Abstract
Endothelin-1 plays a major role in the pathophysiology of hypertension and its associated cardiovascular risk. We tested the hypothesis that chronic nebivolol treatment reduces ET-1-mediated vasoconstrictor tone in adult humans with elevated blood pressure. Furthermore, reducing endothelin-1 vasoconstrictor activity contributes to the improvement in endothelial vasodilator function associated with nebivolol treatment. Forty-two middle-aged adults with elevated blood pressure (systolic ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg) completed a 3-month, double-blind, randomized, placebo controlled trial: 14 received nebivolol (8M/6F; 5 mg/day); 14 received metoprolol succinate (9M/5F; 100 mg/day); and 14 received placebo (9M/5F). Forearm blood flow (plethysmography) responses to selective (BQ-123: 100 nmol/minute; 60 minutes) and nonselective (BQ-123 + BQ-788 [50 nmol/minute]; 60 minutes) ET-1 receptor blockade as well as acetylcholine (4.0, 8.0, 16.0 μg/100 mL tissue/minute) in the absence and presence of non-selective ET-1 receptor blockade were determined before and after each treatment intervention. Forearm blood flow responses to BQ-123 and BQ-123 + BQ-788 were similarly and significantly elevated (~30 and 60%, respectively) from baseline in all three groups. Nebivolol, but not metoprolol or placebo, therapy resulted in a marked (~25 and 45%; P<0.05) reduction in FBF response to BQ-123 and BQ-123 + BQ-788. Moreover, after nebivolol therapy only, vasodilator response to acetylcholine was not significantly increased by ET-1 receptor blockade. These results demonstrate that nebivolol, but not metoprolol, treatment reduces ET-1-mediated vasoconstrictor tone in adult humans with elevated blood pressure. Additionally, nebivolol-induced reduction in endothelin-1-mediated vasoconstrictor tone underlies the favorable effects of this beta-blocker on endothelial vasodilation.
Keywords: endothelin-1, vasoconstriction, blood flow, nebivolol, metoprolol
INTRODUCTION
Endothelin (ET)-1 is a potent vasoconstrictor peptide produced and released by the vascular endothelium 1. In humans, the vascular actions of ET-1 are mediated by two distinct ET receptor subtypes: ETA receptors located exclusively on vascular smooth muscle and ETB receptors located on both the vascular smooth muscle and endothelial surfaces 2–4. In combination with the endothelial vasodilator nitric oxide, ET-1 plays a central role in the regulation of vascular tone 5, 6. In addition to its vasoregulatory actions, there is considerable evidence supporting the involvement of ET-1 in the pathogenesis of atherosclerotic vascular disease 3, 7, 8 and its associated risk factors, most notably elevated blood pressure (BP) 9–11. In a seminal series of studies, Cardillo et al. 9, 12 reported that adults with essential hypertension demonstrate higher forearm vasodilator responses to selective ETA receptor blockade compared with normotensive controls. Moreover, when ETA receptor blockade was combined with ETB receptor blockade there was a further increase in the vasodilator response in the hypertensive adults whereas forearm blood flow remained unchanged in the normotensive controls. Collectively, these results indicated that vasoconstrictor tone to ET-1 is markedly elevated with hypertension and is mediated by both the ETA and ETB receptors. Further, in a follow-up study, blockade of ET-1 receptors improved acetylcholine-induced endothelium-dependent vasodilation in hypertensive patients, indicating that increased ET-1 vasoconstriction contributes to the vasodilator dysfunction associated with hypertension 9. Weil et al. 11 recently reported identical findings in adults with BP in the prehypertensive range (systolic BP: 120–139 mm Hg and/or diastolic BP: 80–89 mm Hg). Thus, clear links have been established between ET-1 system activity and elevations in BP.
Nebivolol, a third generation beta-blocker with high selectivity for β1-adrenergic receptors, has proven to be very effective in treating elevated BP 13–16. A distinguishing feature of nebivolol from other beta-blockers is its hemodynamic profile, specifically the unique ability to enhance both basal and stimulated nitric oxide release resulting in peripheral vasodilation, improved endothelial function and increased myocardial compliance 17–20. Cockcroft et al. 21 demonstrated that the vasodilatory effects of nebivolol were attenuated by the infusion of the nitric oxide synthase inhibitor NG-monomethyl L-arginine; indicating that nebivolol induced improvement in vasodilator function is mediated, in part, by increased nitric oxide bioavailability. However, the favorable vascular effects of nebivolol that contribute to its BP lowering action may not be limited to nitric oxide. Indeed, there are in vitro data to suggest that nebivolol suppresses endothelial ET-1 production 22, but there is currently no in vivo clinical evidence that treatment with nebivolol reduces ET-1-mediated vasoconstrictor tone.
Accordingly, we tested the hypothesis that chronic nebivolol treatment will reduce ET-1-mediated vasoconstrictor tone in adult humans with elevated BP. Moreover, that reducing ET-1 vasoconstrictor activity contributes to the improvement in endothelial vasodilator function associated with nebivolol treatment. To address this hypothesis, we employed a 3-month randomized, double-blind, placebo controlled study to determine the effects of nebivolol, compared with metoprolol and placebo, on ET-1 vasoconstrictor tone in adults with suboptimal BP.
METHODS
Subjects
Forty-two middle-aged adults with elevated BP (systolic BP ≥ 130 and/or diastolic BP ≥ 85 mmHg) participated in a 3-month, double-blind, randomized, placebo controlled trial: 14 received nebivolol (8M/6F; 5 mg/day: Forest Laboratories, Inc.); 14 received metoprolol succinate (9M/5F; 100 mg/day; AstraZeneca LP); and 14 received placebo (9M/5F; 1 gelatin capsule/day; Forest Laboratories, Inc.). The doses of nebivolol and metoprolol were chosen to elicit similar reductions in BP. Resting BP was determined by the average of two or more seated BP readings from two separate visits per American Heart Association guidelines 23. All subjects were free of overt coronary and metabolic disease as assessed by medical history, physical examination, fasting blood chemistries, and electrocardiograms and BP at rest and during incremental exercise performed to exhaustion. In addition, all subjects presented with a resting heart rate > 50 beats per minute. None of the subjects smoked, were taking medications (including vitamins), or performed regular physical exercise for at least 1 year before the start of the study. All of the women were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. After baseline testing, subjects were randomly assigned to 1 of the 3 experimental groups. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. The study was approved by the Institutional Review Board of University of Colorado, Boulder.
Measurements
Blood Pressure
Resting BP measurements were performed in the sitting position on at least two separate days at least one week apart. Subjects were instructed not to ingest caffeine-containing beverages prior to all BP measurements. The recordings were made under quiet, comfortable ambient (~24°C) laboratory conditions. To avoid the possibility of investigator bias, measurements were made with a semi-automated device (Dinamap, Crtikon, FL) that uses an oscillometric technique over the brachial artery. Recordings were made in triplicate in the upright sitting position. All measurements conformed to American Heart Association guidelines as established by the Council for High Blood Pressure Research 24.
Body Composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto, Webb City, MO). Percent body fat was determined by dual energy x-ray absorptiometry (Lunar Radiation Corporation, Madison, WI). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to previously published guidelines 1, 25.
Metabolic Measurements
Fasting plasma lipid, lipoprotein, glucose and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the Clinical Translational Research Center at the University of Colorado at Boulder.
Intra-arterial Infusion Studies
All studies were performed between 7:00 am and 10:00 am after a 10-hour overnight fast in a temperature-controlled room. Under strict aseptic conditions a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm under local anesthesia (1% lidocaine). Heart rate and arterial BP were continuously measured throughout the infusion protocol. Forearm blood flow (FBF) at rest and in response to each pharmacological agent was measured in both the experimental (nondominant) and contralateral (dominant) forearm using strain-gauge venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA), as previously described by our laboratory 26. Baseline FBF was measured for 5 minutes and for 5 minutes before each drug infusion thereafter. Following the measurement of resting blood flow, FBF was assessed in response to infusions of acetylcholine (ACh; IOLAB pharmaceuticals, Duluth, GA) at 4.0, 8.0, and 16.0 μg/100 mL tissue/min and sodium nitroprusside (SNP; Nitroprusside, Abbott Laboratories) at 1.0, 2.0, and 4.0 μg/100 mL tissue/min. Each dose of ACh and SNP was infused for ~5 minutes and sufficient time (~20 minutes) was allowed for FBF to return to resting levels between each vasoactive agent. To avoid an order effect, the sequence of ACh and SNP administration was randomized. After the initial infusion of ACh and SNP and allowing FBF to return to baseline (~20 min), BQ-123 (Clinalfa, AG), a selective ETA receptor antagonist, was infused at a rate of 100 nmol/min for 60 minutes. FBF was measured every 10 minutes throughout the infusion period. The selected dose of BQ-123 has been shown to completely inhibit the vasoconstrictor effect of ET-1 in the human forearm of healthy adults 12, 26. After 60 minutes of BQ-123 infusion, the FBF response to nonselective ET-1 receptor blockade was assessed by the coadministration of BQ-123 and BQ-788 (Clinalfa, AG) for an additional 60 minutes. BQ-788, a specific antagonist of ETB receptors, was infused at a rate 50 nmol/min, a dose demonstrated to effectively inhibit ETB receptors 9. Thereafter, the infusion of BQ-123 and BQ-788 was continued at the same dose and FBF was reassessed during co-administration of ACh as performed earlier.
Statistical Analysis
Differences in subject baseline characteristics were determined by between-groups analysis of variance (ANOVA). Differences in FBF responses to ACh, SNP, BQ-123, BQ-123 + BQ-788 and BQ-123/BQ-788+ACh involving both main effects and interactions (group x intervention) were determined by repeated-measures ANOVA. Post hoc comparisons were performed using the Tukey procedure. There were no significant gender interactions, therefore the data were pooled and presented together. All data are expressed as mean ± SEM. Statistical significance was set a priori at P<0.05.
RESULTS
Selected subject characteristics are presented in Table 1. There were no differences in age, anthropometric, metabolic, or hemodynamic variables between the groups. Table 2 shows the BP responses amongst the groups. There were no differences in resting BP between the nebivolol, metoprolol, and placebo groups. Both nebivolol and metoprolol treatment resulted in similar and significant reductions in systolic (~10%), diastolic (~15%), and mean arterial (~15%) BP. There were no significant changes in BP in placebo group.
Table 1.
Selected Subject Characteristics.
| Nebivolol
|
Metoprolol
|
Placebo
|
||||
|---|---|---|---|---|---|---|
| Variable | Before | After | Before | After | Before | After |
| Sex, M/F | 8/6 | 8/6 | 9/5 | 9/5 | 9/5 | 9/5 |
| Age, yr | 57±1 | 57±1 | 55±1 | 55±1 | 56±1 | 56±1 |
| Body Mass, kg | 77.7±2.7 | 78.4±2.5 | 88.7±4.8 | 90.7±4.7 | 88.7±5.1 | 89.1±5.2 |
| BMI, kg/m2 | 26.6±0.7 | 26.9±0.7 | 29.3±1.4 | 30.1±1.5 | 28.6±1.2 | 28.8±1.2 |
| Body fat, % | 33.6±1.9 | 34.6±1.9 | 34.6±2.1 | 35.7±1.9 | 35.5±2.6 | 35.9±2.6 |
| Waist Circumference, cm | 88.3±2.4 | 89.0±2.4 | 95.9±4.0 | 97.6±3.8 | 94.2±2.9 | 94.5±2.7 |
| VO2 max, mL/kg/min | 28.6±2.2 | 28.0±1.9 | 27.5±1.9 | 27.3±1.8 | 26.5±1.9 | 25.5±1.5 |
| Total cholesterol, mmol/L | 5.2±0.2 | 4.7±0.2 | 5.1±0.2 | 4.5±0.2 | 5.3±0.1 | 4.9±0.2 |
| LDL-cholesterol, mmol/L | 3.1±0.1 | 2.6±0.1* | 3.0±0.2 | 2.8±0.2 | 3.6±0.1 | 3.2±0.3 |
| HDL-cholesterol, mmol/L | 1.4±0.1 | 1.1±0.1 | 1.3±0.1 | 1.1±0.1 | 1.2±0.1 | 1.1±0.1 |
| Triglycerides, mmol/L | 1.5±0.3 | 1.2±0.4 | 1.7±0.2 | 1.4±0.2 | 1.3±0.1 | 1.1±0.1 |
| Glucose, mmol/L | 4.9±0.2 | 4.9±0.2 | 5.1±0.1 | 5.0±0.1 | 5.2±0.1 | 5.0±0.1 |
| Insulin, pmol/L | 62.3±5.6 | 57.2±6.6 | 61.0±7.7 | 67.9±10.1 | 74.6±7.8 | 69.0±7.4 |
BMI, body mass index; VO2 max: maximal oxygen consumption; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Values are mean±SEM.
P<0.05 vs. before intervention.
Table 2.
Subject Heart Rate and Blood Pressure.
| Nebivolol
|
Metoprolol
|
Placebo
|
||||
|---|---|---|---|---|---|---|
| Variable | Before | After | Before | After | Before | After |
| Heart Rate, bpm | 66±1 | 59±2* | 70±2 | 64±2* | 73±2 | 71±2 |
| Systolic BP, mmHg | 144±2 | 126±2* | 140±2 | 125±3* | 139±1 | 134±2 |
| Diastolic BP, mmHg | 89±1 | 77±1* | 90±2 | 77±1* | 86±2 | 83±2 |
| MAP, mmHg | 108±1 | 94±2* | 105±2 | 93±2* | 104±2 | 100±2 |
Bpm, beats per minute; BP, blood pressure; MAP, mean arterial pressure. Values are mean±SEM.
P<0.05 vs. before intervention.
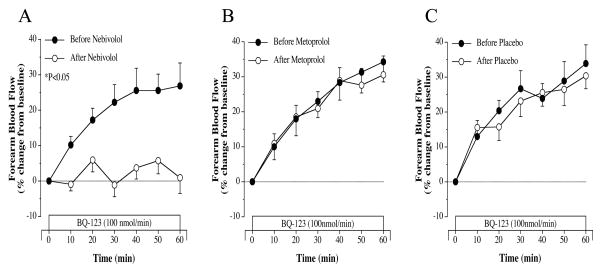
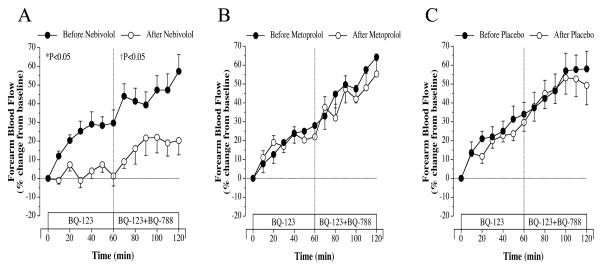
Before randomization to nebivolol/metoprolol/placebo, FBF responses to selective ETA receptor blockade with BQ-123 were similarly and significantly elevated (~30%) from baseline in all three groups. Nebivolol, but not metoprolol or placebo, therapy resulted in a marked (~25%; P<0.05) reduction in FBF response to BQ-123 (Figure 1). The vasodilator response to BQ-123 were almost identical before and after either metoprolol or placebo treatment. The FBF responses to nonselective ETA/B receptor blockade with BQ-123 and BQ-788 were similar amongst the groups prior to treatment. There was a significant increase (~35%) in FBF beyond that of ETA receptor blockade in each group (Figure 2). However, after 3-month of treatment, only nebivolol therapy significantly reduced (~40%) the FBF response to non-selective ETA/B receptor blockade (Figure 2). Neither metoprolol therapy nor placebo significantly altered the FBF responses to BQ-123/BQ-788 infusion (Figure 2). Across the groups the FBF response to BQ-123 and Bq-123/BQ-788 after each intervention was significantly greater in the nebivolol treatment group.
Figure 1.
FBF responses to BQ-123 before and after 3 months of nebivolol (panel A), metoprolol (panel B) and placebo (panel C) intervention. Values are mean±SEM. *P<0.05 refers to the difference in the FBF responses to selective ETA blockade before vs after intervention.
Figure 2.
FBF responses to BQ-123 (100 nmol/min) alone and BQ-123 combined with BQ-788 (50 nmol/min) before and after 3 months of nebivolol (panel A), metoprolol (panel B) and placebo (panel C) intervention. Values are mean±SEM. *P<0.05 refers to the difference in the FBF responses to selective ETA blockade before vs after intervention. †P<0.05 refers to the difference in the FBF response to non-selective ETA/B blockade before vs after intervention.
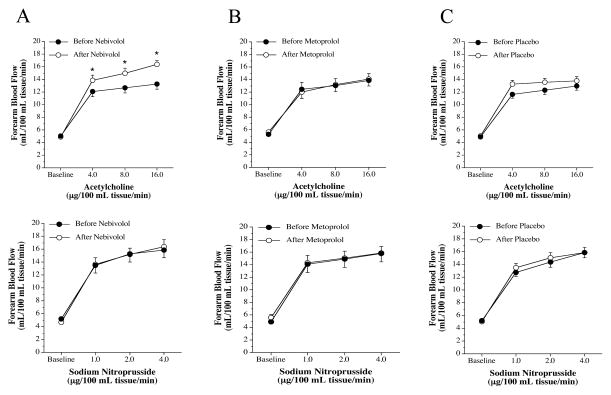
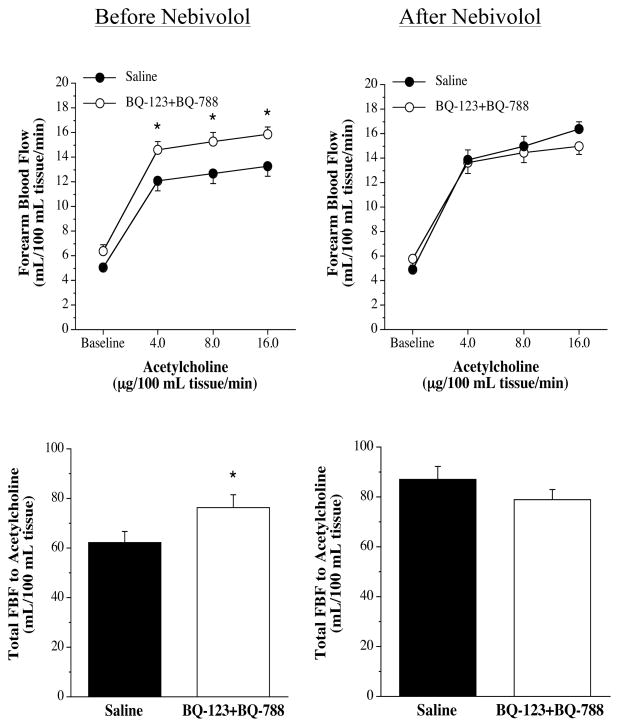
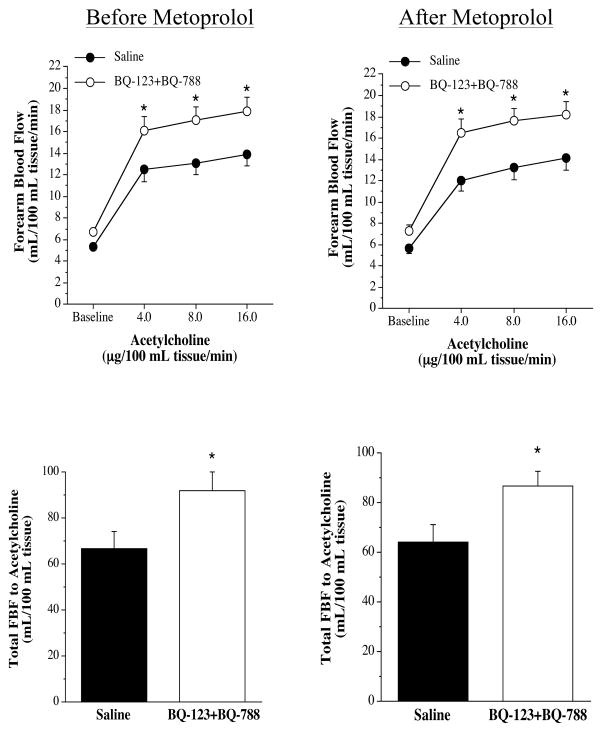
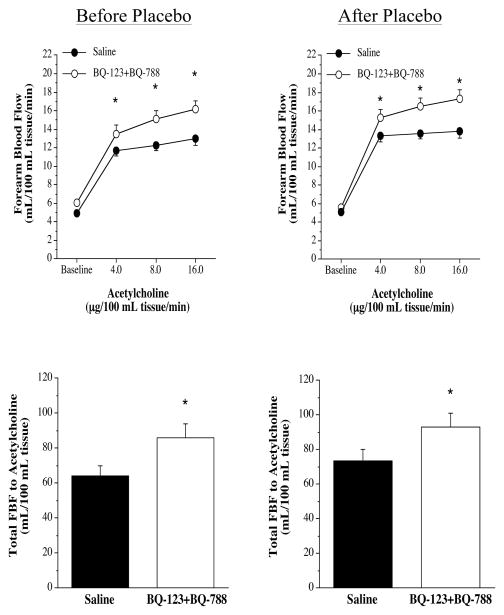
FBF responses to the endothelium-dependent vasodilator ACh were not significantly different between the three groups before intervention (nebivolol: from 5.1±0.3 to 13.3±0.8 mL/100 mL tissue; metoprolol: from 5.3±0.4 to 13.9±1.1 mL/100 mL tissue; and placebo: from 4.9±0.2 to 13.0±0.2 mL/100 mL tissue). Nebivolol treatment resulted in a significant increase (~20%) in the vasodilator response to ACh (from 4.9±0.4 to 16.4±0.6 mL/100 mL tissue) (Figure 3). In stark contrast, there was no change in the FBF responses to ACh in either the metoprolol (from 5.7±0.5 to 14.1±1.1 mL/100 mL tissue) or placebo (from 5.1±0.3 to 13.8±0.7 mL/100 mL tissue) groups after each respective intervention. Across the groups the FBF response to ACh after each intervention was significantly greater in the nebivolol treatment group. The FBF responses to SNP were not affected by each intervention (Figure 3). The co-infusion of ACh with non-selective ETA/B receptor blockade (BQ-123 + BQ-788) resulted in significantly greater (~30%) vasodilator responses in all three groups prior to each intervention (Figure 4). However, after nebivolol therapy (Figure 4), but not metoprolol (Figure 5) or placebo (Figure 6), FBF response to ACh was not significantly increased by the co-infusion of BQ-123 + BQ-788 (Figure 4). In both the metoprolol and placebo groups non-selected ETA/B receptor blockade augmented ACh-mediated vasodilation to similar extent as compared with before each intervention.
Figure 3.
FBF responses to acetylcholine (upper graphs) and sodium nitroprusside (lower graphs) before and after 3 months of nebivolol (panel A), metoprolol (panel B) and placebo (panel C) intervention. Values are mean±SEM. *P<0.05 vs before intervention.
Figure 4.
FBF responses (upper panel) and total FBF (lower panel) to acetycholine in the absence or presence of non-selective ETA/B blockade (BQ-123 +BQ-788) before and after nebivolol intervention. Values are mean±SEM. *P<0.05 vs saline.
Figure 5.
FBF responses (upper panel) and total FBF (lower panel) to acetycholine in the absence or presence of non-selective ETA/B blockade (BQ-123 +BQ-788) before and after metoprolol intervention. Values are mean±SEM. *P<0.05 vs saline.
Figure 6.
FBF responses (upper panel) and total FBF (lower panel) to acetycholine in the absence or presence of non-selective ETA/B blockade (BQ-123 +BQ-788) before and after placebo intervention. Values are mean+SEM. *P<0.05 vs saline.
DISCUSSION
The BP lowering effects of nebivolol are well-established 13, 27, 28. The seminal and novel finding of the present study, however, is that in addition to, and independent of, lowering BP, nebivolol markedly and favorably affects ET-1 system activity. Indeed, the results reported herein demonstrate that chronic nebivolol, but not metoprolol, therapy: 1) reduces ET-1-mediated vasoconstrictor tone in adults with elevated BP; and 2) reductions in ET-1 vasoconstriction underlie nebivolol-induced improvements in endothelium-dependent vasodilation. Diminished ET-1-mediated vasoconstrictor tone may represent an important endovascular pleiotropic effect of nebivolol, contributing to its favorable effect on overall cardiovascular risk 29.
In the present study, there was a similar and significant (~30%) increase in FBF responses to selective ETA receptor blockade in all three groups prior to treatment. In addition, non-selective ETA/B receptor blockade resulted in a further increase (~35%) in FBF in all the groups. These findings are fully consistent with previous studies establishing enhanced ET-1-mediated vasoconstrictor tone in adults with BP in both the hypertensive 12 and prehypertensive 11 range. For example, Cardillo et al. 1, 12 demonstrated almost identical increases (35–55%) in FBF to selective and non-selective ET receptor blockade, to that reported herein, in adults with essential hypertension compared with marginal, non-significant changes in FBF in normotensive adults. Thus, we are confident that ET-1-mediated vasoconstrictor tone was abnormally high in our subjects with elevated BP without a direct comparison to a normotensive control group.
In vitro, nebivolol has been shown to blunt endothelial production, and in turn release, of ET-1 30. The results of the present study compliment and significantly extend these findings by demonstrating that nebivolol reduces ET-1 mediated vasconstrictor tone in vivo in adults with elevated BP. After three months of nebivolol therapy, there was a marked reduction (~25%) in the vasodilator response to both selective ETA and non-selective ETA/B receptor blockade. Of note, the nebivolol-induced reduction in ET-1 vasoconstrictor tone was independent of concomitant reductions in BP. Indeed, BP was equally and significantly reduced in adults randomized to either the nebivolol or metoprolol treatment groups. However, metoprolol therapy had no effect on the vascular responses to either selective or non-selective ET-1 receptor blockade despite, its BP lowering effect. Moreover, there were no significant changes in body composition or cardiometabolic risk factors in response to nebivolol (or metoprolol) treatment. Collectively, this provides further evidence for a direct clinical effect of nebivolol on the ET-1 system. Several mechanisms may underlie this unique feature of nebivolol. Most notably, nebivolol has been shown to ameliorate prepro-ET-1 mRNA production in human coronary endothelial cells 30, 31. Prepro-ET-1 is the peptide transcribed from prepro-ET-1 mRNA that is posttranslationally modified to ET-1 1. Reduction in prepro-ET-1 would ultimately lead to less ET-1 formation. Other contributing factors may include greater nitric oxide bioavailability 32 and reduced oxidative mediators 33, 34. Nebivolol increases nitric oxide bioavailability by enhancing endothelial nitric oxide synthase activity through calcium 35, 36 and non-calcium dependent pathways 37. Nitric oxide, in turn, has a potent inhibitory influence on ET-1 at the level of transcription as well as endothelin converting enzyme activity 38, 39. Regarding oxidative stress, nebivolol has been shown to block NADPH oxidase, a known activator of the ET-1 system 40.
Concurrent with the nebivolol-induced reduction in ET-1-mediated vasoconstrictor tone, we also demonstrate that the nebivolol-induced improvement in endothelium-dependent vasodilation is due, at least in part, to the reduction in ET-1 vasoconstriction. It is important to note that prior to intervention, the FBF responses to acetylcholine in all three groups were similar to that previously reported in prehypertensive 11 and hypertensive 12 adults, supporting diminished endothelium-dependent vasodilation in our study population. Consistent with previous studies 41, we demonstrate that chronic nebivolol therapy significantly improves (~30%) ACh-mediated endothelium dependent vasodilation in adults with elevated BP. In stark contrast, there was no effect of metoprolol therapy (or placebo), on endothelial vasodilator function. It has been suggested that the nebivolol-induced improvement in endothelium-dependent vasodilation is largely due to an increase in nitric oxide bioavailability 32. A seminal finding of the present study is that reduced ET-1 vasoconstrictor tone appears to be a primary contributor to improved endothelial vasodilator function. Indeed, prior to intervention, the co-infusion of non-selective ETA/B receptor blockade resulted in a significant increase (~35%) in ACh-stimulated endothelial vasodilation in all 3 treatment groups. After the 3-month intervention period this effect was unchanged in the metoprolol and placebo groups; however, in the nebivolol group non-selective ETA/B receptor antagonism no longer enhanced the FBF responses to ACh. Although we did not assess whether the nebivolol-induced improvement in ACh-mediated vasodilation was nitric oxide dependent, it is plausible that the previously reported increase in nitric oxide bioavailability with nebivolol is due, in part, to an uncoupling of ET-1 mediated nitric oxide inhibition. Moreover, relieving ET-1-mediated vasoconstriction would allow nitric oxide, and other endothelium-derived relaxing factors, to act without opposition and dilate the vessel appropriately in response to stimulation. Thus, the unique vasomotor properties of nebivolol appear to involve both vasodilator and vascoconstrictor factors. To the best of our knowledge, this is the first study to assess the involvement of the ET-1 system in nebivolol-induced improvements in endothelial vasodilator function.
There are a few experimental considerations regarding the present study that deserve mention. First, given the extended half-life of ET receptor antagonists our study design did not involve the singular administration of the selective ETB receptor antagonist BQ-788 and therefore we cannot comment on the effects of nebivolol (or metoprolol) on the independent vascular actions of the ETB receptor. Secondly, we did not measure circulating plasma levels of ET-1 in the present study. ET-1 produced by the endothelium is predominantly (>80%) released abluminally toward the vascular smooth muscle 42; thus, the pathophysiological significance of circulating ET-1 levels can be variable 43. Circulating plasma concentrations of the peptide may not necessarily reflect local vascular production but rather spillover into, and clearance from, the bloodstream 12. However, elevations in plasma ET-1 concentrations have been linked with ET receptor activity 44. Thirdly, consistent with previous studies we infused BQ-123 for 60 minutes prior to the co-infusion with BQ-788 12, the time course for the slow onset vasodilation with BQ-123 has been shown to maximize by 60 minutes in some studies 45–47 and 90 minutes in another study 48. As a result, we can not rule out the possibility that further increase in FBF noted in the groups in response to the addition of BQ-788 to BQ-123 may involve some residual effects of BQ-123.
PERSPECTIVES
In conclusion, the results of this study indicate that nebivolol, but not metoprolol, treatment reduces ET-1-mediated vasoconstrictor tone in adult humans with elevated BP. Moreover, nebivolol-induced reduction in ET-1-mediated vasoconstrictor tone appears to be an important factor underlying the favorable effects of this beta-blocker on endothelial vasodilation. Importantly, the direct effect of nebivolol on ET-1 system activity is in addition to, and independent of, its established BP lowering effects and may be a key factor contributing to the improvement in endovascular health and reduction in cardiovascular morbidity and mortality 49 associated with chronic nebivolol treatment.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New
The novel and seminal finding of this study is that nebivolol, independent of lowering blood pressure, reduces endothelin-1-mediated vasoconstrictor tone in adults with elevated blood pressure. In addition, reduction in endothelin-1 vasoconstriction underlies the nebivolol-induced improvement in endothelium-dependent vasodilation.
What is Relevant
Endothelin-1 plays a pivotal role in the regulation of vascular tone and the etiology of hypertension and atherosclerotic vascular disease. While both nebivolol and metoprolol are highly effective in lowering blood pressure, nebivolol, but not metoprolol, reduces endothelin-1-mediated vasoconstrictor tone.
Summary
This study is the first to demonstrate significant pleiotropic effects of nebivolol on endothelial vasomotor, both vasoconstrictor and vasodilator, regulation in adult humans with elevated blood pressure.
Acknowledgments
We thank all of the subjects who participated in the study, as well as the clinical staff at the Clinical Translational Research Center, University of Colorado-Boulder for their assistance. Dr. Brunjes current affiliation is Columbia University Medical Center, New York, NY.
SOURCES OF FUNDING
This study was supported by funding from Forest Research Institute, Inc (BYS-MD-57) and National Institute of Health award UL1TR001082. This study is registered on Clinical Trials.gov (# NCT01395329; https://clinicaltrials.gov/ct2/show/NCT01395329?term=NCT01395329&rank=1)
Footnotes
DISCLOSURES
None.
References
- 1.Yanagisawa M, Hurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Haynes W, Strachan F, Gray G, Webb D. Forearm vasoconstriction to endothelin-1 is mediated by ETA and ETB receptors in vivo in humans. J Cardiovasc Pharmacol. 1995;26:S40–43. [PubMed] [Google Scholar]
- 3.Dashwood M, Tsui J. Endothelin-1 and atherosclerossis: potnetial complications associated with endothelin-receptor blockade. Atherosclerosis. 2002;160:297–304. doi: 10.1016/s0021-9150(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 4.Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991;89:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- 5.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Cardillo C, Kilcoyne C, Cannon R, Panza J. Interactions between nitric oxide and endothelin in the regulation of vascular tone in human resistance vessels in vivo. Hypertension. 2000;35:1237–1241. doi: 10.1161/01.hyp.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 7.Hopfner R, Gopalakrishnan V. Endothelin: emerging role in diabetic complications. Diabetologia. 1999;42:1383–1394. doi: 10.1007/s001250051308. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- 9.Cardillo C, Campia U, Kilcoyne C, Bryant M, Panza J. Improved endothelium-dependent vasodilation after blockade of endothelin receptors in patients with essential hypertension. Circulation. 2002;105:452–456. doi: 10.1161/hc0402.102989. [DOI] [PubMed] [Google Scholar]
- 10.Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–459. doi: 10.1161/HYPERTENSIONAHA.108.117366. [DOI] [PubMed] [Google Scholar]
- 11.Weil BR, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Elevated endothelin-1 vasoconstrictor tone in prehypertensive adults. Can J Cardiol. 2012;28:347–353. doi: 10.1016/j.cjca.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Cardillo C, Kilcoyne C, Waclawiw M, Cannon R, Panza J. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JW. Nebivolol: a third-generation beta-blocker for hypertension. Clin Ther. 2009;31:447–462. doi: 10.1016/j.clinthera.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Kuroedov A, Cosentino F, Luscher TF. Pharmacological mechanisms of clinically favorable properties of a selective beta1-adrenoceptor antagonist, nebivolol. Cardiovasc Drug Rev. 2004;22:155–168. doi: 10.1111/j.1527-3466.2004.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin CM, Keam SJ. Nebivolol: in the treatment of hypertension in the US. Am J Cardiovasc Drugs. 2009;9:253–260. doi: 10.2165/1120274-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Cleophas TJ, Agrawal R, Lichtenthal A, Makel W, Fici F. Nationwide efficacy-safety study of nebivolol in mildly hypertensive patients. Am J Ther. 2006;13:192–197. doi: 10.1097/01.mjt.0000149923.39085.44. [DOI] [PubMed] [Google Scholar]
- 17.Kamp O, Metra M, Bugatti S, Bettari L, Dei Cas A, Petrini N, Dei Cas L. Nebivolol: haemodynamic effects and clinical significance of combined beta-blockade and nitric oxide release. Drugs. 2010;70:41–56. doi: 10.2165/11530710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Czuriga I, Riecansky I, Bodnar J, Fulop T, Kruzsicz V, Kristof E, Edes I NEBIS Invesitgators, NEBIS Investigators Group. Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS) Cardiovasc Drugs Ther. 2003;17:257–263. doi: 10.1023/a:1026180325278. [DOI] [PubMed] [Google Scholar]
- 19.Rosei EA, Rizzoni D, Comini S, Boari G. Evaluation of the efficacy and tolerability of nebivolol versus lisinopril in the treatment of essential arterial hypertension: a randomized, multicentre, double-blind study. Blood Press Suppl. 2003;1:30–35. doi: 10.1080/08038020310000104. [DOI] [PubMed] [Google Scholar]
- 20.Van Nueten L, Lacourciere Y, Vyssoulis G, Korlipara K, Marcadet DM, Dupont AG, Robertson JI. Nebivolol versus nifedipine in the treatment of essential hypertension: a double-blind, randomized, comparative trial. Am J Ther. 1998;5:237–243. doi: 10.1097/00045391-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft JR, Chowienczyk PJ, Brett SE, Chen CP, Dupont AG, Van Nueten L, Wooding SJ, Ritter JM. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995;274:1067–1071. [PubMed] [Google Scholar]
- 22.Brehm BR, Heinle H, Risler T, Wolf SC. Chronically elevated endothelin-1 concentrations modulate the beta-adrenergic receptor system in vitro and in vivo. J Cardiovasc Pharmacol. 2000;36:S157–159. doi: 10.1097/00005344-200036051-00049. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jones D, Materson B, Oparil S, Wright J, Roccella E. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.Perloff D, Grim C, Flack J, Frohlich E, Hill M, McDonald M, Morgenstern B. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 25.Lohman T, Roche A, Mortorell R. Athropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 26.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 27.DeCree J, Van Rooy P, Geukens H, Haeverans K, Verhaegen H. The antihypertensive and cardiac hemodynamic effects of nebivolol. Angiology. 1992;43:369–377. doi: 10.1177/000331979204300501. [DOI] [PubMed] [Google Scholar]
- 28.McNeely W, Goa KL. Nebivolol in the management of essential hypertension: a review. Drugs. 1999;57:633–651. doi: 10.2165/00003495-199957040-00011. [DOI] [PubMed] [Google Scholar]
- 29.Basile JN. One size does not fit all: the role of vasodilating beta-blockers in controlling hypertension as a means of reducing cardiovascular and stroke risk. Am J Med. 2010;123:S9–15. doi: 10.1016/j.amjmed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Brehm BR, Bertsch D, von Fallois J, Wolf SC. Beta-blockers of the third generation inhibit endothelin-1 liberation, mRNA production and proliferation of human coronary smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2000;36:S401–403. doi: 10.1097/00005344-200036051-00117. [DOI] [PubMed] [Google Scholar]
- 31.Brehm BR, Wolf SC, Bertsch D, Klaussner M, Wesselborg S, Schuler S, Schulze-Osthoff K. Effects of nebivolol on proliferation and apoptosis of human coronary artery smooth muscle and endothelial cells. Cardiovasc Res. 2001;49:430–439. doi: 10.1016/s0008-6363(00)00253-4. [DOI] [PubMed] [Google Scholar]
- 32.Bakris GL, Basile JN, Giles TD, Taylor AA. The role of nitric oxide in improving endothelial function and cardiovascular health: focus on nebivolol. Am J Med. 2010;123:S2–8. doi: 10.1016/j.amjmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Fratta Pasini A, Garbin U, Nava MC, Stranieri C, Davoli A, Sawamura T, Lo Cascio V, Cominacini L. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J Hypertens. 2005;23:589–596. doi: 10.1097/01.hjh.0000160216.86597.ff. [DOI] [PubMed] [Google Scholar]
- 34.Serg M, Kampus P, Kals J, Zagura M, Zilmer M, Zilmer K, Kullisaar T, Eha J. Nebivolol and metoprolol: long-term effects on inflammation and oxidative stress in essential hypertension. Scand J Clin Lab Invest. 2012;72:427–432. doi: 10.3109/00365513.2012.691991. [DOI] [PubMed] [Google Scholar]
- 35.Broeders MA, Doevendans PA, Bekkers BC, Bronsaer R, van Gorsel E, Heemskerk JW, Egbrink MG, van Breda E, Reneman RS, van Der Zee R. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102:677–684. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- 36.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 37.Pasini AF, Garbin U, Stranieri C, Boccioletti V, Mozzini C, Manfro S, Pasini A, Cominacini M, Cominacini L. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257. doi: 10.1038/ajh.2008.260. [DOI] [PubMed] [Google Scholar]
- 38.Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1288–295. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- 39.Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Fail Rev. 2003;8:107–115. doi: 10.1023/a:1022155206928. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Horke S, Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double-blind, crossover study. Circulation. 2001;104:511–514. doi: 10.1161/hc3001.094207. [DOI] [PubMed] [Google Scholar]
- 42.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 43.Schiffrin E. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens. 2001;14:835–895. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- 44.MacIntyre IM, Dhaun N, Lilitkarntakul P, Melville V, Goddard J, Webb DJ. Greater functional ETB receptor antagonism with bosentan than sitaxsentan in healthy men. Hypertension. 2010;55:1406–1411. doi: 10.1161/HYPERTENSIONAHA.109.148569. [DOI] [PubMed] [Google Scholar]
- 45.Verhaar M, Strachan F, Newby D, Cruden N, Koomans H, Rabelink T, Webb D. Endothelin-a recptor antagonist-mediated vasodilation is attenuated by inhibition of nitric oxide sysnthesis and by endothelin-b receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 46.Newby D, Flint L, Fox K, Boon N, Webb D. Reduced responsiveness to endothelin-1 in peripheral resistance vessels of patients with syndrome x. J Am Coll Cardiol. 1998;31:585–590. doi: 10.1016/s0735-1097(98)00143-0. [DOI] [PubMed] [Google Scholar]
- 47.Taddei S, Virdis A, Ghiadoni L, Sudano I, Magagna A, Salvetti A. Role of endothelin in the control of peripheral vascular tone in human hypertension. Heart Fail Rev. 2001;6:277–285. doi: 10.1023/a:1011400124060. [DOI] [PubMed] [Google Scholar]
- 48.Love MP, Haynes WG, Gray GA, Webb DJ, McMurray JJ. Vasodilator effects of endothelin-converting enzyme inhibition and endothelin ETA receptor blockade in chronic heart failure patients treated with ACE inhibitors. Circulation. 1996;94:2131–2137. doi: 10.1161/01.cir.94.9.2131. [DOI] [PubMed] [Google Scholar]
- 49.de Boer RA, Voors AA, van Veldhuisen DJ. Nebivolol: third-generation beta-blockade. Expert Opin Pharmacother. 2007;8:1539–1550. doi: 10.1517/14656566.8.10.1539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.