Abstract
Human neutrophils have been known to release neutrophil extracellular traps (NETs), antimicrobial DNA structures capable of capturing and killing microbes. Recently, a similar phenomenon has been reported in macrophages infected with various pathogens. However, a role for macrophages extracellular traps (METs) in host defense responses against Mycobacterium massiliense (M. mass) has yet to be described. In this study, we show that M. mass, a rapid growing mycobacterium (RGM), also induces the release of METs from PMA-differentiated THP-1 cells. Intriguingly, this process is not dependent on NADPH oxidase activity, which regulates NET formation. Instead, M. mass-induced MET formation partially depends on calcium influx and requires phagocytosis of high bacterial load. The METs consist of a DNA backbone embedded with microbicidal proteins such as histone, MPO and elastase. Released METs entrap M. mass and prevent their dissemination, but do not have bactericidal activity. Instead, they result in enhanced bacterial growth. In this regard, METs were considered to provide interaction of M. mass with cells and an environment for bacterial aggregation, which may facilitate mycobacterial survival and growth. In conclusion, our results demonstrate METs as an innate defense response against M. mass infection, and suggest that extracellular traps play a multifaceted role in the interplay between host and bacteria.
Introduction
The Mycobacterium abscessus (M. abscessus) complex is a group of rapidly growing nontuberculous mycobacteria (NTM) that cause inflammatory disease ranging from localized abscess to disseminated infection in patients with immune deficiency such as cystic fibrosis or immunocompromised patients [1, 2, 3]. The M. abscessus complex comprises of three genetically related species: M. abscessus (sensu stricto), M. massiliense, and M. bolletii [4]. M. abscessus and M. massiliense (M. mass) are often isolated from patients with respiratory disease, and are known to be hard to treat and take longer to cure because they often acquire resistance to multiple antibiotics [5].
Various infectious agents such as viruses, bacteria and fungi have been known to cause release of extracellular DNA structures from neutrophils, macrophages, eosinophils, and mast cells [6, 7, 8]. The structure, termed neutrophil extracellular traps (NETs) as they were first found in neutrophils, is known to entrap and expose microorgnisms to clearance by other immune cells [6, 9]. Extracellular traps comprise of DNA, histones, and antimicrobial enzymes that have bactericidal effects [6, 10]. Activated neutrophils eject chromatin with granular and nuclear proteins by a phagocytosis-independent mechanism before undergoing NETosis, a cell death process that is morphologically distinct from apoptosis and necrosis [11, 12, 13].
Although extracellular traps have beneficial effects, their ineffective clearance is known to cause host pathology. For example, impaired clearance of NETs is associated with aggravation of atherosclerosis [14], rheumatoid arthritis [15], and systemic lupus erythematosus (SLE) [16, 17, 18]. These responses are mediated by cytotoxic effect of NETs on host tissues. For example, extracellular histones and peptides in NETs were reported to induce epithelial and endothelial cell death [19]. Furthermore, the prolonged presence of NETs in the lungs may trigger autoimmune responses [20].
Recently, it was reported that macrophages also release extracellular traps [21, 22, 23, 24]. Macrophage extracellular traps (METs) were shown to capture and mediate clearance of bacteria, fungi and parasites [21, 24, 25]. MET formation by macrophages of various species was examined in the context of infection with different microbes. Human macrophages differentiated from peripheral blood monocytes induced extracellular trap formation against Strongyloides stercoralis [25]. Histophilus somni and Mannheimia haemolytica infection induced MET formation by bovine and murine macrophages [21, 22]. In these studies, MET formation was shown to be associated with activation of elastase or NADPH oxidase. In addition to direct contact with microbes, bacterial exotoxins also induce the formation of extracellular traps from macrophages [21]. For example, bovine monocyte-derived macrophages exposed to leukotoxin of Mannheimia haemolytica released METs that entrapped and killed bacteria.
A recent study reported that M. tuberculosis, a slow growing virulent mycobacteria, triggers not only cell death but also extracellular trap formation in neutrophils and macrophages [23, 26]. In this study, we show that M. mass, a rapid growing mycobacteria (RGM), also induces the release of extracellular traps from PMA-differentiated THP-1 macrophages. We examined the composition of M. mass-induced METs, and demonstrate that the mechanism of MET formation is dependent on calcium influx and phagocytosis. Our present results suggest that these METs not only entrap bacteria, but also perform complex roles in the interaction between host and rapidly growing, virulent mycobacteria.
Materials and Methods
Bacterial strains
A clinically isolated rough strain of M. mass (M. mass R), reported as M. mass Asan 50594 belonging to the Type II genotype, was used in this study [27, 28, 29]. M. mass type II genotype was reported to show loss of genes related to glycopeptidolipid (GPL) biosynthesis and rough type due to an irreversible genetic factor [29]. M. mass strain CIP 108297 (M. mass CIP) was obtained from CIP (Collection of Institut Pasteur, Paris, France). M. mass were cultured in Middlebrook 7H9 medium (BD Biosciences, Franklin Lakes, NJ, USA) supplemented with 10% OADC (BD Biosciences, Franklin Lakes, NJ, USA), 0.2% glycerol and 0.05% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA). Cultured bacteria were collected by centrifugation. Then, collected mycobacteria were stored at -70°C until use. To prepare single cells of M. mass, cultured bacteria were homogenized with 23G syringe needle and sonicated in 10 min after soft spin centrifugation to exclude bacterial clumping. Nonsonicated M. mass aggregates were prepared by performing only soft spin centrifugation of the same bacterial cultures. The number of viable bacteria in stored bacterial vials was counted on Luria-Bertani (LB) agar (BD Biosciences, Franklin Lakes, NJ, USA).
Cell culture and reagents
The human acute monocytic leukemia THP-1 cell line was maintained in RPMI media supplemented with 10% FBS (Gibco, Carlsbad, California, USA). Differentiation of THP-1 cells into macrophages was performed by incubation with 25 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA) for 2 days at 37°C in a humidified atmosphere with 5% CO2. In these experiments, THP-1 cells were grown in 24-well tissue culture plates (Corning, Corning, NY, USA), and PMA-differentiated THP-1 macrophages were selected by keeping only the adherent cells.
Quantification of extracellular trap
PMA-differentiated THP-1 cells were cultured on 12-mm glass cover slides in 24 well plates (2×105 cells/well), and infected by M. mass R or CIP at multiplicity of infection (MOI) of 5, 10 or 20 (bacteria per cell) with or without 50 units/ml DNase I (Sigma-Aldrich, St. Louis, MO, USA), or stimulated with 1 mM hydrogen peroxide or 10 μg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA) for 24 hr. To examine the responses of THP-1 macrophages infected with only intracellular M. mass R or CIP, infected cells were washed twice at 3hr after infection to eliminate extracellular bacteria, and cultured for 24hr. For fluorescence staining of M. mass R, the Vybrant CFDA-SE (CFSE) cell tracer kit (Thermo Fisher, Waltham, MA, USA) was used. To visualize extracellular traps, cells were stained with 1 μM TO-PRO-3 (Thermo Fisher, Waltham, MA, USA) for 30 min at 37°C, and examined using fluorescence microscope CTR6000 (LEICA, Wetzlar, GE). Macrophages releasing extracellular DNA structures were considered as producing METs. For quantification of MET production, the total number of macrophages and the number of macrophages releasing METs per field of view were counted in 4 or 5 individual images per sample. At least 400 cells per sample were examined and expressed as a percentage.
Scanning Electron Microscopy (SEM)
M. mass CIP-infected cells on cover slips were washed, fixed with 2.5% glutaraldehyde overnight, and washed with 0.1 M phosphate buffer twice and post-fixed with 1% osmium tetroxide for 70 min. The samples were subsequently dehydrated with a graded ethanol series (30%, 50%, 70%, 80%, 90%, 100%), and then prepared as previously described [12]. The samples were examined using a scanning electron microscope (JEOL JSM-7401f, Japan).
Immunofluorescence microscopy
To perform immunofluorescence staining, M. mass R-infected cells were fixed with 4% paraformaldehyde (PFA) for 15 min, and permeabilized with 0.1% Triton X-100 for 5 min and blocked in 1% BSA/PBS for 1 hr at room temperature. The samples were subsequently incubated with rabbit anti-histone H4, rabbit anti-myeloperoxidase (MPO) or rabbit anti-elastase antibody (Santa cruz, Dallas, TX, USA) at 1:50–1:100 dilution for 90 min at room temperature. They were then washed and incubated with Alexa 488-conjugated anti-rabbit IgG (Thermo Fisher, Waltham, MA, USA) at 1:200 dilution for 90 min. Lastly, the slides were stained with 1μM TO-PRO-3 for 30 min and examined using fluorescence.
Cytokine treatment of macrophage
Differentiated THP-1 macrophages were pretreated with 10ng/ml IFN-γ (ProSpec, East Brunswick, NJ, USA) and TNF-α (R&D Systems, NE Minneapolis, MN, USA) or untreated for 24 hr. The cells were washed and infected with M. mass R or CIP (MOI 5) for 24 hr. Then, MET formation in each sample was examined.
Cytokine analysis
For cytokine analysis, the supernatants of THP-1 macrophages infected with M. mass R or CIP (5 MOI) were harvested at 24hr post infection, and stored at -80°C until use. The levels of inflammatory cytokines (TNF-α, IL-1β and IL-6) were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Roche, Basel, Switzerland). The assays are performed according to the manufacturer’s instructions.
Polymerase chain reaction (PCR)
After extracellular traps were induced by infection with M. mass R for 24 hr, released DNA in the supernatants of infected cells and uninfected control were purified using QIAamp MinElute Media Kit (Qiagen, Venlo, Netherlands). PCR analysis was performed on purified DNA using two nuclei-specific primers (Gapdh, Forward: 5’-AAT CCC ATC ACC ATC TTC CA-3’, reverse: 5’-TGG ACT CCA CGA CGT ACT CA-3’. β-actin, Forward: 5’-GTT GCT ATC CAG GCT GTG-3’, reverse: 5’- TGA TCT TGA TCT TCA TTG TG-3’) and two mitochondria-specific primers (Atp6, Forward: 5’-ATA CAC AAC ACT AAA GGA ACC T-3’, reverse: 5’-GAG GCT TAC TAG AAG TGT GAA AAC G-3’. Nds1, Forward: 5’-GCA TTC CTA ATG CTT ACC GAAC-3’, reverse: 5’-AAG GGT GGA GAG GTT AAA GGA G-3’). PCR reaction was carried out using a MyCyclerTM (Biorad, Hercules, CA, USA). Initial denaturation at 95°C for 10 min was followed by 28 cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 40 sec, and extension at 72°C for 90 sec, with a final incubation for extension at 72°C for 7 min. The PCR products were analyzed by 1.5% agarose gel electrophoresis.
Reactive Oxygen Species (ROS) Detection
Differentiated THP-1 macrophages were pretreated with 20 μM DPI or left untreated for 30 min before stimulation with 1 mM hydrogen peroxide or M. mass R (MOI 5) for 24 hr. The cells were washed and stained with intracellular indicator, 5 μM 2’, 7 dichlorofluorescein diacetate (H2DCF-DA) (Thermo Fisher, Waltham, MA, USA) for 30 min. After washing, labeled cells were treated with 5 mM EDTA for 15 min, and collected. The samples were subsequently washed and analyzed using BD FACSCalibur and data analysis was done using FlowJo software (BD Biosciences, Franklin Lakes, NJ, USA).
Inhibition of macrophage elastase by N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (AAPV)
Differentiated THP-1 macrophages were untreated or pretreated with AAPV (0.1 or 1 μM) (Sigma-Aldrich, St. Louis, MO, USA) for 30 min before infection with M. mass R (MOI 5) for 24 hrs. The MET formation in each sample was quantified as previously described.
Measurement of intracellular calcium influx
For monitoring intracellular calcium influx, THP-1 cells were plated at 5 x 104 cell/well in 96-well black microplates (Corning, Corning, NY, USA) and differentiated by PMA. Differentiated THP-1 cells were washed and loaded with the Ca2+-sensitive indicator, 1 μM Fluo-4 AM (Thermo Fisher, Waltham, MA, USA) for 20 min. Then, Fluo-4-labelled cells were untreated or pre-treated with 20 μM 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) (Thermo Fisher, Waltham, MA, USA) or 1 μM ethylene glycol tetraacetic acid (EGTA) for 30 min, and subsequently infected with M. mass R (MOI 5) or stimulated with 1 μM ionomycin (Sigma-Aldrich, St. Louis, MO, USA) as positive control. Fluo-4-labelled cells were washed at 3 hr post infection and measured at 494nm/516nm using a microplate reader (Tecan infinite 200, San Jose, CA, USA). Fluorescence intensity was measured at 20 sec intervals over 10 min.
Assessment of bacterial survival and phagocytosis
To assess bacterial survival in the presence or absence of METs, cells were pre-treated with 50 unit/ml DNase I (Sigma-Aldrich, St. Louis, MO, USA) for 30 min, and then infected by M. mass R (MOI 10) for 24 hr to induce MET release. The supernatants were collected to recover extracellular bacteria, and attached cells with METs were gently washed and lysed with PBS containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) to recover cell-associated bacteria. To determine the extent of phagocytosis of bacteria, cells were or were not pre-treated with 5 μM Cytochalasin D (Sigma-Aldrich, St. Louis, MO, USA) for 30 min, and then infected by M. mass R or CIP (MOI 1, 3 or 10) for 24 hr to induce MET release. Infected cells were washed several times and lysed to recover only intracellular bacteria. The supernatants or released bacterial suspension was diluted in PBS, and plated on LB agar for CFU determination. The summation of CFUs recovered from cells and supernatants reflect the total quantities of surviving bacteria in each well. To examine M. mass within macrophages, acid-fast bacilli (AFB) stain kits (BD Biosciences, Franklin Lakes, NJ, USA) were used.
Lactate dehydrogenase (LDH) release assay
To assess cell lysis in infected cells, PMA-differentiated THP-1 cells were cultured in 24 well plates (2×105 cells/well), and infected by M. mass R (MOI 1, 3, or 10) for 24 hr. The supernatants were collected and Lactate dehydrogenase (LDH) release detection was performed using cytotoxicity detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Annexin V and Propidium iodide staining for flow cytometry
To determine necrotic cell death, differentiated THP-1 macrophages were infected with M. mass R (MOI 5) or left uninfected for 1 day. Collected cells were washed and then stained with Annexin V FITC (BD Biosciences, Franklin Lakes, NJ, USA) and Propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) for 15 min. Stained cells were analyzed using BD LSR Fortessa (BD Biosciences, Franklin Lakes, NJ, USA), and data analysis was done using FlowJo software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analyses
ANOVA and Student’s t-test were performed to determine statistically significant differences between groups using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). A P value of < 0.05 was deemed to be statistically significant and is indicated in the figures by an asterisk. P values of <0.01 and <0.001 are indicated by two and three asterisks, respectively.
Results
Microscopic examination of MET formation induced by M. mass infection
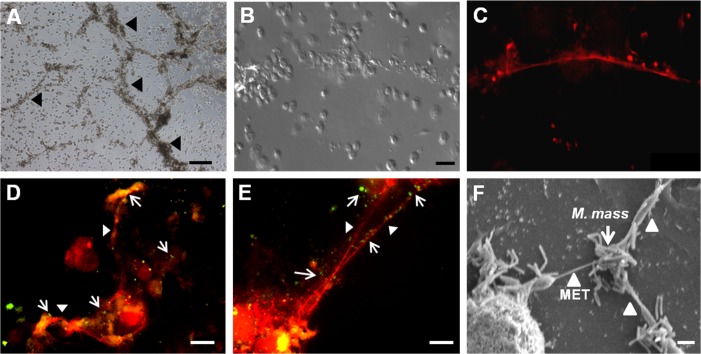
In a previous study, M. abscessus infection induced the formation of DNA extracellular trap structures by human peripheral blood mononuclear cells (PBMC) [30]. Similarly, when PMA-differentiated THP-1 macrophages were infected by M. mass cells released extracellular fiber structures that merged together (Fig 1A). These structures were positively stained by a DNA-staining dye, TO-PRO, and were morphologically similar to DNA extracellular traps (Fig 1B and 1C). We observed similar structures in PBMC-derived human macrophages infected with M. mass (data not shown). To confirm whether these structures can function like METs and entrap bacteria, we infected macrophages with CFSE-stained M. mass. Fluorescence microscopic examination revealed CFSE-stained M. mass co-localization with extracellular DNA fibers 1 day after infection, suggesting that extracellular M. mass was entrapped and bound to the MET structures (Fig 1D and 1E). In addition, we used scanning electron microscopy (SEM) to examine the ultrastructure of the METs induced by M. mass (Fig 1F). In SEM examination, most of extracellular M. mass were shown to be attached to macrophages. Some mycobacteria were linked to METs released from adjacent macrophages. MET structures were more common near clustered M. mass rather than single or a few bacilli. Thus, we confirmed that M. mass infection induces the formation of DNA extracellular traps from differentiated THP-1 macrophages and that M. mass-induced METs are morphologically similar to the extracellular structures released by activated neutrophils and other immune cells.
Fig 1. Microscopic examination of macrophage extracellular trap (MET) formation induced by M. mass infection.
Differentiated THP-1 macrophages were infected with M. mass R or CIP at a MOI of 5 for 24 hr, and stained by DNA-binding dye, TO-PRO-3 (Red) to visualize extracellular trap-like structure. (A) Microscopic examination of extracellular trap-like structures in M. mass R-infected THP-1 macrophages. Bar, 200μm. (B, C) Microscopic examination (B) and TO-PRO staining (C) of extracellular trap-like structures induced by M. mass R infection. Bar, 50μm. (D, E) Fluorescence micrographs of CFSE-stained M. mass R (Green) entrapped by METs. Bar, 20μm. (F) Scanning electron microscopy (SEM) images of METs induced by M. mass CIP. Bar, 1μm. Arrow: Mycobacteria. Arrow heads: Extracellular trap-like structures.
M. mass with rough colony morphology strongly induces MET formation
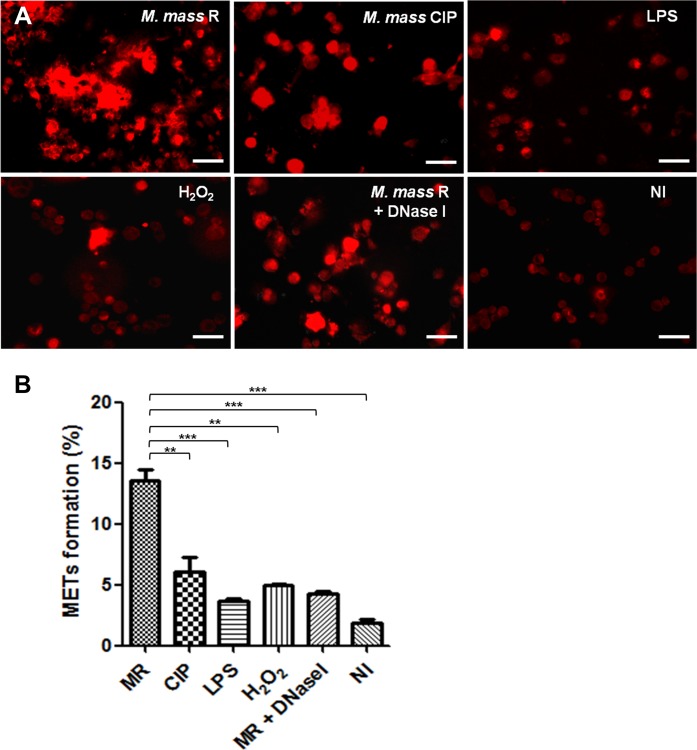
Various stimuli have been shown to induce neutrophil extracellular traps including PMA, LPS, hydrogen peroxide, etc [6, 31]. We examined the effects of such stimuli on inducing MET formation by PMA-differentiated THP-1 macrophages (Fig 2A). LPS (10 μg/ml) and hydrogen peroxide (1 mM) treatments for 1 day did not induce extracellular traps from macrophages, and high concentrations of stimuli (LPS ≥ 100 μg/ml; H2O2 ≥ 10mM) caused extensive cell death. In addition, we infected THP-1 macrophages with two different strains of M. mass, those that form rough and smooth colonies, to determine whether colony type affects MET formation or not. We infected the cells with each M. mass at 20 MOI, to induce extreme responses by each colony type for 1 day and compare them with the effects of other stimuli. After infecting macrophages with each strain of M. mass, the sample infected with the rough strain, M. mass R, showed MET formation in 14% of the cells (Fig 2B). On the other hand, less than 6% of the macrophages infected by the smooth strain, M. mass CIP, formed METs. This result is similar to that of previous study using human monocytes infected with M. abscessus [30]. We examined whether only intracellular infection without extracellular bacteria induces MET formation. The sample with only intracellular infection showed much lower levels of MET formation than the sample infected with both intracellular and extracellular bacteria (S1 Fig). DNase treatment during infection readily dissolved the extracellular traps, but M. mass-induced cell death during MET formation was still detected.
Fig 2. Rough strain of M. mass strongly induces MET formation in differentiated THP-1 macrophages.
(A) Differentiated THP-1 macrophages were stimulated with M. mass R (MOI 20) with or without DNase I (50 units/ml), M. mass CIP (MOI 20), LPS (10 μg/ml) or hydrogen peroxide (1 mM) for 24 hr, and then stained by TO-PRO-3 to examine METs. Bar, 50μm. (B) MET formation (%) of each sample was quantified by calculating percentages of MET-positive cells to total cells count. MR: M. mass R. Data are representative of three independent experiments with similar results. *, p<0.05; **, p<0.01 compared to M. mass R-infected group by one-way ANOVA with Bonferroni’s post-test.
M. mass R significantly induced MET formation. However, when THP-1 macrophages were infected with M. mass R at MOI of 20, severe cell death was detected 24 hr after infection. So we performed the following experiments using M. mass R or CIP at MOI of 5. To examine the effect of bacterial aggregation on MET formation, we infected THP-1 macrophages with sonicated single cells or nonsonicated aggregates of M. mass. Colony-forming unit (CFU) of nonsonicated M. mass were measured half as many as that of sonicated M. mass. When macrophages were infected by 5 MOI of sonicated or 2.5 MOI of nonsonicated M. mass R, nonsonicated aggregates of M. mass R induced more MET formation and cell death than sonicated single bacteria did. However, nonsonicated M. mass CIP did not enhance the levels of MET formation or cell death, comparing with sonicated M. mass CIP (S2 Fig). Previous studies reported that rough strains of M. mass induce higher levels of proinflammatory cytokines than smooth strains. Our study also showed that M. mass R induce higher levels of TNF-α, IL-1β and IL-6 than M. mass CIP (S3A Fig). However, pretreatment of macrophages with IFN-γ or TNF-α did not enhance MET formation by infection with M. mass R or CIP (S3B Fig). These results suggest that DNA extracellular trap production by macrophages is not due to simple inflammatory or oxidative stimuli, but is dependent on the colony type of M. mass, indicating that interactions with distinct antigens or surface structures of M. mass may cause the release of extracellular traps from macrophages.
M. mass R-induced METs contain mitochondrial DNA, nuclear DNA, and microbicidal proteins
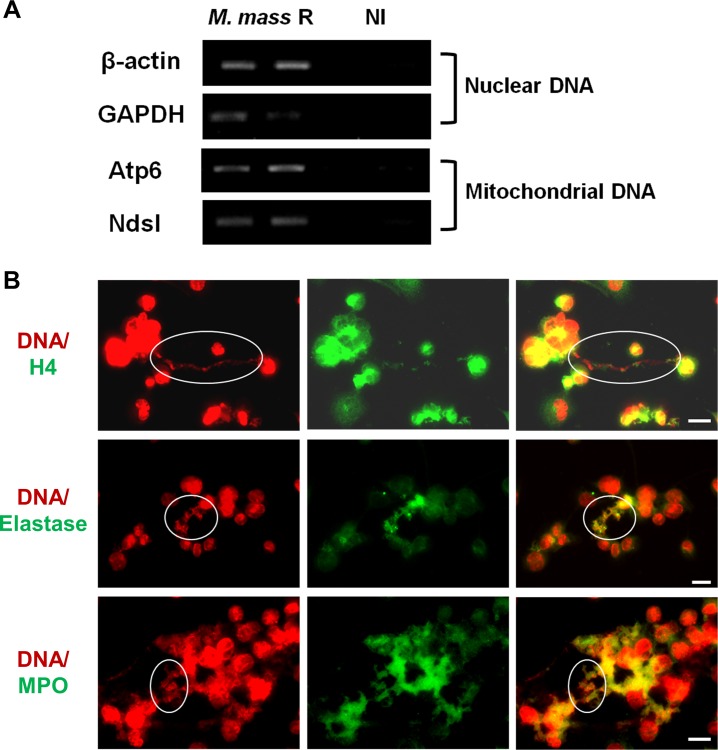
Extracellular traps formed by different immune cells are known to consist of nuclear or mitochondrial DNA embedded with histones and various enzymatic peptides [6, 16, 32]. To investigate the origin of the DNA released by macrophages infected with M. mass, we infected THP-1 cell with M. mass R and performed PCR analysis to detect nuclear signals (β-Actin and Gapdh) and mitochondrial signals (Atp6 and Nds1). PCR analysis of the supernatants from M. mass R-infected cells, containing visible MET structures, showed that the extracellular DNA contained sequences of both nuclear and mitochondrial genes (Fig 3A). It is possible that the extracellular DNA in supernatants is due to the release of cell contents from cell death associated with M. mass R-induced MET production. In addition, we performed immunofluorescence staining to identify the DNA-embedded components in M. mass R-induced METs (Fig 3B). We used specific antibodies to test for potential candidates such as histones and antimicrobial enzymes (MPO and elastase), and found that histones colocalized with the extracellular DNA of METs while antimicrobial enzymes colocalized with METs. The latter suggests that M. mass R-induced METs also have enzyme-mediated bactericidal functions. However, we also observed that portions of METs did not contain histones or enzymes. Collectively, we show that M. mass R-induced METs have a backbone of nuclear and mitochondrial DNA that is embedded with histones and enzymatic proteins, suggesting they have similar functions in host defense against foreign pathogens to extracellular traps of other immune cells.
Fig 3. M. mass R-induced METs induce release of mitochondrial as well as nuclear DNA, and contain Histone, MPO and Elastase.
(A) PCR analysis was performed for nuclear (β-actin, Gapdh) and mitochondrial genes (Atp6, Nds1) using DNA isolated from the supernatants of M. mass R-infected THP-1 macrophages and uninfected control samples. Both nuclear and mitochondrial genes were detected in the infected samples. Data are representative of three independent experiments. (B) M. mass R-infected THP-1 macrophages were fixed and processed for histone 4, elastase and MPO staining. METs were stained by TO-PRO-3. Circles indicate co-localization of METs with each component. Data are representative of three independent experiments. H4: histone 4. Bar, 20μm.
M. mass R-induced MET formation does not depend on NADPH oxidase, but rather calcium influx
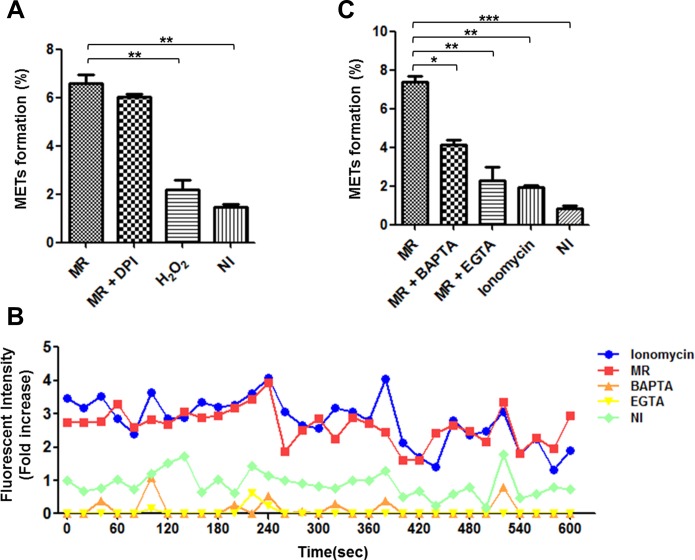
Extracellular trap formation is known to be closely associated with the activation of NADPH oxidase [6, 33, 34]. To investigate whether NADPH oxidase is required for M. mass R-induced MET formation, we treated infected macrophages with diphenylene iodonium (DPI), an inhibitor of NADPH oxidase enzymes (Fig 4A). Although treatment of infected macrophages with DPI reduced ROS production, M. mass R infection did not augment ROS production compared to uninfected cells (S4 Fig). Microscopic examination showed that DPI treatment of M. mass R-infected cells did not significantly reduce MET formation, suggesting that inhibition of ROS generation by NADPH oxidase does not affect extracellular trap release from macrophages by M. mass R infection. Although hydrogen peroxidase treatment enhanced ROS generation in macrophages, it did not induce MET formation. In a previous study using M. tuberculosis, MET formation was reported to be associated with elastase activity [26]. We tested using various concentrations of N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (AAPV), elastase inhibitor, but it did not affect M. mass R-induced MET formation (S5 Fig).
Fig 4. M. mass R-induced MET formation is not dependent on NADPH oxidase activity, but does depend on calcium influx.
After staining THP-1 macrophages with TO-PRO-3, the MET formation (%) in each sample was determined by quantification of MET-positive cells to total cell count. (A) Differentiated THP-1 macrophages were stimulated with hydrogen peroxide (1 mM), M. mass R (MOI 5) with or without DPI (20 μM) for 24 hr. (B) Fluo-4-labeled THP-1 macrophages were untreated or pretreated with BAPTA (20 μM) or EGTA (1 mM) for 30 min and then infected with M. mass R (MOI 5). Ionomycin (1 μM) was used as positive control. Intracellular calcium transients of labeled cells were recorded in a microplate reader at 6 hr after infection. (C) Determination of MET formation (%] in each sample. MR: M. mass R. Data are representative of three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001 compared to M. mass R-infected group by one-way ANOVA with Bonferroni’s post-test.
Recent studies have reported that calcium influx also triggers the formation of extracellular traps via an NADPH oxidase-independent mechanism [35, 36]. To investigate the role of calcium influx in M. mass R-induced MET formation, we measured calcium influx in infected and uninfected THP-1 macrophages by staining cells with Fluo-4 AM (Fig 4B). Fluorescence intensity measurement showed that M. mass R infection triggers a slow increase in the level of intracellular calcium that apparently exceeds uninfected cells at 6 hr after infection. The chelation of intracellular or extracellular calcium using BAPTA or EGTA, respectively, decreased calcium influx in M. mass R-infected macrophages. To evaluate the effects of calcium influx on METs formation, we monitored the formation of extracellular traps by M. mass R-infected macrophages after pretreatment with BAPTA or EGTA (Fig 4C). Although both treatment with BAPTA and EGTA reduced intracellular calcium levels of infected cells at early stages, however, BAPTA and EGTA did not completely abolish MET formation. Conversely, although treatment with ionomycin increased calcium influx, it could not trigger significant release of METs. Collectively, these results suggest that production of extracellular traps is partially affected by calcium-mediated inflammatory signals induced by M. mass R infection, and not calcium influx, in an NADPH oxidase-independent manner.
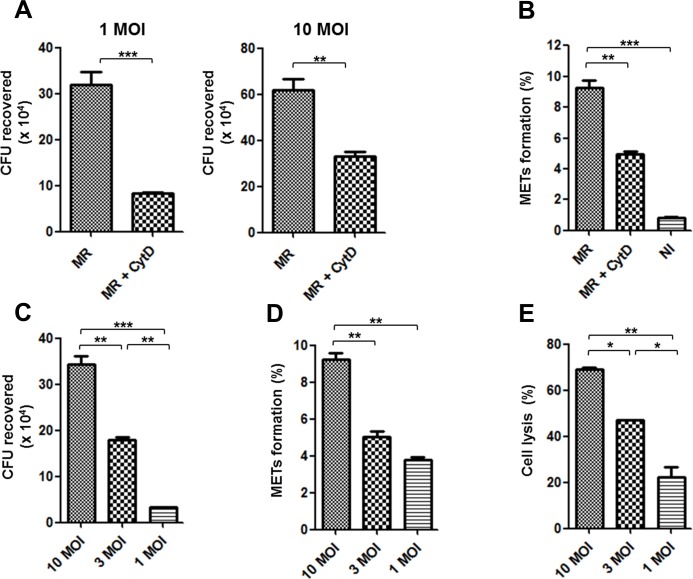
MET formation is dependent on phagocytosis of M. mass R
Considering the previous results, we supposed that an intracellular direct interaction between macrophages and M. mass R may contribute to MET formation. To analyze the dependency of MET formation on phagocytosis of M. mass R, we pretreated THP-1 macrophages with a phagocytosis blocker, cytochalasin D (CytD), prior to M. mass R infection and analyzed changes in MET formation. Treated cells showed decreased phagocytosis, represented by decreased intracellular CFUs, compared to untreated cells (Fig 5A). This was correlated with reduced MET formation in CytD-treated cells, suggesting that induction of MET is dependent on phagocytosis of M. mass R (Fig 5B). To corroborate this, we investigated MET formation after phagocytosis of different numbers of bacteria (Fig 5C). We infected the cells with M. mass R at 10, 3 and 1 MOI, to examine gradually induced MET formation and cell lysis for 1 day. Indeed, the extent of MET formation depended on the initial amount of bacteria that was phagocytosed (Fig 5D). In addition, LDH release data showed that cell lysis occurred after infection with M. mass R in a manner that is also correlated with bacterial burden (Fig 5E). Necrotic cell death of macrophages was confirmed by Annexin V and PI staining (S6 Fig). To examine whether M. mass CIP induce similar responses after cell lysis, we infected macrophages with M. mass CIP at 10, 3 and 1 MOI. M. mass CIP did not induce a significant increase in MET formation and cell lysis for 1 day (data not shown). At 2 day after infection, while M. mass R-infected cells are almost dead (data not shown), M. mass CIP-infected macrophages underwent gradual cell death responses depending on MOIs (S7A Fig). However, the samples infected with M. mass CIP for 2 days showed lower levels of MET production, comparing with the samples infected with the same amount of M. mass R for 1 day (S7B Fig). Taken together, these results suggest that MET formation is associated with macrophage phagocytosis of M. mass R, rather than an extracellular interaction with bacteria. Moreover, M. mass R induces more active cell necrosis and MET formation than M. mass CIP, and the subsequent responses associated with cell necrosis seem to contribute to the release of DNA.
Fig 5. MET formation is dependent on phagocytosis and cell lysis.
(A) CFUs of M. mass R recovered from infected macrophages with or without cytochalasin D (5 μM) for 24 hr. (B) MET formation (%) in the macrophages infected with M. mass R (MOI 10) after pretreatment with or without cytochalasin D. (C) CFUs recovered from macrophages infected with M. mass R at various MOI at 4 hr post infection. (D) Determination of MET formation (%) in each sample at 24 hr post infection. (E) Supernatants were collected from macrophages infected with M. mass R at various MOIs at 24 hr post infection. The level of cell lysis of each group was determined by LDH release assay. MR: M. mass R. Data are representative of three independent experiments. ns, non-significant; *, p<0.05; **, p<0.01; ***, p<0.001 by Student’s t-test (A) or one-way ANOVA with Bonferroni’s post-test (B-E).
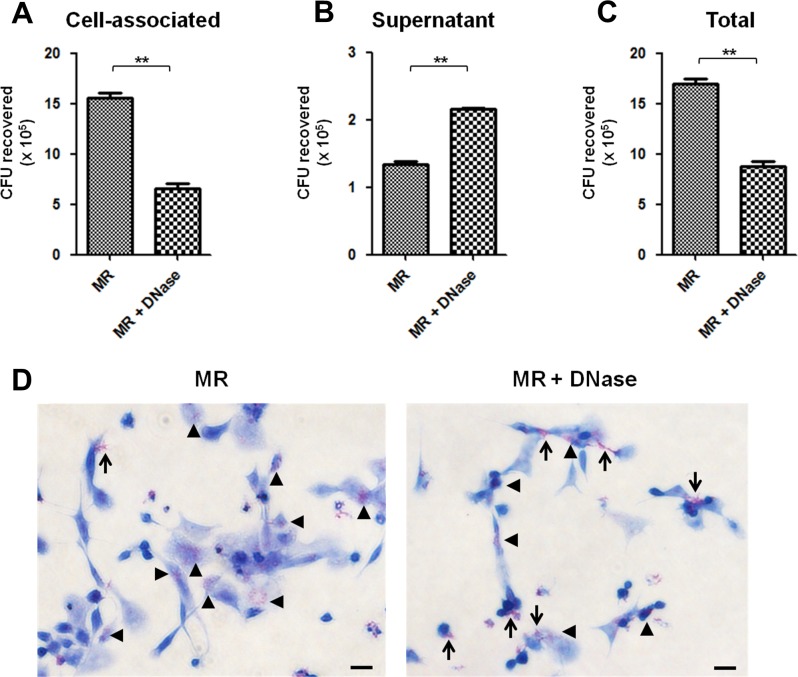
METs have no bactericidal effects on M. mass R
Extracellular traps released from neutrophils or other immune cells are known to be microbicidal by entrapping microbes [6, 8, 21]. To determine whether METs induced by M. mass R infection have anti-bacterial activity, we examined bacterial survival after treatment of infected macrophages with DNase to degrade METs. Interestingly, disintegration of METs by DNase caused a decrease in cell-associated M. mass R compared to untreated macrophages (Fig 6A). On the other hand, there was an increase in extracellular bacteria recovered from culture supernatant in DNase-treated samples (Fig 6B). These results suggest that METs have a role in entrapping microbes and thereby reduce extracellular CFUs in the supernatant, but may not have direct bactericidal activity on M. mass R. The collective amounts of M. mass R recovered from DNase-treated groups were significantly higher than those of untreated groups (Fig 6C). A previous study investigating METs induced by M. tuberculosis reported similar results that bacterial aggregation and growth increased as MET formation was enhanced [23]. To test whether M. mass R aggregation was induced by METs, we performed AFB stain on infected cells (Fig 6D). Indeed, we observed intracellular growth and aggregation of M. mass R were decreased in DNase-treated samples. Instead, extracellular bacteria attached to cell surface were detected more often in the same samples. These data indicate that METs have no bactericidal activity on M. mass R, but rather may increase MET-mediated interaction of bacteria with cell and facilitate stable aggregation of entrapped M. mass R.
Fig 6. Released METs have no bactericidal effects on M. mass R.
To determine the bactericidal effect of METs on M. mass R, macrophages with METs-degrading DNase I 30 min before infection. (A) Cell-associated (intracellular or METs-bound), (B) Extracellular, (C) Total number of bacteria recovered from the samples infected by M. mass R (MOI 5) for 24 hr, with or without DNase (50 units/ml). (D) AFB staining of THP-1 macrophages infected by M. mass R with or without DNase for 24 hr. Bar, 20μm. Data are representative of three independent experiments with similar results. **, p<0.01 by Student’s t-test.
Discussion
Macrophages are distributed through-out many body tissues and contribute to tissue homeostasis and immune defense against foreign agents. Upon infection by pathogens, macrophages generally perform the first innate immune responses, engulfing and killing pathogens, and evoking subsequent responses by other immune cells. Recent studies report that macrophages can generate extracellular trap structures consisting of DNA as a backbone with microbicidal proteins, and entrap extracellular bacteria, fungi, and parasites [22, 24, 25]. Similar to extracellular traps of other immune cells, METs are known to be induced by various stimuli, such as microbes, microbial products or cytokines [21, 37]. However, the characteristics and host defense mechanisms of METs remain to be elucidated.
Investigations on the interactions of macrophages with mycobacteria previously focused on intracellular responses after phagocytosis [38, 39]. However, M. tuberculosis was recently shown to induce extracellular trap formation by human PBMC-derived macrophages, providing evidence of a novel role of macrophages in extracellular immune defense against mycobacterial infection [23]. In this study, we showed that M. mass, a rapid growing virulent mycobacterium, also induces the formation of extracellular traps from PMA-differentiated THP-1 macrophages. Notably, M. mass R, a rough isolate of M. mass, significantly induced more MET release than a smooth isolate of M. mass (Fig 2). The main difference between the rough and smooth strains is the loss of glycopeptidolipid (GPL) in cell surface, caused by loss or mutation of the genes related to GPL biosynthesis [29]. Rough strains are known to be more virulent and associated with acute respiratory disease [40, 41, 42]. Because loss of GPL unmasks bioactive cell wall lipids, rough strains induce higher inflammatory reponses than smooth strains [43, 44]. This mechanism is considered to be associated with MET formation. Similarly, in a previous study, the cord-forming rough isolate of M. abscessus was found to mainly induce extracellular trap formation by human monocytes [30]. In addition, nonsonicated aggregates of M. mass R was shown to induce more MET formation comparing sonicated single bacteria. This response was also examined in human macrophages infected with M. tuberculosis [23]. Collectively, these results indicate colony type and bacterial structures modulate interaction with macrophages and extracellular trap formation.
Previous study using M. tuberculosis showed IFN-γ pretreatment of human macrophages readily induced rapid necrotic death and MET formation [23]. These responses were strictly dependent on ESX-1. However, our results showed that pretreatment of THP-1 macrophages with IFN-γ or TNF-α do not enhance MET formation. Because M. mass do not have ESX-1 secretion system, M. mass-induced MET formation may be affected by not pretreatment with proinflammatory cytokines but other mechanisms. In addition, chemical agents, such as PMA, LPS or hydrogen peroxide, did not stimulate MET release by human THP-1 macrophages (Fig 2), indicating that extracellular trap release from macrophages is closely associated with signal transductions mediated by specific microbes or antigens [21, 23, 24].
The formation of extracellular traps by neutrophils or other immune cells is known to mainly depend on NADPH oxidase [6, 33, 34]. However, our study revealed that the activity of NADPH oxidase did not affect M. mass-induced MET formation (Fig 4A). A previous study reported similar results that E. coli and Candida albicans induce MET production by murine macrophages in an NADPH oxidase-independent manner [24]. On the other hand, another study using bovine macrophages showed that Mannheimia haemolytica-induced MET formation is dependent on the activation of NADPH oxidase [21]. Collectively, these data indicate that the requirements for NADPH oxidase and therefore mechanisms for NET and MET extracellular trap formation differ depending on the stimulus [45].
Recent studies suggest that calcium influx is a major regulator of NOX-independent NETosis and that calcium activated NETosis is induced faster than NOX-dependent NETosis [35, 36]. M. mass infection indeed increased the levels of intracellular calcium and MET formation was partially dependent on calcium influx. These results seem to be consistent with M. tuberculosis, which caused calcium influx and extracellular trap formation in neutrophil by secretory antigen, ESAT-6-mediated cytolysis [46]. In addition, calcium-activated NET formation is known to be associated with mitochondrial ROS and activation of the MAPK pathway [35]. However, such mechanisms have yet to be confirmed in MET formation induced by bacterial infection. Further studies are needed to elucidate the molecular mechanism of calcium-mediated MET formation.
Neutrophils generally release extracellular traps toward extracellular microbes, such as Shigella flexneri and Staphylococus aureus, in a phagocytosis-independent manner [6]. However, a recent study reported M. tuberculosis-induced NET formation depends on phagocytosis [47]. In the study, M. tuberculosis-infected neutrophils after pretreatment with CytD closely resembled the uninfected control. Similarly, M. mass-induced MET formation was reduced by inhibition of phagocytosis with CytD, suggesting that phagocytosis of M. mass by macrophages is required for the production of extracellular traps. A previous study reported similar results that CytD-treated bovine macrophages showed a decrease in METs formation in response to M. haemolytica infection [21]. It was also shown that infection of human macrophages with high burden of M. tuberculosis is linked to more extracellular trap formation [23]. These results suggest that extracellular trap production by macrophages mainly depends on inflammatory responses following phagocytosis of M. mass.
NET-associated cell death, designated as NETosis, is a process that is morphologically and functionally distinct from apoptosis or necrosis [11, 13]. A previous study reported that neutrophils release nuclear DNA while maintaining membrane integrity and phagocytic responses without cell lysis [13]. However, unlike NETosis, necrotic cell death was observed during MET formation after phagocytosis of M. mass R. Similarly, M. tuberculosis also induced MET formation via their secretory system ESX-1-dependent macrophage necrosis [23]. However, although M. mass CIP induced macrophage cell death, infected cells showed low levels of MET formation. Collectively, these results reveal that M. mass-induced MET formation is accompanied by necrotic cell death, which is different from NETosis and may be affected by specific antigens related to bacterial strains [21, 23, 24].
Embedded histones and antimicrobial enzymes in NETs are known to have microbicidal effects on entrapped microbes [6, 48, 49]. However, a recent study reported that some microbes, such as Streptococcus pneumoniae or S. pyogenes, have mechanisms to evade entrapment and destruction by extracellular traps [50, 51]. In our study, METs released from macrophages did not show bactericidal activity on M. mass, but rather enhanced bacterial growth. Previous study investigating METs induced by C. albicans also report similar result [24]. In another study, survival and growth of M. tuberculosis were not inhibited by METs, and bacterial aggregation and burden increased as extracellular trap formation from human macrophages was enhanced by stimulation with IFN-γ [23]. We also found that M. mass growth was enhanced in macrophages with METs. A recent study reported macrophages are able to process extracellular trap via the phagocytosis route [52]. In this regard, we consider entrapping by METs may enhance phagocytosis of M. mass by macrophages and bacterial aggregation, which may facilitate mycobacterial survival and growth. However, the mechanism for evading the bactericidal action of METs and for enhancing mycobacteria survival is not yet fully understood.
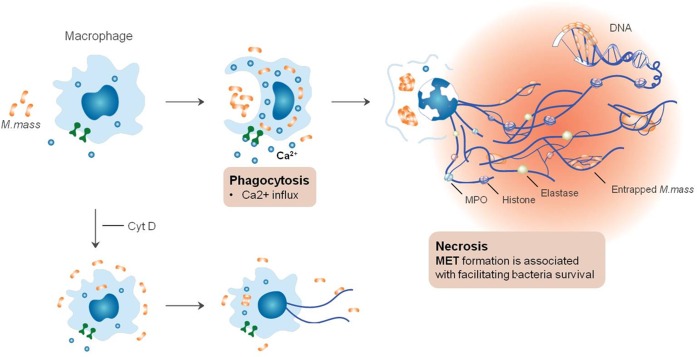
In conclusion, our study revealed that rapid growing virulent mycobacteria M. mass infection causes differentiated THP-1 macrophages to release extracellular DNA traps (Fig 7). M. mass-induced METs have the distinct characteristics compared to NETs. Phagocytosis of M. mass by macrophages induces calcium influx and intracellular bacterial growth. And increase in intracellular bacterial number is followed by macrophage cell necrosis and subsequent METs release. In addition, instead of killing entrapped bacteria, METs may enhance intracellular growth of M. mass. This response may be closely related to sequelae of extracellular traps, such as chronic inflammation or autoimmune diseases [53, 54, 55]. However, METs retain the primary functions of entrapping M. mass and preventing bacterial spread. Furthermore, considering the importance of adaptive immune responses against M. mass, METs may enhance immune responses, priming microbes to be targeted by other immune cells and evoking subsequent immune responses including T cell recruitment and activation [56, 57, 58]. Thus, our data suggest that METs have complex functions on host interactions with mycobacteria, and more studies are required for elucidating their functions in the context of various immune cells in vivo.
Fig 7. METs formation mediated by entry of M. mass into macrophage.
(A) Phagocytosis of M. mass by macrophages induces an increase in the level of intracellular calcium. Then, accompanying necrosis, METs are released and prevent bacterial dissemination by entrapping M. mass. The METs comprise of histones and antimicrobial enzymes (elastase and MPO) but rather facilitate bacterial survival and growth without bactericidal effects. (B) Treatment with cytochalasin D (Cyt D) prevents phagocytosis of M. mass by macrophage, thereby resulting in decreased METs production.
Supporting Information
THP-1 macrophages were infected with M. mass R (5 MOI). For intracellular infection, infected cells were washed after 3hr of infection to eliminate extracellular bacteria. Then, the samples were cultured for 1 day and MET formation was quantified. MR, M. mass R; MR-in, intracellular M. mass R. ns, non-significant; **, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
THP-1 macrophages were infected with sonicated single cells or nonsonicated aggregates of M. mass R or CIP (5 MOI) for 1 day, and MET formation was quantified. MR, M. mass R single cells; MR-Ag, M. mass R aggregates; CIP, M. mass CIP single cells; CIP-Ag, M. mass CIP aggregates. ns, non-significant; *, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
A) Proinflammatory cytokines (TNF-α, IL-1β and IL-6) secreted from THP-1 macrophages infected with M. mass R or CIP. B) THP-1 macrophages were stimulated by IFN-γ and TNF-α (10ng/ml) for 24hr, and infected with M. mass R or CIP (5 MOI). The METs formation was examined at 1 day post infection. MR, M. mass R; CIP, M. mass CIP. ns, non-significant; *, p<0.05; **, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
The quantification of ROS production in differentiated THP-1 macrophages stimulated with hydrogen peroxide or, M. mass with or without DPI by staining with ROS probe, H2DCF-DA. The mean fluorescence intensity (MFI) of the ROS probe was measured by flow cytometry analysis. The data are representative of three independent experiments. ns, non-significant by one-way ANOVA with Bonferroni’s post-test.
(TIF)
Differentiated THP-1 cells were untreated or pretreated with AAPV (0.1 or 1 μM) and infected with M. mass R (MOI 5) for 24 hrs. The MET formation was quantified as previously described in materials & methods. ns, non-significant; ***, p<0.001 compared to M. mass R-infected group by one-way ANOVA with Bonferroni’s post-test.
(TIF)
Flow cytometry analysis of (A) uninfected or (B) infected THP-1 macrophages with M. mass (MOI 5) by staining with Annexin V (AV) and Propidium iodide (PI) at 1 day post infection. The numbers indicate the percentages of cells in each quadrant (lower left: AV-/PI-, live cells; lower right: AV+/ PI-, apoptotic cells; upper left: AV-/ PI+, necrotic cells; upper right: AV+/ PI +, late apoptotic or secondary necrotic cells). The data are representative of three independent experiments.
(TIF)
(A) Cell lysis in THP-1 macrophages infected with M. mass CIP (MOI 10, 3, 1) at 2 days post infection. (B) MET formation in THP-1 macrophages infected with M. mass CIP for 2 days comparing with M. mass R-induced MET formation for 1 day. *, p<0.05; **, p<0.01; ***, p<0.001 by one-way ANOVA (A) or two-way ANOVA (B) with Bonferroni’s post-test.
(TIF)
Acknowledgments
The images in this research were generated using instruments and services at the Seoul National University College of Medicine.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning [NRF-2014R1A1A1008012] (http://www.msip.go.kr/web/main/main.do). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Griffith DE, Girard WM, Wallace RJ Jr. (1993) Clinical features of pulmonary disease caused by rapidly growing mycobacteria. Am Rev Respir Dis 147: 1271–1278. [DOI] [PubMed] [Google Scholar]
- 2.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee J, Zhang Y, et al. (2003) Nontuberculous mycobacteria: I: Multicenter prevalence study in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 167(6): 828–834. [DOI] [PubMed] [Google Scholar]
- 3.Esther CR, Esserman DA, Gilligan P, Kerr A, Noone PG. (2010) Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. Journal of Cystic Fibrosis 9(2): 117–123. 10.1016/j.jcf.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, et al. (2009) Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol 47(7): 1985–1995. 10.1128/JCM.01688-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh W, Jeon K, Lee NY, Kim B, Kook Y, Lee SH, et al. (2011) Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. American Journal of Respiratory and Critical Care Medicine 183(3): 405–410. 10.1164/rccm.201003-0395OC [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663): 1532–1535. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14(9): 949–953. 10.1038/nm.1855 [DOI] [PubMed] [Google Scholar]
- 8.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. (2008) Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111(6): 3070–3080. 10.1182/blood-2007-07-104018 [DOI] [PubMed] [Google Scholar]
- 9.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. (2006) Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8(4): 668–676. [DOI] [PubMed] [Google Scholar]
- 10.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5(10): e1000639 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. (2010) Neutrophil extracellular traps: How to generate and visualize them. J Vis Exp (36). pii: 1724. doi(36): 10.3791/1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remijsen Q, Kuijpers T, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. (2011) Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death & Differentiation 18(4): 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, et al. (2012) Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125(13): 1673–1683. 10.1161/CIRCULATIONAHA.111.046755 [DOI] [PubMed] [Google Scholar]
- 15.Rohrbach AS, Hemmers S, Arandjelovic S, Corr M, Mowen KA. (2012) PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther 14(3): R104 10.1186/ar3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann V, Zychlinsky A. (2012) Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol 198(5): 773–783. 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan MJ, Radic M. (2012) Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol 189(6): 2689–2695. 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. (2010) Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107(21): 9813–9818. 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One 7(2): e32366 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13(4): 463–469. [DOI] [PubMed] [Google Scholar]
- 21.Aulik NA, Hellenbrand KM, Czuprynski CJ. (2012) Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect Immun 80(5): 1923–1933. 10.1128/IAI.06120-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellenbrand KM, Forsythe KM, Rivera-Rivas JJ, Czuprynski CJ, Aulik NA. (2013) Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb Pathog 54: 67–75. 10.1016/j.micpath.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong KW, Jacobs WR Jr. (2013) Mycobacterium tuberculosis exploits human interferon gamma to stimulate macrophage extracellular trap formation and necrosis. J Infect Dis 208(1): 109–119. 10.1093/infdis/jit097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Wu X, Liao C, Liu X, Du J, Shi H, et al. (2014) Escherichia coli and Candida albicans induced macrophage extracellular trap-like structures with limited microbicidal activity. PLoS One 9(2): e90042 10.1371/journal.pone.0090042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonne-Année S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, Lok JB, et al. (2014) Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microb Infect 16(6): 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Kichik V, Mondragón-Flores R, Mondragón-Castelán M, Gonzalez-Pozos S, Muñiz-Hernandez S, Rojas-Espinosa O, et al. (2009) Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis 89(1): 29–37. 10.1016/j.tube.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Kim BJ, Yi S, Shim T, Do SY, Yu H, Park YG, et al. (2012) Discovery of a novel hsp65 genotype within Mycobacterium massiliense associated with the rough colony morphology. PloS One 7(6): e38420 10.1371/journal.pone.0038420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim BJ, Kim BR, Hong SH, Seok SH, Kook YH, Kim BJ. (2013) Complete genome sequence of Mycobacterium massiliense clinical strain asan 50594, belonging to the type II genotype. Genome Announc 1(4): 10.1128/genomeA.00429-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BJ, Kim BR, Lee SY, Kook YH, Kim BJ. (2013) Rough colony morphology of Mycobacterium massiliense type II genotype is due to the deletion of glycopeptidolipid locus within its genome. BMC Genomics 14: 890-2164-14-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jönsson BE, Bylund J, Johansson BR, Telemo E, Wold AE. (2013) Cord‐forming mycobacteria induce DNA meshwork formation by human peripheral blood mononuclear cells. Pathogens and Disease 67(1): 54–66. 10.1111/2049-632X.12007 [DOI] [PubMed] [Google Scholar]
- 31.Zawrotniak M, Rapala-Kozik M. (2013) Neutrophil extracellular traps (NETs)-formation and implications. Acta Biochim Pol 60(3): 277–284. [PubMed] [Google Scholar]
- 32.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon H. (2009) Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death & Differentiation 16(11): 1438–1444. [DOI] [PubMed] [Google Scholar]
- 33.Remijsen Q, Berghe TV, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, et al. (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21(2): 290–304. 10.1038/cr.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114(13): 2619–2622. 10.1182/blood-2009-05-221606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douda DN, Khan MA, Grasemann H, Palaniyar N. (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A 112(9): 2817–2822. 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta AK, Giaglis S, Hasler P, Hahn S. (2014) Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. Plos One 9(5): e97088 10.1371/journal.pone.0097088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohanan S, Horibata S, McElwee JL, Dannenberg AJ, Coonrod SA. (2013) Identification of macrophage extracellular trap-like structures in mammary gland adipose tissue: A preliminary study. Frontiers in Immunology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong JA, Hart PD. (1971) Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med 134(3 Pt 1): 713–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst JD. (1998) Macrophage receptors for Mycobacterium tuberculosis. Infect Immun 66(4): 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catherinot E, Clarissou J, Etienne G, Ripoll F, Emile JF, Perronne C, et al. (2007) Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect Immun 75(2): 1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cullen AR, Cannon CL, Mark EJ, Colin AA. (2000) Mycobacterium abscessus infection in cystic fibrosis: Colonization or infection? American Journal of Respiratory and Critical Care Medicine 161(2): 641–645. [DOI] [PubMed] [Google Scholar]
- 42.Jonsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. (2007) Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J Clin Microbiol 45(5): 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. (2009) Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J Immunol 183(3): 1997–2007. 10.4049/jimmunol.0802181 [DOI] [PubMed] [Google Scholar]
- 44.Davidson LB, Nessar R, Kempaiah P, Perkins DJ, Byrd TF. (2011) Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HbetaD2 gene expression and IL-8 release. PloS One 6(12): e29148 10.1371/journal.pone.0029148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. (2012) Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 92(4): 841–849. 10.1189/jlb.1211601 [DOI] [PubMed] [Google Scholar]
- 46.Francis R, Butler R, Stewart G. (2014) Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death & Disease 5(10): e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braian C, Hogea V, Stendahl O. (2013) Mycobacterium tuberculosis-induced neutrophil extracellular traps activate human macrophages. J Innate Immun 5(6): 591–602. 10.1159/000348676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, et al. (2010) NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microb Infect 12(12): 928–936. [DOI] [PubMed] [Google Scholar]
- 49.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, et al. (2011) Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: Evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 6(9): e23637 10.1371/journal.pone.0023637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. (2006) An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Current Biology 16(4): 401–407. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, et al. (2006) DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Current Biology 16(4): 396–400. [DOI] [PubMed] [Google Scholar]
- 52.Farrera C, Fadeel B. (2013) Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol 191(5): 2647–2656. 10.4049/jimmunol.1300436 [DOI] [PubMed] [Google Scholar]
- 53.Cheng OZ, Palaniyar N. (2013) NET balancing: A problem in inflammatory lung diseases. Frontiers in Immunology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. (2011) Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of Influenza pneumonitis. The American Journal of Pathology 179(1): 199–210. 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, et al. (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15(6): 623–625. 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rottman M, Catherinot E, Hochedez P, Emile JF, Casanova JL, Gaillard JL, et al. (2007) Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect Immun 75(12): 5898–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantovani A, Cassatella MA, Costantini C, Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology 11(8): 519–531. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 58.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. (2015) Inflammation. neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349(6245): 316–320. 10.1126/science.aaa8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
THP-1 macrophages were infected with M. mass R (5 MOI). For intracellular infection, infected cells were washed after 3hr of infection to eliminate extracellular bacteria. Then, the samples were cultured for 1 day and MET formation was quantified. MR, M. mass R; MR-in, intracellular M. mass R. ns, non-significant; **, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
THP-1 macrophages were infected with sonicated single cells or nonsonicated aggregates of M. mass R or CIP (5 MOI) for 1 day, and MET formation was quantified. MR, M. mass R single cells; MR-Ag, M. mass R aggregates; CIP, M. mass CIP single cells; CIP-Ag, M. mass CIP aggregates. ns, non-significant; *, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
A) Proinflammatory cytokines (TNF-α, IL-1β and IL-6) secreted from THP-1 macrophages infected with M. mass R or CIP. B) THP-1 macrophages were stimulated by IFN-γ and TNF-α (10ng/ml) for 24hr, and infected with M. mass R or CIP (5 MOI). The METs formation was examined at 1 day post infection. MR, M. mass R; CIP, M. mass CIP. ns, non-significant; *, p<0.05; **, p<0.01 by one-way ANOVA with Bonferroni’s post-test.
(TIF)
The quantification of ROS production in differentiated THP-1 macrophages stimulated with hydrogen peroxide or, M. mass with or without DPI by staining with ROS probe, H2DCF-DA. The mean fluorescence intensity (MFI) of the ROS probe was measured by flow cytometry analysis. The data are representative of three independent experiments. ns, non-significant by one-way ANOVA with Bonferroni’s post-test.
(TIF)
Differentiated THP-1 cells were untreated or pretreated with AAPV (0.1 or 1 μM) and infected with M. mass R (MOI 5) for 24 hrs. The MET formation was quantified as previously described in materials & methods. ns, non-significant; ***, p<0.001 compared to M. mass R-infected group by one-way ANOVA with Bonferroni’s post-test.
(TIF)
Flow cytometry analysis of (A) uninfected or (B) infected THP-1 macrophages with M. mass (MOI 5) by staining with Annexin V (AV) and Propidium iodide (PI) at 1 day post infection. The numbers indicate the percentages of cells in each quadrant (lower left: AV-/PI-, live cells; lower right: AV+/ PI-, apoptotic cells; upper left: AV-/ PI+, necrotic cells; upper right: AV+/ PI +, late apoptotic or secondary necrotic cells). The data are representative of three independent experiments.
(TIF)
(A) Cell lysis in THP-1 macrophages infected with M. mass CIP (MOI 10, 3, 1) at 2 days post infection. (B) MET formation in THP-1 macrophages infected with M. mass CIP for 2 days comparing with M. mass R-induced MET formation for 1 day. *, p<0.05; **, p<0.01; ***, p<0.001 by one-way ANOVA (A) or two-way ANOVA (B) with Bonferroni’s post-test.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.