Abstract
Tropical forests have long been recognized for their biodiversity and ecosystem services. Despite their importance, tropical forests, and particularly those of central Africa, remain understudied. Until recently, most forest inventories in Central Africa have focused on trees ≥10 cm in diameter, even though several studies have shown that small-diameter tree population may be important to demographic rates and nutrient cycling. To determine the ecological importance of small-diameter trees in central African forests, we used data from a 25-ha permanent plot that we established in the rainforest of Gabon to study the diversity and dynamics of these forests. Within the plot, we censused 175,830 trees ≥1 cm dbh from 54 families, 192 genera, and 345 species. Average tree density was 7,026 trees/ha, basal area 31.64 m2/ha, and above-ground biomass 369.40 Mg/ha. Fabaceae, Ebenaceae and Euphorbiaceae were the most important families by basal area, density and above-ground biomass. Small-diameter trees (1 cm ≥ dbh <10 cm) comprised 93.7% of the total tree population, 16.5% of basal area, and 4.8% of the above-ground biomass. They also had diversity 18% higher at family level, 34% higher at genus level, and 42% higher at species level than trees ≥10 cm dbh. Although the relative contribution of small-diameter trees to biomass was comparable to other forests globally, their contribution to forest density, and diversity was disproportionately higher. The high levels of diversity within small-diameter classes may give these forests high levels of structural resilience to anthropogenic/natural disturbance and a changing climate.
Introduction
Tropical forests have long been recognized for their biodiversity and ecosystem services such as carbon sequestration, habitat provision for vertebrates and invertebrates, and non-timber resources [1–3]. The tropical forest biome extends across three floristic regions—Central and South America, the southeastern Asian-Pacific, and equatorial Africa, all with high biodiversity. The African tropical rainforest has been least studied, and information is lacking regarding tree species diversity and the distribution of structural elements responsible for carbon storage and other ecosystem functions. Because small-diameter trees (here defined as those with less than 10 cm diameter at breast height [dbh, 1.3 m above the ground] and greater than or equal to 1 cm in diameter) are such a visually prevalent element of these forests (Fig 1), we predict that as in other African forests, they would be a much more important component of the forest diversity, structure and biomass than other forests globally.
Fig 1. Representative photos of the four strata of the forest canopy.

(A) Treelets (<10 m tall). (B) Understory trees (10 m to 20 m tall), (C) lower canopy trees (20 m to 30 m tall) and (D) upper canopy trees (≥30 m tall). (E) The plot also features a stream and 11 stumps from past logging of valuable trees (F).
The small-diameter tree population has often been overlooked because it is not important to timber extraction, and is a much smaller constituent of forest biomass than the larger trees (e.g., [4]). Until recently, most studies in Africa concentrated on large-diameter trees (here defined as those with dbh ≥10 cm; e.g., [5–8]. However, recent studies have shown that the small-diameter tree population may be important to demographic rates and nutrient cycling (e.g., [9]), and that the relative contribution of smaller diameter trees to forest biomass may be higher in tropical Africa than in other tropical and temperate forests (e.g., [10]). Small-diameter trees may also contribute disproportionately to woody plant diversity [11], as many taxa that reach 1 cm dbh are not present at the 10 cm diameter class—potentially much more so in tropical Africa than in other forests.
We, therefore, sought to establish a permanent research plot where we could study the unique contributions of smaller diameter trees to the distribution and abundance of all trees, and also to compare the Rabi forest with others in the Smithsonian Center for Tropical Forest Science ForestGEO network of long-term permanent plots [12], including those also located in the Congo basin rainforest—Ituri in Democratic Republic Congo [13], Korup in Cameroon [14], and at Rabi in Gabon (the present study). Previous results from Ituri and Korup showed that tropical African forests have some of the highest proportions of small-diameter trees in the world, both in terms of forest density and contributions to overall biomass [13,15,16]. Similarly, Lin et al. [17] showed that small-diameter trees (1 cm ≤ dbh <10 cm) contributed 10.4% of the biomass in a subtropical forest in China, and Vincent et al. [18] showed that the presence of small-diameter trees contributes to higher local levels of aboveground biomass. Our objectives were to characterize the composition and structure of the forests of Rabi and examine the relative contribution of small-diameter trees to tree abundance, basal area, above-ground biomass, and diversity. We hypothesized that the relative importance of small-diameter trees would be higher than in other forests globally and, therefore, that a comprehensive understanding of tropical African forests requires sampling of small-diameter trees in addition to the current emphasis on larger diameter individuals.
Methods
The research authorization to carry out this study was granted by the Government of Gabon, through the Centre National de la Recherche Scientifique et Technologique (CENAREST).
Study site
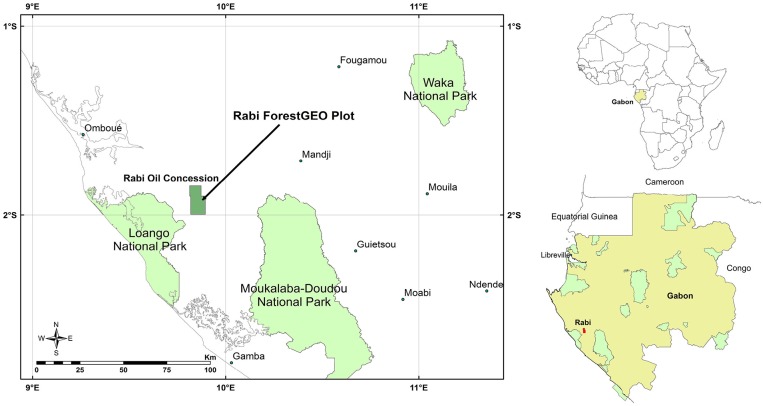
The study site is in the Gamba Complex of Protected Areas (1°50′ to 3°10′ S; 9° 15′ to10° 50′ E) in southwestern Gabon (Fig 2). This region is the southern portion of Guineo-Congolian forest type [14,19–21], which includes swamp and mixed moist semi-evergreen forest types [22]. The Gamba Complex includes two national parks, Loango National Park on the west and Moukalaba Doudou National Park on the east. This area is the largest protected area in Gabon, covering 267,667 km2 or about 4% of the total area of Gabon [20].
Fig 2. Map of the Rabi Forest Monitoring Plot in relation to the Gamba Complex of Protected Areas and the Rabi Oil Concession (Africa and Gabon inset, right).
Rabi is in the northern part of the Gamba Complex between the two national parks. The vegetation is characterized by the abundance of the tree species such as Dichostemma glaucescens, Diogoa zenkeri, Klaineanthus gaboniae, Coula edulis, Crudia gabonensis and Odyendea gabonensis. The most abundant families are Fabaceae, Euphorbiaceae and Olacaceae [21,23]. Family classification follows the Angiosperm Phylogeny Group III [24].
Soils are characterized as ferralitic and hydromorphic, with mostly sandy clay (approximately 25% clay) to clay sand (approximately 35% clay) [20]. Temperature throughout the year is almost invariant between 24°C and 28°C [21]. Annual precipitation averages 2299 mm. There are two main seasons, a dry season from June to September with precipitations averaging 24 mm/month and a wet season between October and May when precipitations average 269 mm/month. November and March with averages of 406 mm and 303 mm of rain respectively are the wettest months of the year, while July with an average of 11 mm is the driest month of the year (Shell Gabon unpublished data, 1985 to 2015).
The 25-ha permanent plot (plot center 1°55′S, 9°52′W) is orientated southeast—northwest (Fig 3). Plot elevation varies between 32 m and 62 m, and the plot is bisected by a stream surrounded by gentle slopes and three ridges (Fig 3). The forest in the area underwent selective logging, mostly of Lophira alata (Azobe), prior to 1990; there are 11 stumps from felled trees inside the plot (Fig 1F). In addition to the selective logging, seismic assessments were conducted in the area in the 1980s. Seismic assessments involve the temporary use of heavy equipment in localized areas resulting in removal of smaller statured vegetation [25,26]. The forest canopy is generally divided into four strata—treelets, understory, lower canopy trees and upper canopy (Fig 1).
Fig 3. Topographic map of the 25-ha Rabi plot with 1-m contour intervals.
The river flows at the lowest elevations, specifically between the 32-m and 36-m contours.
Data collection
Field methods followed the standards of the Smithsonian Center for Tropical Forest Science [27]. The 25-ha was divided into 625 quadrats of 20 m × 20 m each. For tree enumeration, each quadrat was in turn divided into 16 subquadrats of 5 m × 5 m. Within each subquadrat, the main stem of every tree ≥1 cm in diameter at breast height (dbh; 1.3 m above ground level) was mapped (subsidiary stems of the same individual were not mapped). If the tree had either an irregularity or a buttress at 1.3 m height, the height of measurement was moved (and recorded) to avoid the irregularity or the buttress.
Tree enumeration (diameter measurement, tagging and mapping) was carried out from June 2010 to June 2012. Tree identification was conducted in the field and in herbaria. In the field, trees were grouped into morphospecies—morphologically identical entities—mostly on the basis of vegetative characters. To ensure consistency in the groupings, up to 15 voucher specimens were collected for each morphospecies and compared side-by-side. Over time (2012–2014), flowering and fruiting material were collected for 85% of the morphospecies for further taxonomic identifications at herbaria in Libreville, Leiden and Brussels. Voucher specimens for the Rabi plot are deposited at the National Herbarium of Gabon, the Naturalis Biodiversity Center in Leiden, the Missouri Botanical Garden and the Smithsonian Museum of Natural History.
Maximum height at maturity
The classification of Rabi species into life-forms followed Kenfack et al. [14]. Each species was assigned to one of the following four life-forms according to the maximum height that they attain at maturity, using information from the literature and our field observations. Trees that do not normally reach 10 m in height and are <10 cm dbh were classified as treelets; understory trees were those 10 m to 20 m tall and 10 cm to 30 cm dbh; lower canopy included trees 20 m to 30 m tall and 30 cm to 60 cm dbh; and finally, upper canopy trees were defined as those reaching >30 m in height and >60 cm dbh.
Data Analyses
Because most forest inventory plots in Central Africa are 1 ha in area and include only trees ≥10 cm dbh, for comparison we calculated abundances, basal area, Fisher’s diversity and aboveground biomass (AGB) per hectare and for two diameter classes; 1 cm ≤ dbh <10 cm and ≥10 cm. Diversity was analyzed at the levels of families, genera and species. Aboveground biomass was estimated for each individual tree (including all stems for multi-stemmed trees) using the dbh and the wood density allometric equation
from Chave et al. [28], where AGB is aboveground dry biomass (in kg), ρ the wood density (g/cm3), D the dbh (in cm), ln the natural logarithm, and exp the exponential function. Frequency was calculated based on the 20 m × 20m quadrats that make up the plot. Abundance-diameter relationships were calculated with 1 cm bins. All analyses were performed using version 3.4.2 of R [29] and the CTFS R package (http://ctfs.arnarb.harvard.edu/Public/CTFSRPackage/index.php/web).
Results
Floristics and diversity
We found 345 morphospecies within the 25-ha plot, of which 294 (85.2%) were identified to species and the remaining 51 (14.8%) to genus level. Six of the species identified to genus level have been confirmed to be new to science, and we expect that others will be confirmed as new to science when fertile material can be collected for these morphospecies. Three species in the plot were recorded in Gabon for the first time, Okoubaka aubrevillei Pellegr (Santalaceae) & Normand, Magnistipula multinervia Burgt (Chrysobalanaceae), and Beilschmiedia auriculata Robyns & R. Wilczek (Lauraceae). Because field identification was carried out for some portions of the plot several months after tree enumeration and mapping, a total of 2,109 individual trees were dead when they were revisited and not identified nor included in the analysis.
The 345 morphospecies belong to 54 families and 192 genera. Rubiaceae, with 57 species in 32 genera, was the most diverse family, followed by Fabaceae that had 43 species in 26 genera. Other diverse families in the plot included Annonaceae (17 species in 9 genera), Phyllanthaceae (16 species in 10 genera) and Anacardiaceae (16 species in 2 genera) (see S1 Appendix for a complete listing of species). The genera Trichoscypha and Diospyros were the richest with 14 and 12 species respectively, followed by Beilschmiedia (8), Maesobotrya, Memecylon, and Xylopia, each with seven species. Of these genera, Trichoscypha, Beilschmiedia and Memecylon had the highest number of unidentified species (S1 Appendix).
The small-diameter tree group comprised 333 species (96% of the total) in 185 genera and 52 families, while 233 species in 137 genera and 44 families were recorded among large-diameter trees. There were 207 species/ha for all trees in the plot, 201 species/ha for small-diameter trees, as compared to only 84 species/ha for large-diameter trees (Table 1). Fisher’s α for all trees was 40.1 per ha, almost the same (39.4/ha) for small-diameter trees, but lower for large diameter-trees (30.8/ha).
Table 1. Comparison of the small-diameter (1 cm ≥ dbh <10 cm) and large-diameter (dbh ≥10cm) tree contribution to structure and diversity of the Rabi 25-ha plot.
Numbers in parenthesis represent standard deviation or percentages.
| ≥ 1 cm | <10 cm | ≥ 10 cm | |
|---|---|---|---|
| All families | 54 | 52 (96.3%) | 44 (81.5%) |
| All genera | 194 | 187 (96.4%) | 139 (71.6%) |
| All species | 345 | 333 (96.5%) | 234 (67.8%) |
| All trees | 175,660 | 164,491 (93.6%) | 11,170 (6.4%) |
| Total Basal area (m2) | 791.23 | 130.73 (16.5%) | 660.60 (83.5%) |
| Total aboveground biomass (Mg) | 9,235.18 | 458.32 (5.0%) | 8,777.01 (95.0%) |
| Mean density per ha | 7026 (660) | 6,580 (644) | 447 (31) |
| Mean number of species per ha | 207 (12) | 201 (12) | 84 (8) |
| Mean Fishers α per ha | 40.1 (3.3) | 39.4 (3.4) | 30.8 (4.6) |
| Mean basal area per ha | 31.65 (4.11) | 5.23 (0.55) | 26.42 (3.94) |
| Mean AGB per ha | 369.41 (82.30) | 18.33 (2.11) | 351.08 (81.83) |
Abundances
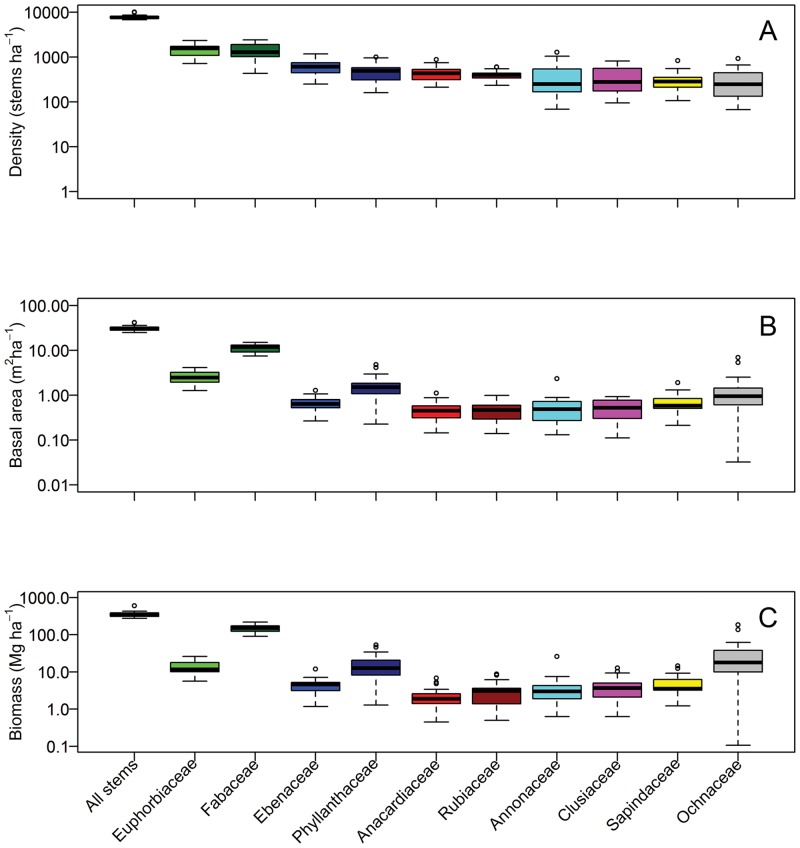
In the 25-ha plot, a total of 175,830 trees were recorded, with an average density of 7,026 individuals/ha. Small-diameter trees were 14 times more abundant than large-diameter trees. There were 164,491 small-diameter trees (93.6% of all stems), averaging 6580 individuals/ha, while large-diameter trees had a density of only 447 individuals/ha (Table 1). The families Euphorbiaceae, and Fabaceae were the most abundant, with densities >1000 individuals/ha, followed by Ebenaceae, Anacardiaceae, Phyllanthaceae, Rubiaceae, and Clusiaceae (Fig 4). Among genera, Dichostemma and Crotonogyne, both of the family Euphorbiaceae, were the most abundant, with >570 individuals/ha, followed by Diospyros, Garcinia, Trichoscypha, Tetraberlinia, Gilbertiodendron and Campylospermum (Table 2).
Fig 4. The Density (A), basal area (B), and aboveground live biomass (C) of the ten most abundant tree families in the Rabi plot.
Whiskers indicate the 2.5% and 97.5% values of the 25, 100 m × 100 m individual hectares of the plot.
Table 2. The 20 most abundant tree genera in the 25-ha Rabi plot ranked by density.
Basal area and above-ground biomass (AGB) rank indicated in parenthesis.
| Genus | Density (trees/ha) | Basal area (m2/ha) | AGB (Mg/ha) |
|---|---|---|---|
| Dichostemma | 683.9 | 1.4 (2) | 5.7 (23) |
| Crotonogyne | 573.1 | 0.2 (42) | 0.4 (84) |
| Diospyros | 552.4 | 0.7 (12) | 4.5 (26) |
| Garcinia | 348.1 | 0.5 (24) | 2.5 (35) |
| Trichoscypha | 294.5 | 0 (186) | 1.2 (57) |
| Tetraberlinia | 290.5 | 0.9 (9) | 32.3 (1) |
| Gilbertiodendron | 266.5 | 1.6 (1) | 18.8 (3) |
| Campylospermum | 250.2 | 0.2 (41) | 0.9 (63) |
| Pancovia | 175.8 | 0.5 (26) | 2.7 (34) |
| Piptostigma | 131.2 | 0.2 (51) | 0.4 (86) |
| Warneckea | 126.5 | 0.1 (179) | 1.9 (44) |
| Maesobotrya | 123.6 | 0.2 (45) | 0.9 (64) |
| Calpocalyx | 122.8 | 0.4 (30) | 2.7 (33) |
| Sorindeia | 121.8 | 0.3 (37) | 1.2 (56) |
| Protomegabaria | 119.8 | 0.5 (27) | 3.1 (31) |
| Dialium | 109.6 | 0.6 (19) | 6.9 (17) |
| Diogoa | 100.7 | 0.6 (20) | 5.1 (24) |
| Didelotia | 82.1 | 0.9 (8) | 11.3 (7) |
| Dactyladenia | 81.1 | 0.6 (18) | 6.6 (21) |
| Anisophyllea | 72.4 | 0.6 (15) | 6.8 (20) |
At species level, Dichostemma glaucescens and Crotonogyne gabonensis were the most abundant with densities > 500 individuals/ha, followed by Garcinia smeathmannii and Campylospermum congestum with densities >200 individuals/ha (Table 3). See S1 Appendix for complete listings of density, basal area and above ground biomass by family, genus, and species.
Table 3. The 20 most abundant tree species in the 25-ha Rabi plot ranked by density.
Basal area and above-ground biomass (AGB) rank indicated in parenthesis.
| Species | Density (trees/ha) | Basal area (m2/ha) | AGB (Mg/ha) |
|---|---|---|---|
| Dichostemma glaucescens | 683.9 | 1.4 (1) | 5.7 (21) |
| Crotonogyne gabonensis | 573.1 | 0.2 (43) | 0.4 (107) |
| Garcinia smeathmannii | 304.3 | 0.4 (28) | 1.9 (46) |
| Campylospermum congestum | 213.8 | 0.1 (96) | 0.7 (80) |
| Diospyros obliquifolia | 191.1 | 0.1 (149) | 0.3 (128) |
| Pancovia sp. nov. | 175.8 | 0.5 (23) | 2.7 (38) |
| Diospyros sp. nov. | 158.3 | 0.1 (151) | 0.2 (145) |
| Tetraberlinia moreliana | 149.7 | 0 (306) | 19.8 (2) |
| Gilbertiodendron ogoouense | 135.8 | 0.7 (9) | 7.0 (15) |
| Piptostigma multinervium | 131.2 | 0.2 (54) | 0.4 (114) |
| Tetraberlinia bifoliolata | 123.3 | 0.9 (6) | 9.2 (9) |
| Calpocalyx dinklagei | 121.9 | 0.3 (33) | 1.5 (54) |
| Protomegabaria stapfiana | 119.7 | 0.5 (24) | 3.1 (36) |
| Warneckea floribunda | 109.3 | 0 (336) | 1.4 (56) |
| Sorindeia gabonensis | 103.8 | 0.2 (55) | 1.1 (64) |
| Diogoa zenkeri | 100.7 | 0.6 (13) | 5.1 (23) |
| Gilbertiodendron unijugum | 79.0 | 0.4 (29) | 2.9 (37) |
| Diospyros hoyleana | 75.5 | 0.2 (47) | 0.9 (68) |
| Trichoscypha sp.8 | 69.9 | <0.1 (327) | 0.2 (158) |
| Amanoa strobilacea | 65.5 | 0.9 (5) | 11.1 (6) |
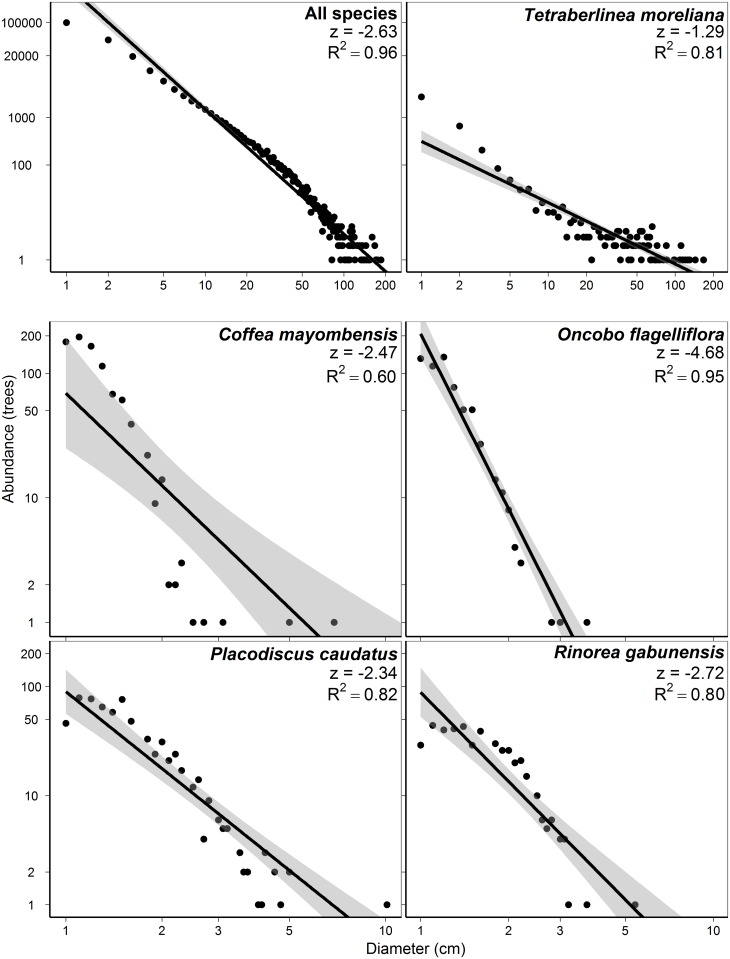
Tree abundances declined rapidly with increasing diameter. The forest structure at Rabi follows a declining exponential distribution, with steeper declines at diameters >50 cm dbh (Fig 5). For all species and all diameters, abundance decreased with a slope of -2.63 on a log-log scale (Fig 5) although some species (e.g., Tetraberlinia moreliana) declined more gradually. Small-diameter trees, especially treelet lifeforms, declined rapidly within the 1 cm to 10 cm dbh range (Fig 5).
Fig 5. Abundance-diameter relationships for trees within the 25-ha Rabi plot.
The relationship between diameter and abundance for all trees (A) has a linear decline in log-log coordinates of z = -2.63 (R2 = 0.96). An abundant canopy tree, Tetraberlinia moreliana (B), which includes the largest individual tree in the plot, and is well represented in all diameter classes, declines less sharply (z = -1.29, R2 = 0.81), and four treelet species, Coffea mayombensis (C; R2 = 0.60), Oncoba flagelliflora (D; R2 = 0.95), Placodiscus caudatus (E; R2 = 0.82), and Rinorea gabunensis (F; R2 = 0.80), have very steep declines in abundance with increasing diameter. One individual of Coffea mayombensis (dbh = 14.9 cm) and three individuals of Placodiscus caudatus (dbh = 10.1 cm, 10.2 cm, and 13.4 cm) not shown, but included in calculations. All relationships P <0.001.
Species with the greatest frequency of occurrence were the understory trees Garcia smeathmannii (91% of 625 quadrats) and Pancovia sp. (82%); the lower canopy trees Diogoa zenkeri (89%), and the treelet Crotonogyne gabonensis (84%). The high number of small trees is driven by four treelet species, Coffea mayombensis (Rubiaceae), Oncoba flagelliflora (Salicaceae), Rinorea gabunensis (Violaceae), and Placodiscus caudatus (Sapindaceae) (Fig 4). A total of 104 species (30%) had densities of less than one individual per ha, of which 32 species were represented by only one individual in the 25-ha plot.
Basal Area
Total basal area was 791.23 m2 (31.65± 4.11 m2/ha) and was dominated by Fabaceae (11.37 m2/ha; 35.9% of total), followed by Euphorbiaceae, Phyllanthaceae, Ochnaceae, Simaroubaceae and Burseraceae. The dominant genera by basal area were Tetraberlinia, Gilbertiodendron, Odyendea, Dischostemma, Eurypetalum and Lophira. At species level, Tetraberlinia moreliana was the most important, followed by Odyendyea gabonensis, Dichostemma glaucescens, Eurypetalum tessmannii, Lophira alata and Tetraberlinia bifoliolata (Table 3). Small-diameter trees contributed 16.5% of the total basal area.
Aboveground biomass
Total aboveground biomass for the 25-ha plot was 9235.18 Mg, averaging 369.41±82.3 Mg/ha for all trees ≥1cm dbh. Fabaceae had the highest AGB (150 Mg/ha), followed by Ochnaceae (33 Mg/ha), Phyllanthaceae (16 Mg/ha), Burseraceae (14 Mg/ha) and Euphorbiaceae (14 Mg/ha). At genus level, Tetraberlinia and Lophira were the most important (Table 4). Lophira alata was the most important species in terms of aboveground biomass, followed by Tetraberlina moreliana, Eurypetalum tessmannii and Librevillea klainei. The aboveground biomass of small-diameter trees was 18 Mg/ha, approximately 5% of the total biomass (Table 4). AGB for large-diameter trees was 351 Mg/ha.
Table 4. Comparison of the number of species, abundances, basal area and aboveground biomass (percent of total in parentheses) between small-diameter trees (1 cm ≥ dbh <10 cm) and large-diameter trees with (dbh ≥10cm) per tree life form in the Rabi plot.
| ≥ 1 cm | <10 cm | ≥ 10 cm | |
|---|---|---|---|
| Species | |||
| Treelets | 105 (30.4%) | 105 (30.4%) | 14 (4.1%) |
| Understory | 96 (27.8%) | 95 (27.5%) | 88 (25.5%) |
| Lower canopy | 83 (24.1%) | 79 (22.9%) | 78 (22.6%) |
| Upper canopy | 61 (17.7%) | 54 (15.7%) | 54 (15.7%) |
| Totals | 345 | 333 (96.5%) | 234 (67.8%) |
| Abundance | |||
| Treelets | 38,474 (22.1%) | 38,413 (22.1%) | 61 (<0.1%) |
| Understory | 55,605 (32.0%) | 53,084 (30.6%) | 2,520 (1.5%) |
| Lower canopy | 53,331 (30.7%) | 48,558 (28.0%) | 4,769 (2.8%) |
| Upper canopy | 26,311 (15.1%) | 22,632 (13.0%) | 3,674 (2.1%) |
| Totals | 173,721 | 162,687 (93.6%) | 11,024 (6.4%) |
| Basal Area (m2) | |||
| Treelets | 15.63 (2.0%) | 14.8 (1.9%) | 0.83 (0.1%) |
| Understory | 91.42 (11.7%) | 44.98 (5.8%) | 46.44 (5.9%) |
| Lower canopy | 221.99 (28.4%) | 46.29 (5.9%) | 175.7 (22.5%) |
| Upper canopy | 452.83 (57.9%) | 23.28 (3.0%) | 429.56 (55.0%) |
| Totals | 781.87 | 129.35 (16.6%) | 652.53 (83.5%) |
| Aboveground Biomass (Mg) | |||
| Treelets | 55.39 (0.6%) | 50.26 (0.6%) | 5.13 (0.1%) |
| Understory | 549.14 (6.0%) | 171.04 (1.9%) | 378.1 (4.1%) |
| Lower canopy | 2,019.66 (22.1%) | 154.02 (1.7%) | 1,865.64 (20.4%) |
| Upper canopy | 6,512.31 (71.3%) | 89.71 (1.0%) | 6,422.60 (70.3%) |
| Totals | 9,136.50 | 465.03 (5.1%) | 8,671.47 (94.9%) |
Forest structure by lifeform
Among the 345 species in the Rabi plot, there were 105 treelet species, with diameters mostly restricted to <10 cm. These accounted for 22% of the total number of individuals in the plot, but only 2% of basal area and 0.6% of aboveground biomass respectively (Table 4). Three of the 20 most abundant species in the plot were treelets, Crotonogyne gabonensis, Campylospermum congestum and Diospyros sp. nov. (Table 4). The remaining 240 species comprised 96 understory, 83 lower canopy and 61 upper canopy species, all of which reach more than 10 m in height and dbh ≥10 cm at maturity. These species represented 78% of the total number of trees, 98% of the total basal area and 99% of the total aboveground biomass.
Small-diameter trees comprised all 105 species of treelets, but also 95%, 91% and 86% of understory, lower canopy and upper canopy species respectively. Treelets accounted for 23.6% of the total individuals, 11.5% of the basal area and 10.8% of the aboveground biomass in this diameter size class. Understory and canopy species had at least 88% of their species among these small-diameter trees, and accounted for 78.4% of all trees, 85% of the basal area and 89% of the aboveground biomass of small-diameter trees. Among trees with dbh ≥10 cm, there were only 14 species of treelets, representing a tiny fraction of their total individuals, total basal area and of the aboveground biomass in this diameter class. Conversely upper canopy species with 33% of the individual accounted for 66% of the basal area and 74% of the aboveground biomass in this diameter class (Table 4).
Discussion
Although there have been some concerted effort in the recent past towards long-term monitoring of tropical African forests (e.g., the African Tropical Rainforest Observation Network; http://www.afritron.org), studies that include small-diameter trees are still uncommon, especially in the Congo Basin. The 25-ha Rabi plot is only the third large continuous patch of forest in Africa within which all trees with dbh ≥1cm are censused. Our results show clear differences in species diversity, abundances, basal area and aboveground biomass between small- and large-diameter trees. As in Korup and Ituri forests [13–15], lowering the sampling diameter to 1 cm in the Rabi plot increased the interpretation of the total diversity and the density of species.
Small-diameter trees were more diverse than large-diameter trees in the Rabi plot based on Fisher’s α. At least 30% of the species in the plot were treelets that achieve reproductive maturity in the forest understory and never attain 10 cm dbh. In addition to these treelets, small-diameter trees comprised saplings of all other understory and canopy tree species that do regenerate.
Tree density in the Rabi plot was comparable to other tropical African and temperate forests (Table 5). The African plots have generally higher proportions (92% to 95%) of small-diameter trees compared to other tropical plots, but the plot at Sinharaja, Sri Lanka was comparable. Temperate plots or dry tropical plots have much lower proportions (30% to 70%) of small-diameter trees, perhaps because of the combination of climatic limitations and repeated low intensity disturbance from fire or herbivory. The Rabi plot was notable in the steepness of the decline in the abundance-diameter relationship (Fig 5, Table 5). When only large-diameter trees are considered, the Rabi forest is dominated by Fabaceae, Olacaceae and Euphorbiaceae, which is in agreement with previous forest inventories in the area that used smaller plots (0.1 ha) with a 5 cm minimum diameter [21,23]. As in the Korup plot in Cameroon, Dichostemma glaucescens was the most abundant canopy species [14]. The abundance of small-diameter trees in the Rabi forest is not unique. Indeed, the high density of small trees has been reported in the Congo Basin [13,14] and in other tropical forests worldwide [16,30–32], and stands in contrast to densities in temperate plots (e.g., [33,34]). For example, within the Rabi plot, small-diameter trees account for 93.6% of all trees while in the 50-ha plot in Korup, they make up 92.4%. Small-diameter trees in Rabi were predominantly composed of saplings of understory and canopy tree species (76.4% of all trees in this size class). Saplings are more vulnerable to environmental fluctuations as well as damaging agents such as browsing herbivores and being crushed by windfalls. Long-term monitoring of this life stage is crucial to understanding the demography of canopy species as well as possible changes to forest composition driven by global change [12].
Table 5. Comparison of the Rabi, Gabon plot to other African CTFS plots and to tropical plots in South America and Asia, as well as two temperate plots in the USA.
African CTFS plots have a higher proportion of small-diameter trees than other tropical plots, and many more than temperate plots. Z represents the negative exponent of the abundance-diameter relationship (see Methods).
| CTFS-ForestGEO Site | Country | Area (ha) | Census year | Trees dbh≥ 1cm (Ind./ha) | Trees dbh<10 cm (Ind./ha) | Trees dbh≥ 10 cm (Ind./ha) | Trees dbh<10 cm (%) | Z |
|---|---|---|---|---|---|---|---|---|
| Rabi | Gabon | 25 | 2013 | 7,026.4 | 6,579.7 | 446.8 | 93.6 | 2.6 |
| Ituri-Lenda | DR Congo | 20 | 1995 | 6,843.6 | 6,486.0 | 357.6 | 94.8 | 2.1 |
| Ituri-Edoro | DR Congo | 20 | 1995 | 8,112.1 | 7,673.5 | 438.6 | 94.6 | 2.1 |
| Sinharaja | Sri Lanka | 25 | 1995 | 8,215.0 | 7,537.5 | 677.5 | 91.8 | 2.1 |
| Korup | Cameroon | 50 | 1999 | 6,580.6 | 6,070.7 | 509.9 | 92.3 | 2.0 |
| Lambir | Malaysia | 52 | 1997 | 6,915.5 | 6,277.5 | 638.0 | 90.8 | 2.0 |
| BCI | Panama | 50 | 2000 | 4,276.1 | 3,852.0 | 424.1 | 90.1 | 1.9 |
| Pasoh | Malaysia | 50 | 2000 | 6,118.9 | 5,553.3 | 565.6 | 90.8 | 1.9 |
| Yasuni | Ecuador | 25 | 1997 | 6,094.2 | 5,392.3 | 701.9 | 88.5 | 1.9 |
| Mudumalai | India | 50 | 2000 | 360.5 | 109.0 | 251.6 | 30.2 | 1.2 |
| Yosemite | USA | 25.6 | 2010 | 1,346.1 | 818.1 | 528.0 | 60.8 | 2.0 |
| Wind River | USA | 25.6 | 2011 | 1,209.9 | 884.7 | 325.3 | 73.1 | 1.8 |
That forest basal area and biomass is disproportionately carried in the large-diameter trees could make the forest at Rabi less resilient to short-term environmental change or disturbance. If the large-diameter individuals were killed, either through disturbance, disease, or human agency, the higher Z, relative to other forests (Table 5) suggests that it could take a relatively longer time for smaller-diameter trees to advance to the overstory. However, the diversity and abundance of the small-diameter trees suggests that the forest could have the ability to respond to disturbances in general. The ability of the forest to recover from disturbances affecting small-diameter trees through the regeneration of small-diameter trees suggests a high level of structural resilience—the perpetration of the existing diameter distribution within the forest. Small-diameter trees. Aboveground biomass in Rabi averaged 351 Mg/ha for trees ≥ 1cm which is lower than the estimated African mean of 395.7 Mg [32]. This lower value is probably due to the prevalence of small-diameter trees in this forest, and the limited selective logging of large-diameter trees that occurred two decades previously (site examination suggests that only the 11 large trees were removed). Small-diameter trees in the Rabi plot stored 5.0% of the total biomass while in the Korup plot, they accounted for 5.7% of the total biomass. This result is in agreement with the assumption that in mature tropical forests, the biomass of small-diameter trees is approximately 5% of the total aboveground biomass [25,33,34]. Our study also confirms that large-diameter trees store the bulk of biomass in tropical forests. In Rabi, 95% of the biomass is stored in trees with dbh ≥ 10 cm. Within this diameter class, upper canopy trees, comprised only 2% of the total individual trees in the plot, but stored 70% of the total biomass. Therefore, the contribution of small-diameter trees to biomass may be approximated based on forest type averages and forest AGB can be estimated from the few large trees [4,33], thereby avoiding the labor intensive work of censusing the small-diameter trees. However the demography of small-diameter trees is important to predicting the long-term change in above-ground biomass of the forest. For example, in subtropical evergreen broad leaved forests of China, Lin et al. [17] showed that small-diameter trees contributed 10.4% of the total above ground biomass, more than trees ≥50 cm dbh. Therefore the dynamics of the small-diameter trees in this forest could be relatively more important to overall ecosystem function—an opposite conclusion from many studies emphasizing large-diameter trees (e.g., [4,30,34]).
As the Congo Basin forest is considered to be the second most important forested region on earth after the Amazon, it is crucial that more comprehensive long-term studies including small diameter trees be implemented throughout its range.
Supporting Information
(DOCX)
Acknowledgments
We thank the Government of Gabon for authorizing us to conduct the study; Shell Gabon for funding, logistical support, and permission to carry out this project; the Compagnie des bois du Gabon (CBG) for permission to establish the plot in the its forest concession; the Smithsonian Tropical Research Institue through the Center for Tropical Forest Science—Forest Global Earth Observatory for funding and technical advice; and finally, the Institut de Recherche en Ecologie Tropicale (IRET) and the Herbier National through the Centre National de la Recherche Scientifique et Technologique (CENAREST) for their support and contributions. We also thank particularly Gauthier Moussavou, Etienne Moumoulossi, Landry Tchignoumba, Prince Biessemou, Wilfried Mbading-Mbading, Gorky Villa, and all other technicians who were involved in fieldwork. Special thanks to Diosdado Nguema for his invaluable contribution to the botanical identifications. We thank Suzanne Lao, Rick Condit, Kristina Anderson-Teixeira, and Helene Muller-Landau of the Smithsonian CTFS-ForestGEO for their help with database development and discussions, Franck Chatar for providing the Rabi precipitation data, and Igor Akengue Aken for cartography. This is contribution #148 of the Gabon Biodiveristy Program.
Data Availability
The Rabi 25-hectare Forest Monitoring Plot data belongs to the plot PIs and is managed by the CTFS-ForestGEO network. Data are available under request at (http://www.ctfs.si.edu/site/Rabi).
Funding Statement
Shell Gabon provided financial support for field work, especially to HRM and LK. Shell Gabon also provided logistical support including flights, lodging and food to LK, HRM, DK and 14 other field staff. Shell Gabon had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Smithsonian Tropical Research institute through the Forest Global Earth Observatory (ForestGEO) provided funding for the salaries of 12 field staff including HRM, and also provided funding to DK for training and technical advice. The Rabi plot was established with the protocols developed by ForestGEO, and DK is African Program Coordinator for ForestGEO. The data was analyzed by HRM, DK and JL during a series of analytical workshops funded by ForestGEO in China, US and Panama.
References
- 1.Myers N (1988) Threatened biotas: “Hot Spots” in tropical forests. Environmentalist 8(3): 187–208. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier J, Kirby KR, Potvin C (2012) Significance of carbon stock uncertainties on emission reductions from deforestation and forest degradation in developing countries. Forest Policy and Economics 24: 3–11. 10.1016/j.forpol.2010.05.005 [DOI] [Google Scholar]
- 3.Wright SJ, Kitajima K, Kraft NJB, Reich PB, Wright IJ, Bunker DE, et al. (2010) Functional traits and the growth—mortality trade-off in tropical trees. Ecology 91: 3664–3674. [DOI] [PubMed] [Google Scholar]
- 4.Bastin J-F, Barbier N, Réjou-Méchain M, Fayolle A, Gourlet-Fleury S, Maniatis D, et al. (2015) Seeing central African forests through their largest trees. Scientific Reports 5: 13156 10.1038/srep13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doucet J-L (2003) L’alliance délicate de la gestion forestière et de la biodiversité dans les forêts du centre du Gabon. Dissertation, Faculté Universitaire des Sciences Agronomiques de Gembloux, Belgique.
- 6.Engone Obiang NL (2011) Dynamique des espèces héliophiles dans les forêts non perturbées du Gabon. Rapport scientifique Aire Sud N 7148.
- 7.Medjibe VP, Putz FE, Starkey MP, Ndouna AA, Memiaghe HR (2011) Impacts of selective logging on above-ground forest biomass in the Monts de Cristal in Gabon. Forest Ecology and Management 262: 1799–1806. [Google Scholar]
- 8.Chisholm RA, Muller-Landau HC, Abd. Raman K, Bebber DP, Bin Y, Bohlman SA, et al. (2013) Scale-dependent relationships between species richness and ecosystem function in forests. Journal of Ecology 101: 1214–1224. [Google Scholar]
- 9.Green PT, Harms KE, Connell JH (2014) Nonrandom, diversifying processes are disproportionately strong in the smallest size classes of a tropical forest. PNAS 111: 18649–18654. 10.1073/pnas.1321892112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert GS, Howard E, Ayala-Orozco B, Bonilla-Moheno M, Cummings J, Langridge S, et al. (2010) Beyond the tropics: forest structure in a temperate forest mapped plot. Journal of Vegetation Science 21: 388–405. [Google Scholar]
- 11.Erickson DL, Jones FA, Swenson NG, Pei N, Bourg NA, Chen W, et al. (2014) Comparative evolutionary diversity and phylogenetic structure across multiple forest dynamics plots: a mega-phylogeny approach. Frontiers in Genetics fgene.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson-Teixeira KJ, Davies SJ, Bennett AC, Gonzalez-Akre EB, Muller-Landau HC, Wright SJ, et al. (2015) CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Global Change Biology 21(2): 528–549. 10.1111/gcb.12712 [DOI] [PubMed] [Google Scholar]
- 13.Makana J-R, Ewango CN, McMahon SM, Thomas SC, Hart TB, Condit R (2011) Demography and biomass change in monodominant and mixed old-growth forest of the Congo. Journal of Tropical Ecology 27: 447–461. [Google Scholar]
- 14.Kenfack D, Thomas DW, Chuyong G, Condit R (2007) Rarity and abundance in a diverse African forest. Biodiversity Conservation 16: 2045–2074. [Google Scholar]
- 15.Chuyong GB, Kenfack D, Harms KE, Thomas DW, Condit R, Comita LS (2011) Habitat specificity and diversity of tree species in an African wet tropical forest. Plant Ecology 212: 1363–1374. [Google Scholar]
- 16.Muller-Landau HC, Condit RS, Harms KE, Marks CO, Thomas SC, Bunyavejchewin S, et al. (2006) Comparing tropical forest tree size distributions with the predictions of metabolic ecology and equilibrium models. Ecology Letters 9: 589–602. [DOI] [PubMed] [Google Scholar]
- 17.Lin D, Lai J, Muller-Landau HC, Mi X, Ma K (2012) Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS ONE 7: e48244 10.1371/journal.pone.0048244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JB, Henning B, Saulei S, Sosanika G, Weiblen GD (2015) Forest carbon in lowland Papua New Guinea: local variation and the importance of small trees. Austral Ecology 40(2): 151–159. 10.1111/aec.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White F (1983) Vegetation of Africa. UNESCO, Paris, France. [Google Scholar]
- 20.Thibault M, Fisher BL, Goodman SM (2004) Description of Monts Doudou, Gabon, and the 2000 Biological Inventory of the Reserve In: Fisher BL (Ed) Monts Doudou, Gabon: A Floral and Faunal Inventory with Reference to Evational Variation. Number 28, Memoire of California Academy of Sciences, Sans Francisco, CA. [Google Scholar]
- 21.Lee ME, Alonso A, Dallmeier F, Campbell P, Pauwells Olivier SG (2006) The Gamba Complex of Protected Areas: an Illustration of Gabon’s biodiversity In: Alonso A, Lee ME, Campbell P, Pauwels OSG, Dallmeier F (eds) Gamba, Gabon: Biodiversity of an equatorial African rainforest. Bulletin of the Biological Society of Washington, No. 12. [Google Scholar]
- 22.Bonnefille R (2011) Rainforest responses to past climatic changes in tropical Africa In Bush MB, Flenley JR, Gosling WD (eds) Tropical rainforest responses to climatic change (2nd Edn). Springer-Verlag; Berlin Heidelberg: 2011. [Google Scholar]
- 23.Campbell P, Rivera P, Thomas D, Bourobou-Bourobo H, Nzabi T, Alonso A, et al. (2006) Floristic structure, composition and diversity of equatorial forest in Gabon In Alonso A, Lee ME, Campbell P, Pauwels OSG, Dallmeier F (Eds) Gamba, Gabon: Biodiversity of an equatorial African rainforest. Bulletin of the Biological Society of Washington, No. 12. [Google Scholar]
- 24.Bremer B, Bremer K, Chase MW, Fay MF, Reveal JL, Soltis DE, et al. (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. [Google Scholar]
- 25.Zabbey N (2004) Impacts of extractive industries on the biodiversity of the Niger Delta region, Nigeria. Paper presented at National Workshop on Coastal and Marine Biodiversity Management, Calabar, Cross-River State, 7–9 September.
- 26.Kafada AA (2012) Environmental Impacts of Oil Exploration and Exploitation in the Niger Delta of Nigeria. Global Journal of Science Frontier Research Environment and Earth Sciences 12, Online ISSN: 2249-4626 and Print ISSN: 0975-5896. [Google Scholar]
- 27.Condit R (1998) Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots. Springer-Verlag. [Google Scholar]
- 28.Chave J, Brown S, Cairns MA, Chambers JQ, Eamus D, Fölster H, et al. (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145(1): 87–99. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 30.LaFrankie JV, Ashton PS, Chuyong GB, Co L, Condit R, Davies SJ, et al. (2006) Contrasting structure and composition of the understory in species-rich tropical rain forests. Ecology 87: 2298–2305. [DOI] [PubMed] [Google Scholar]
- 31.Bohlman SA (2015) Species diversity of canopy versus understory trees in a neotropical forest: Implications for forest structure, function and monitoring. Ecosystems 18: 658–670. [Google Scholar]
- 32.Deb JC, Roy A, Wahedunnabi MD (2015) Structure and composition of understory treelets and overstory trees in a protected area of Bangladesh. Forest Science and Technology 11: 76–85. [Google Scholar]
- 33.Lutz JA, Larson AJ, Swanson ME, Freund JA (2012) Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 7(5): e36131 10.1371/journal.pone.0036131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutz JA, Larson AJ, Freund JA, Swanson ME, Bible KJ (2013) The importance of large-diameter trees to forest structural heterogeneity. PLOS ONE 8(12): e82784 10.1371/journal.pone.0082784 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The Rabi 25-hectare Forest Monitoring Plot data belongs to the plot PIs and is managed by the CTFS-ForestGEO network. Data are available under request at (http://www.ctfs.si.edu/site/Rabi).