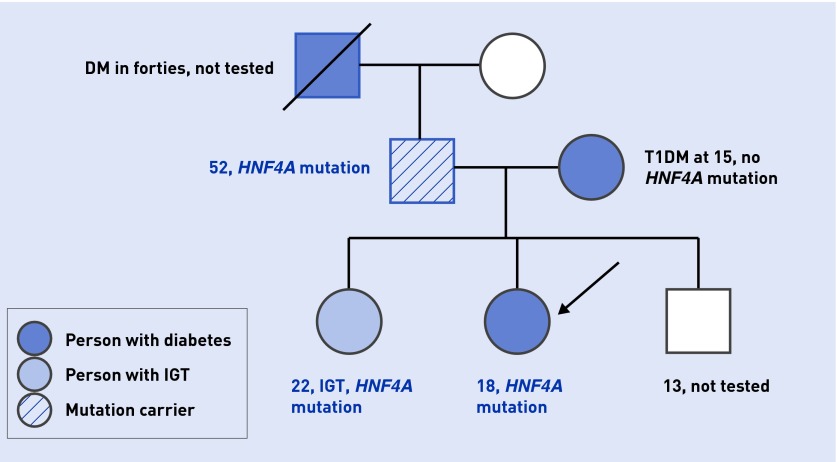
Referral to the monogenic diabetes clinic was made for investigation. Her BMI was 17 kg/m2 with no signs of insulin resistance. The commonest form of pancreatic beta-cell antibodies, glutamic acid decarboxylase (GAD) antibodies, were negative. C-peptide (a marker of endogenous insulin production, co-secreted with insulin) was 0.35 nmol/L (reference range 0.27–1.28 nmol/L), lipids were in normal range, and HbA1c was 75 mmol/mol (9%, normal range 20–42 mmol/mol or 4–6%). Birth weight was 4.2 kg, with no history of neonatal hypoglycaemia. A genetic test was requested. Twenty milligrams of gliclazide daily was commenced with good response and regular contact maintained. A mutation in HNF4A was identified, confirming a diagnosis of HNF4A-MODY within 6 weeks of diabetes onset. Her mother had absent C-peptide and was negative for the mutation (truly T1DM), whereas her sister with impaired glucose tolerance and their normoglycaemic father had the same mutation (Figure 1).
Figure 1.
The family tree of the patient with HNF4A-MODY. The proband is marked with an arrow. Family members with diabetes are in blue: the darkest shade used for T1DM; pale blue for IGT ; and stripes for HNF4A mutation carrier. DM = diabetes mellitus. IGT = impaired glucose tolerance. T1DM = type 1 diabetes mellitus.
Case 2
A 24-year-old male presented to his GP with lethargy and no other symptoms. His fasting glucose was 6.7 mmol/L, his HbA1c 49 mmol/mol (6.6%), and he had negative GAD antibodies. His BMI was 23 kg/m2. His father had recently been diagnosed with diabetes at age 47; the diabetes was controlled with diet. The patient was referred to the monogenic diabetes clinic for consideration of MODY. A mutation in GCK was suspected and confirmed on genetic testing. The patient was reassured that he did not require any treatment and discharged. His father is waiting for genetic testing for the same mutation.
DIAGNOSIS
A typical patient with MODY presents with diabetes in the second to fourth decade of life and does not fit the clinical features of either T1DM or T2DM (Table 1). Patients with MODY have a subacute or incidental presentation without ketosis, unlike in T1DM. They are usually non-obese without features of insulin resistance such as dyslipidaemia, hypertension, or fatty liver, so do not resemble type 2 diabetes. Frequently, diabetes is present in at least two consecutive generations, although this is not a good discriminator from young T2DM.
Table 1.
Comparison of main types of monogenic diabetes with type 1 and type 2 diabetes
| Feature | HNF1A-/4A-MODY | HNF1B-MODY | GCK-MODY | T1DM | T2DM |
|---|---|---|---|---|---|
| Typical age at diagnosis | 10–45 years | 10–45 years | Birth onwards | 1–30 years | After 25 years |
| Ketonaemia/diabetic ketoacidosis | Very rare | Very rare | Would not be predicted to occur | Common | Rare except in ketosis-prone subtype |
| First-degree relative with DM | Reported in 50–60% | Around 50% (high rate of spontaneous mutations) | Often undiagnosed or reported as IGT/GDM | 2–4% | Around 50% |
| Insulin resistance/obesity | Same as non-diabetic population | Same as non-diabetic population | Same as non-diabetic population | Same as non-diabetic population | Common |
| Beta-cell antibodies | Negative | Negative | Negative | Positive at diagnosis in 80–90% | Negative |
| C-peptide after 3 years from diagnosis | Normal range | Normal range | Normal range | Low or undetectable in majority | High normal |
| First-line drug treatment | Low-dose sulphonylurea | Metformin if renal function allows | None | Insulin | Metformin |
DM = diabetes mellitus. GCK = glucokinase. GDM = gestational diabetes mellitus. IGT = impaired glucose tolerance. MODY = maturity-onset diabetes of the young. T1DM = type 1 mellitus. T2DM = type 2 diabetes mellitus.
HNF1A-MODY and HNF4A-MODY have very similar clinical features and result in a progressive beta-cell dysfunction. Affected individuals are normoglycaemic in childhood, but develop diabetes as young adults. Patients with HNF1A-MODY have decreased renal glucose threshold, resulting in glycosuria often before diagnosis of diabetes, whereas HNF4A-MODY have hyperinsulinaemia in utero with macrosomia at birth (>4 kg) and transient neonatal hypoglycaemia.3 Hypoglycaemia on normal starting doses of sulphonylureas (SUs) suggests HNF1A/4A-MODY. GCK mutations cause a resetting of the glucose threshold for insulin secretion, leading to mild hyperglycaemia without a significant postprandial glucose increment. The hyperglycaemia in GCK-MODY is present throughout life, but is usually asymptomatic and detected when blood glucose is measured incidentally (for example, during pregnancy).
In some cases other clinical features in combination with diabetes should prompt consideration of a unifying diagnosis, for example, HNF1B mutations are associated with renal cysts and genitourinary abnormalities4 and mitochondrial diabetes with deafness.5
Investigation of young adults
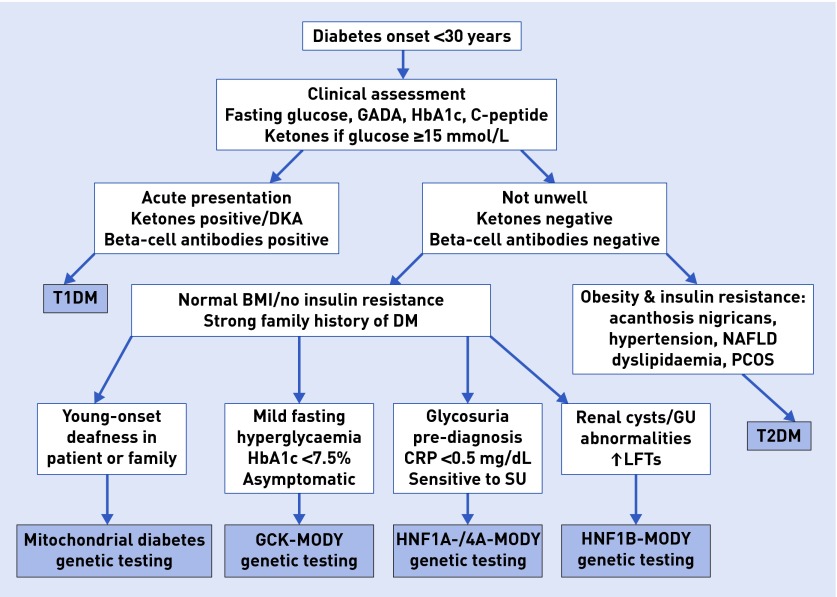
Initial blood tests should include glucose, HbA1c, and beta-cell antibodies, for example, GAD antibodies, if available (Figure 2). If glucose is >15 mmol/L, capillary ketones ought to be checked but are usually negative in MODY. C-peptide is a helpful test in those on insulin, as it indicates endogenous insulin secretion that becomes negative in T1DM after the honeymoon period (the first 1–3 years post-diagnosis). In MODY C-peptide remains in the normal range and beta-cell antibodies are negative. The lipid profile is normal. Patients with HNF1B-MODY may have elevated liver enzymes or abnormal renal function. Very low C-reactive protein (CRP) of <0.5 mg/dL is characteristic for HNF1A-MODY.6 Mild fasting hyperglycaemia and HbA1c <7.5% point towards GCK-MODY.
Figure 2.
Clinical algorithm for assessment of young adult diabetes. BMI = body mass index. CRP = C-reactive protein. DKA = diabetic ketoacidosis. DM = diabetes mellitus. GADA = glutamic acid dehydrogenase antibodies. GCK = glucokinase. GU = genitourinary. LFTs = liver function tests. NAFLD = non-alcoholic fatty liver disease. PCOS = polycystic ovary syndrome. SU = sulphonylurea. T1DM = type 1 diabetes mellitus. T2DM = type 2 diabetes mellitus.
MANAGEMENT
At presentation of diabetes, if the differential diagnosis includes T1DM, close monitoring of blood glucose with ketones and regular contact is required. Insulin may be commenced for safety while investigation results are awaited. A referral to a monogenic diabetes clinic should be made early (for example, once antibodies are known to be negative) as only a genetic test gives a definitive diagnosis and allows firm treatment decisions to be made.
A small dose of a SU is the recommended first-line treatment in HNF1A-/HNF4A-MODY. The typical starting dose close to onset of diabetes is 20 mg of gliclazide or 1.25 mg of glibenclamide once a day. Most patients respond well to this treatment, with HbA1c remaining at target for many years. There is no evidence base for second-line treatment, but metformin is a sensible choice, followed by other oral agents for T2DM. The only exception for HNF1A-MODY would be to avoid sodium/glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin, as HNF1A deficiency already decreases SGLT2 expression. Patients with HNF1B-MODY are not sensitive to SUs and usually require insulin earlier than other subtypes of MODY. No specific treatment is recommended for GCK-MODY, and treatment that has been previously commenced can be stopped. Observational studies have shown that those with GCK-MODY do not develop diabetic complications7 and HbA1c does not change with pharmacological treatment.
In any type of MODY, diabetic relatives should be offered genetic testing for the same mutation as they could also benefit from treatment changes. Unaffected first-degree family members should be offered diabetes screening. In the UK, the network of genetic diabetes nurses/monogenic diabetes clinics can offer assistance with screening of family members (www.diabetesgenes.org).
CONCLUSION
It is important to consider monogenic diabetes in young patients with non-acute presentation of diabetes, absence of beta-cell autoimmunity, and no signs of insulin resistance. Careful monitoring and rapid referral for genetic testing can establish optimal treatment and avoid insulin use.
Acknowledgments
The authors would like to thank both patients for their consent.
Patient consent
The patients gave their consent for publication of this article.
Funding
A Juszczak is a Diabetes UK George Alberti Clinical Fellow. KR Owen receives funding from the Oxford NIHR Biomedical Research Centre.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Shields BM, Hicks S, Shepherd MH, et al. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504–2508. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- 2.Thanabalasingham G, Pal A, Selwood MP, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35(6):1206–1212. doi: 10.2337/dc11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson ER, Pruhova S, Tack CJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48(5):878–885. doi: 10.1007/s00125-005-1738-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen YZ, Gao Q, Zhao XZ, et al. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin Med J (Engl) 2010;123(22):3326–3333. [PubMed] [Google Scholar]
- 5.Murphy R, Turnbull DM, Walker M, Hattersley AT. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med. 2008;25(4):383–399. doi: 10.1111/j.1464-5491.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 6.Thanabalasingham G, Shah N, Vaxillaire M, et al. A large multi-centre European study validates high-sensitivity C-reactive protein (hsCRP) as a clinical biomarker for the diagnosis of diabetes subtypes. Diabetologia. 2011;54(11):2801–2810. doi: 10.1007/s00125-011-2261-y. [DOI] [PubMed] [Google Scholar]
- 7.Steele AM, Shields BM, Wensley KJ, et al. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014;311(3):279–286. doi: 10.1001/jama.2013.283980. [DOI] [PubMed] [Google Scholar]