Abstract
Background
Several metals have been reported to be associated with childhood asthma. However, the results on relationships between metals and risk of childhood asthma are inconclusive, and the research on adult asthma in the Chinese general population is rare.
Objectives
To investigate potential associations between levels of urinary metals and adult asthma.
Methods
A case-control study of 551 adult asthma cases and 551 gender- and age-matched controls was conducted in Wuhan, China. Demographic information was obtained, and lung function was assessed. The urinary concentrations of 22 metals were measured by inductively coupled plasma mass spectrometry.
Results
After adjusting for other metalsand other covariates, urinary cadmium, molybdenum, chromium, copper, uranium and selenium were positively associated with asthma, with odds ratios (95% CI) of 1.69 (1.00, 2.85), 3.76 (2.30, 6.16), 4.89 (3.04, 7.89), 6.06 (3.27, 11.21), 6.99 (4.37, 11.19) and 9.17 (4.16, 20.21), respectively. By contrast, urinary lead, barium, iron, zinc, nickel, manganese and rubidium were negatively associated with asthma, with odds ratios (95% CI) of 0.48 (0.29, 0.80), 0.44 (0.27, 0.71), 0.41 (0.26, 0.64), 0.40 (0.24, 0.66), 0.30 (0.22, 0.41), 0.23 (0.14, 0.39) and 0.07 (0.03, 0.15), respectively. When comparing urinary metals in different subgroups of cases with those in matched controls, the associations of above 13 metals with asthma prevalence were nearly the same.
Conclusions
Our results suggested that asthma prevalence in the Chinese adults was positively associated with urinary chromium, chromium, selenium, molybdenum, cadmium, and uranium, and negatively associated with urinary manganese, iron, nickel, zinc, rubidium, barium and lead. Additional research with larger populations in different regions is required to support our findings.
Introduction
Asthma, characterized by airway hyper-responsiveness (AHR) and recurrent episodes of airway obstruction and wheezing, is one of the most common chronic inflammatory lung diseases. In recent decades, the prevalence of asthma has dramatically increased, and it affects approximately 235 million individuals worldwide [1]. In China, approximately 30 million individuals suffered from asthma, and the prevalence was 1.24% in 2011[2].
Current research has indicated that the elevated prevalence of asthma is attributable partly to increased exposures to environmental and industrial agents [3]. Along with the process of industrialization and urbanization, humans are ineluctably exposed to metals present in air, water, food, and domestic materials. Absorbed metals accumulate in tissues and organs, with half-lives ranging from several months to decades. Some metals were reported to play an important role in the expressions of inflammatory cytokines or oxidant/antioxidant balance. Since inflammatory responses and oxidative stress were the possible pathogenesis of asthma, concerns have been raised as to whether certain metals were associated with asthma prevalence [4].
In the occupational setting, metal exposure was considered as one of the primary risk factors for occupational asthma [5]. Jaakkola et al. found that the metal work was the second strongest determinant of asthma among male-dominated occupations [6]. In a case-control study in Taiwan, Wang et al. found that nonatopic occupational asthma was significantly associated with exposure to metal sensitizers and fumes [7]. Mar Ferna´ndez-Nieto also reported that chromium (Cr) and nickel (Ni) fumes could give rise to occupational asthma in exposed workers [8]. However, in the general population, levels of metal exposures are lower than in the occupational population, and studies investigating the association of asthma with metals have yielded inconsistent results. For example, Carneiro reported that low concentrations of selenium (Se) were associated with asthma [9], but a cohort study in New Zealand found no association between Se levels and asthma [10]. In addition, Ulrike Gehring reported that high levels of zinc (Zn) and iron (Fe) in ambient particulate matter were positively associated with the increased risk of asthma and allergy in schoolchildren [11], while lower concentrations of Zn and Fe in blood were observed in asthmatics [12, 13]. Hence, the exact relationships between asthma and metal exposure require more research.
With rapid process of industrialization and urbanization, China is confronted with serious metal pollution problems. Over last 30 years, the domestic emissions of cadmium (Cd), Cr, and Ni from anthropogenic sources have tripled, and the average concentrations of atmospheric arsenic (As), manganese (Mn), Cr, Ni and Cd are all beyond the limits indicated by the WHO [14]. The prevalence of asthma is relatively higher in highly industrialized cities [15]. Several epidemiological studies have focused on the dose-response association of childhood asthma with exposure to metals in China [16]. But little was known about the association between adult asthma and exposure to metals in the Chinese adult population. Adult-onset asthma is largely under investigated and far from completely understood. Compared with childhood-onset asthma, adult-onset asthma is more likely among nonatopic females and features a greater fall in lung function [17]. Moreover, previous studies have reported that source and extend of exposure, gender, age, and geography could influence the exposure levels to metals [18–20]. The role of metals in adult-onset asthma should not be extrapolated from the studies in children.
As the main route of metal excretion, urine is the preferred non-invasive matrix for metal biomonitoring, especially for surveys where large numbers of participants are involved [18]. Urinary concentrations of metals could reflect long- and/or short-term exposure to metals from all sources besides air pollution [21]. Therefore, we used the levels of metals in urine as personal metal internal exposure. In the present study, we conducted a case-control study in Wuhan, which was an industrial city and had an increasing asthma prevalence [22]. The 22 metal elements (aluminum (Al), vanadium (V), Cr, Mn, Fe, cobalt (Co), Ni, Cu, Zn, As, Se, rubidium (Rb), strontium (Sr), molybdenum (Mo), Cd, tin (Sn), antimony (Sb), barium (Ba), tungsten (W), thallium (Tl), lead (Pb), uranium (U)), part of which were reported to play some biological or pathological roles in the development of cardiopulmonary disease [23], were simultaneously determined. The objective of this study was to explore the potential associations of adult asthma with 22 urinary metals. We also compare the levels of asthma related metals in different subgroups of cases with those in the matched controls. Moreover, we investigated the correlations between urinary metals and lung function.
Materials and Methods
Subjects
Asthma cases were enrolled from a general hospital in Wuhan, China, from October 2010 through January 2012. All subjects were older than 18 years and lived in the communities for more than 5 years. They were outpatients and diagnosed by the qualified physicians if they had symptoms such as episodic breathlessness, wheezing, cough, and chest tightness, and/or spirometry demonstrating an increase in forced expiratory volume in 1 s (FEV1) of at least 12% and at least 200 ml from the prebronchodilator value according to the Global Initiative for Asthma (GINA) guidelines [24]. Adult-onset asthma has been defined as from young as 16 years [25].We excluded subjects who had any known infection, heart failure, and complications of other diseases, such as hypertension, diabetes and cerebral vessel disease at the time of study. A total of 1009 eligible adults with asthma participated in this study and the urine samples of 582 subjects had been collected. Thirty one subjects were excluded for their inadequate urine sample volume for metals assays. Thus, 551 subjects with asthma were involved in final analyses, including 312 subjects newly diagnosed as asthmatics. All subjects were subdivided into two categories by severity of asthma (intermittent, persistent) due to that small amounts of subjects had moderate persistent asthma (n = 71) or severe persistent asthma (n = 15) [24]. The numbers of subjects with intermittent and persistent asthma were 310 (56.3%) and 231 (43.7%), respectively.
Throughout the study period, we recruited the non-asthmatic controls living in the same residential areas as asthma cases for more than 5 years. A total of 3053 community residents aged 18 to 80 years agreed to participate in the study, and health examinations for the controls were performed by the same physicians. We excluded 1094 controls from this study for the history of any known infection, lung diseases, heart failure, and complications of other diseases, such as hypertension, diabetes and cerebral vessel disease. In addition, 282 controls were excluded for inadequate urine samples for metal determination. Among the remaining eligible subjects, 551 controls were matched 1:1 with asthma cases for the age (±1 year) and gender.
The study was approved by the Ethnics and Human Subject Committees of Tongji Medical School at the Huazhong University of Science and Technology (2011–17). Written informed consents were obtained from all subjects.
Questionnaire, anthropometric measures and spirometry
Information regarding work history, educational level, occupational dust exposure, family history of asthma, tobacco smoking, physical activity, pet ownership and flower gardening was obtained by trained staff during face-to-face interviews using uniform questionnaires. For asthma cases, basic information before the diagnosis was collected. Educational level was defined as low (below senior high school), middle (senior high school to technical school), or high (college degree or higher). Family history of asthma was defined as whether the direct relatives had asthma. Work history information including job titles and beginning and ending times of jobs were collected. Occupational dust exposure was defined as self-report history of exposure to dust in the occupational setting. Individuals who had smoked more than one cigarette per day over the previous 6 months were considered as current smokers. Individuals who had drunk alcohol beverage more than once a week over the previous 6 months were considered as current drinkers. Physical activity was defined as regular exercise of more than 30 minutes in each session, at least once a week during previous 6 months.
Standing height and weight were measured without shoes before physical examinations. The body mass index (BMI) was calculated by dividing weight (kilogram) by the square of height (meter).
All subjects underwent spirometry with portable spirometers (HI-101, Chestgraphy, Japan) in sitting position with a nose clip according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [26]. The spirometers were calibrated every morning before assessments according to the manufacturer’s instruction. Each subject was required to perform three satisfactory blows and the data of the best one of 3 measurements were used for analyses. The results were presented as expiratory volume (L) and percentage of the predicted values of individuals with similar characteristics (gender, age and height). FEV1 and forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio were obtained.
Determination of urinary metals and creatinine
On the morning of recruitment days, spot urine samples were collected, divided into aliquots, and subsequently stored in polyethylene tubes at -20°C until further processing. We determined concentrations of 22 metals using inductively coupled plasma mass spectrometry (ICP-MS) with an Agilent 7700x instrument (Agilent Technologies, Waldbronn, Germany). We followed the previously reported method with minor modifications [27]. Standard reference material (SRM) 2670a (Toxic Elements in Urine) containing low and high concentration levels and 1640a (Trace Elements in Natural Water) purchased from NIST (National Institute of Standards and Technology, Gaithersburg, Maryland, USA) were used for quality control. Accuracy was estimated by comparing the difference between the certified values available and the measured values with their uncertainty according to the calculation method reported elsewhere [28]. The results measured by this method were in line with the SRM 2670a certified values. SRM 1640a was used to monitor the accuracy of procedure for each analytic batch. The mean of three replicate measurements for every metal was reported. The limits of quantification (LOQ) for 22 metals ranged from 0.0004 to 0.3934 μg/L. For metal concentrations below the LOQ, we assigned half the LOQ for the calculation. We measured urinary creatinine concentrations using a fully automated clinical chemistry analyzer (Mindray Medical International Ltd., Shenzhen, China). The concentrations of metals were adjusted by creatinine and were presented as micrograms per gram of creatinine.
Statistical analysis
Chi-square test or Student’s t-test was used to compare basic characteristics between asthma cases and controls as appropriate. We compared the urinary concentrations of the 22 metals between the two groups using a Wilcoxon signed-rank test. The correlations among the 22 metals were analyzed by using Spearman’s rank correlation analysis. To improve the normalization of concentrations of metals, we transformed them by natural logarithm transformation [29]. We used conditional logistic regression models to analyze relationships between individual metals and asthma after adjusting for asthma-related factors such as educational level, occupational dust exposure, family history of asthma, tobacco smoking, pet ownership, flower gardening, physical activity and BMI. Due to multicollinearity, we subsequently constructed logistic regression models including all metals related to asthma in single-metal models and the above confounders and used a backward elimination procedure to retain the metals that predicted the outcome at p<0.05. The correlation analyses between urinary concentrations of asthma-related metals in multiple-metal models and lung function were also performed by the partial Spearman’s correlation analysis with adjustment for age, gender, occupational dust exposure, family history of asthma, tobacco smoking, physical activity, and BMI. In addition, we used the false discovery rate (FDR) to adjust p values for multiple testing [30]. The level of significance was set at p<0.05. We performed statistical analyses using SAS software (version 9.1; SAS Institute Inc., USA).
Results
General characteristics
A total of 551 asthma cases and 551 matched non-asthmatic controls were included in the analysis. The participants aged ranged from 18 to 78 years, and mean age was 42.41±12.80 years for the asthma cases and 42.74±12.56 years for controls. The age at diagnosis of asthma for cases varied from 16 to 72 years. Table 1 presents the characteristics, lifestyle and clinical features of cases and controls. Compared with controls, the percentage of asthma cases with occupational dust exposure or family history of asthma was significantly higher (p<0.05, p = 0.0001, respectively). The distributions of tobacco smoking were significantly different between the two groups (p = 0.0001). Pet ownership was more prevalent among the asthma cases than among the controls (p<0.0011). The mean FEV1% (88.07±13.76) and FEV1/FVC ratio (87.90±8.80) for the controls were significantly higher than those for asthma cases (82.85±24.00 and 71.40±14.09, respectively) (both p<0.0001). The mean BMI of the asthma cases (23.13±4.21) was lower than that of the controls (24.00±3.66) (p = 0.0002).
Table 1. Demographic characteristics, lifestyle and clinical features of asthma cases and controls.
| Characteristic | Control (n = 551) | Case (n = 551) | p-valuea |
|---|---|---|---|
| Male, n (%) | 237 (43.01) | 237 (43.01) | |
| Age (years, Mean±SD) | 42.74±12.56 | 42.41±12.80 | 0.3769 |
| Educational level, n (%) | 0.0005 | ||
| Low | 260 (47.19) | 308 (55.90) | |
| Middle | 197 (35.75) | 138 (25.05) | |
| High | 94 (17.06) | 105 (19.06) | |
| Exposure to dust, n (%) | 69 (12.52) | 96 (17.42) | 0.0224 |
| Family history of asthma, n (%) | 11 (2.00) | 62 (11.25) | 0.0001 |
| Tobacco smoking, n (%) | <0.0001 | ||
| Nonsmokers | 392 (71.14) | 434(78.77) | |
| Former smokers | 27 (4.90) | 42(7.62) | |
| Current smokers | 132 (23.96) | 75(13.61) | |
| Physical activity, n (%) | 188 (34.12) | 126 (22.87) | 0.0001 |
| Pet ownership, n (%) | 56 (10.16) | 93 (16.88) | 0.0011 |
| Flower gardening, n (%) | 169 (30.67) | 108 (19.60) | <0.0001 |
| BMI level, n (%) | <0.0001 | ||
| Underweight, <18.5 kg/m2 | 22 (3.99) | 48 (8.71) | |
| Normal weight, 18.5–24 kg/m2 | 267 (48.46) | 309 (56.08) | |
| Overweight, 24–28 kg/m2 | 198 (35.93) | 151 (27.40) | |
| Obese, ≥28 kg/m2 | 64 (11.62) | 43 (7.80) | |
| BMI (kg/m2, Mean±SD) | 24.00±3.66 | 23.13±4.21 | 0.0002 |
| Spirometric indices (Mean±SD) | |||
| FEV1 (L) | 2.54±0.64 | 2.33±0.84 | <0.0001 |
| FEV1(% predicted) | 88.07±13.76 | 82.85±24.00 | <0.0001 |
| FEV1/FVC (%) | 87.90±8.80 | 71.40±14.09 | <0.0001 |
Abbreviation: SD, standard deviation; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
aStudent’s t-test for continuous variables and Chi-square test for categorical variables.
Urinary metal concentrations
Table 2 compares the creatinine-adjusted urinary metal concentrations (μg/g creatinine) between asthma cases and controls. The urinary concentrations of Sn, Ni, V, Pb and W were < LOQ in 26.50%, 15.97%, 8.35%, 5.17% and 2.09% of the samples, respectively. Less than 1% of the samples were < LOQ for Cr, Mn, Fe, Co, Cu, Sb and U. The geometric mean urinary concentrations of eleven metals (Cr, Cu, As, Se, Sr, Mo, Cd, Sn, Sb, W and U) were significantly higher in the asthma cases than in the controls (p<0.01). In contrast, the geometric mean urinary concentrations of ten metals (Al, V, Mn, Fe, Ni, Zn, Rb, Ba, Tl and Pb) were significantly lower in the asthma cases (p<0.05). We observed no significant differences in urinary Co levels between the two groups (p = 0.872).
Table 2. Comparison of metal concentrations (μg/g creatinine) in urine [geometric means (25th, 75th percentiles)] between asthma cases and controls.
| Metal | Control (n = 551) | Case (n = 551) | p-value | n (%)<LOQ | Reference valuesa |
|---|---|---|---|---|---|
| Al | 21.54 (12.53, 33.91) | 14.73 (7.55, 25.10) | <0.0001 | 0(0.00) | |
| V | 0.33 (0.20, 0.52) | 0.27 (0.20, 0.97) | <0.0001 | 92(8.35) | |
| Cr | 0.94 (0.53, 1.52) | 1.52 (0.81, 2.77) | <0.0001 | 1(0.09) | |
| Mn | 1.48 (0.88, 2.42) | 0.58 (0.27, 1.21) | <0.0001 | 7(0.64) | |
| Fe | 47.94 (25.66, 85.24) | 14.15 (6.99, 26.12) | <0.0001 | 5(0.45) | |
| Co | 0.18 (0.10, 0.34) | 0.15 (0.10, 0.31) | 0.8720 | 2(0.18) | 0.29 (0.26, 0.31) |
| Ni | 1.45 (0.87, 2.37) | 0.38 (0.10, 1.31) | <0.0001 | 176(15.97) | |
| Cu | 4.71 (3.13, 6.27) | 5.81 (3.69, 8.35) | <0.0001 | 0(0.00) | |
| Zn | 165.67 (118.40, 248.60) | 115.58 (73.46, 180.60) | <0.0001 | 0(0.00) | |
| As | 16.44 (11.18, 22.87) | 19.11 (12.79, 24.90) | <0.0001 | 0(0.00) | 8.30 (7.19, 9.57) |
| Se | 4.81 (3.34, 6.75) | 7.69 (5.58, 10.67) | <0.0001 | 0(0.00) | |
| Rb | 1187.97 (853.76, 1747.47) | 1074.92 (775.47, 1501.06) | 0.0002 | 0(0.00) | |
| Sr | 77.48 (51.74, 122.10) | 93.69 (56.19, 153.2) | <0.0001 | 0(0.00) | |
| Mo | 27.11 (18.01, 40.89) | 42.95 (26.51, 66.03) | <0.0001 | 0(0.00) | 35.90 (34.00, 38.00) |
| Cd | 0.49 (0.31, 0.76) | 0.62 (0.40, 0.90) | <0.0001 | 0(0.00) | 0.26 (0.24, 0.28) |
| Sn | 0.17 (0.11, 0.27) | 0.20 (0.12, 0.33) | 0.0018 | 292(26.50) | |
| Sb | 0.10 (0.07, 0.14) | 0.11 (0.07, 0.17) | 0.0026 | 1(0.09) | |
| Ba | 2.56 (1.49, 4.13) | 1.82 (0.95, 3.07) | <0.0001 | 0(0.00) | 1.34 (1.19, 1.56) |
| W | 0.08 (0.05, 0.14) | 0.11 (0.06, 0.19) | <0.0001 | 23(2.09) | |
| Tl | 0.36 (0.25, 0.55) | 0.32 (0.21, 0.48) | <0.0001 | 0(0.00) | 0.15 (0.13, 0.16) |
| Pb | 1.93 (1.25, 3.01) | 1.39 (0.90, 2.05) | <0.0001 | 57(5.17) | 0.63 (0.58, 0.67) |
| U | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.04) | 0.0019 | 5(0.45) |
Abbreviation: LOQ, limits of quantification.
aThe values were from The Fourth National Report on Human Exposure to Environmental Chemicals (U.S. 2009) for 20 years and older.
Correlation analysis showed that the 22 metals were significantly correlated with one another except for Mn vs. Se (p = 0.34), Mn vs. Mo (p = 0.53), Fe vs. Mo (p = 0.39), Fe vs. W (p = 0.23) and Ni vs. Mo (p = 0.08) (Table 3).
Table 3. Correlation coefficients of urinary metals (μg/g creatinine) in the entire study population.a.
| Al | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Rb | Sr | Mo | Cd | Sn | Sb | Ba | W | Tl | Pb | U | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1.00 | |||||||||||||||||||||
| V | 0.43 | 1.00 | ||||||||||||||||||||
| Cr | 0.43 | 0.69 | 1.00 | |||||||||||||||||||
| Mn | 0.73 | 0.12 | 0.35 | 1.00 | ||||||||||||||||||
| Fe | 0.57 | 0.07 | 0.21 | 0.73 | 1.00 | |||||||||||||||||
| Co | 0.38 | 0.31 | 0.37 | 0.39 | 0.33 | 1.00 | ||||||||||||||||
| Ni | 0.47 | 0.27 | 0.13 | 0.46 | 0.48 | 0.47 | 1.00 | |||||||||||||||
| Cu | 0.46 | 0.34 | 0.64 | 0.48 | 0.35 | 0.41 | 0.26 | 1.00 | ||||||||||||||
| Zn | 0.38 | 0.09 | 0.14 | 0.46 | 0.46 | 0.23 | 0.41 | 0.42 | 1.00 | |||||||||||||
| As | 0.14 | 0.30 | 0.21 | 0.09 | 0.09 | 0.31 | 0.11 | 0.24 | 0.22 | 1.00 | ||||||||||||
| Se | 0.13 | 0.38 | 0.53 | 0.03 | -0.06 | 0.31 | -0.1 | 0.39 | 0.14 | 0.53 | 1.00 | |||||||||||
| Rb | 0.22 | 0.22 | 0.21 | 0.26 | 0.23 | 0.31 | 0.16 | 0.25 | 0.18 | 0.47 | 0.41 | 1.00 | ||||||||||
| Sr | 0.32 | 0.36 | 0.38 | 0.23 | 0.17 | 0.38 | 0.16 | 0.33 | 0.24 | 0.37 | 0.39 | 0.16 | 1.00 | |||||||||
| Mo | 0.08 | 0.26 | 0.25 | 0.02 | -0.03 | 0.34 | 0.05 | 0.28 | 0.11 | 0.52 | 0.47 | 0.28 | 0.38 | 1.00 | ||||||||
| Cd | 0.21 | 0.20 | 0.23 | 0.13 | 0.09 | 0.41 | 0.14 | 0.39 | 0.23 | 0.48 | 0.45 | 0.43 | 0.34 | 0.43 | 1.00 | |||||||
| Sn | 0.39 | 0.23 | 0.27 | 0.28 | 0.22 | 0.30 | 0.18 | 0.32 | 0.27 | 0.28 | 0.34 | 0.29 | 0.38 | 0.30 | 0.37 | 1.00 | ||||||
| Sb | 0.44 | 0.31 | 0.29 | 0.33 | 0.24 | 0.36 | 0.25 | 0.44 | 0.32 | 0.42 | 0.35 | 0.37 | 0.46 | 0.42 | 0.46 | 0.55 | 1.00 | |||||
| Ba | 0.62 | 0.28 | 0.34 | 0.58 | 0.47 | 0.34 | 0.38 | 0.39 | 0.35 | 0.15 | 0.08 | 0.13 | 0.63 | 0.11 | 0.22 | 0.39 | 0.50 | 1.00 | ||||
| W | 0.23 | 0.27 | 0.18 | 0.09 | 0.04 | 0.25 | 0.14 | 0.19 | 0.14 | 0.32 | 0.30 | 0.19 | 0.36 | 0.40 | 0.26 | 0.39 | 0.53 | 0.27 | 1.00 | |||
| Tl | 0.32 | 0.24 | 0.27 | 0.33 | 0.29 | 0.39 | 0.21 | 0.29 | 0.22 | 0.38 | 0.32 | 0.75 | 0.33 | 0.28 | 0.40 | 0.36 | 0.40 | 0.33 | 0.22 | 1.00 | ||
| Pb | 0.54 | 0.25 | 0.30 | 0.54 | 0.47 | 0.35 | 0.37 | 0.35 | 0.42 | 0.25 | 0.20 | 0.35 | 0.47 | 0.21 | 0.33 | 0.46 | 0.55 | 0.61 | 0.32 | 0.49 | 1.00 | |
| U | 0.41 | 0.18 | 0.14 | 0.27 | 0.18 | 0.21 | 0.20 | 0.33 | 0.25 | 0.23 | 0.10 | 0.22 | 0.37 | 0.28 | 0.34 | 0.48 | 0.71 | 0.49 | 0.46 | 0.31 | 0.45 | 1.00 |
a p<0.05 for the correlations between all the metals, except for Mn vs. Se (p = 0.34), Mn vs. Mo (p = 0.53), Fe vs. Mo (p = 0.39), Fe vs. W (p = 0.23) and Ni vs. Mo (p = 0.08).
Associations between urinary metals and asthma
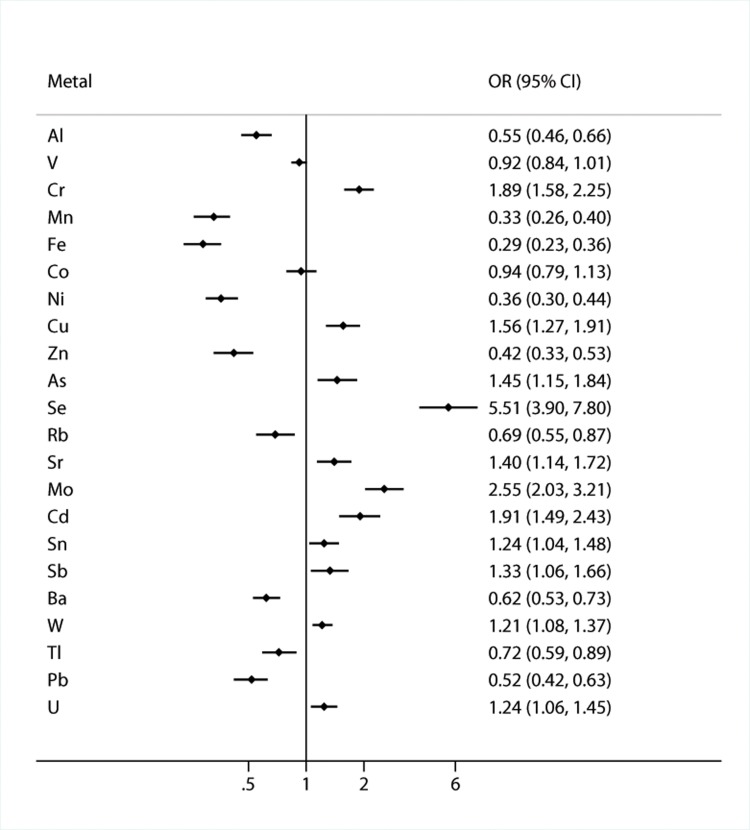
When considered as continuous variables, 20 metals were associated with asthma (Fig 1). The elevated urinary levels of eleven metals (Cr, Cu, As, Se, Sr, Mo, Cd, Sn, Sb, W and U) were significantly positively associated with the prevalence of asthma, with odds ratios (ORs) ranging from 1.21 to 5.51. Furthermore, we observed significantly negative associations between nine metals (Al, Mn, Fe, Ni, Zn, Rb, Ba, Tl and Pb) and asthma, with ORs ranging from 0.29 to 0.72. We found no associations of V and Co with asthma. When metal concentrations were divided into quartiles, the associations between the above mentioned 20 metals and asthma were unchanged, except that for Sn (Table 4). Compared with the lowest quartiles, the ORs (95% confidential interval (CI)) for the highest quartiles of urinary Se and Mo were 11.40 (6.82, 19.05) and 5.67 (3.63, 8.85), respectively. The ORs of the highest quartiles of urinary Mn, Fe and Ni were less than 0.10 when compared with the lowest quartiles. In all, through single-metal models, we observed that 19 metals were significantly associated with asthma. We got similar results when analyzing the associations of asthma with single metal element by excluding the subjects whose concentration of the metal element with metal levels was below the limit of detection.
Fig 1. Adjusted odds ratio and 95% confidential interval for asthma by considering urinary concentrations of metals as continuous variables.
The models were adjusted for educational level, occupational dust exposure, family history of asthma, tobacco smoking, pet ownership, flower gardening, physical activity, and body mass index.
Table 4. Adjusted odds ratio and 95% confidential interval for asthma by considering urinary concentrations of metals as categorical variables.
| Metal | quartile 1 | quartile 2 | quartile 3 | quartile 4 | p-value for trend | p-value for trend a |
|---|---|---|---|---|---|---|
| Al | 1 (ref) | 0.41 (0.27, 0.64) | 0.24 (0.16, 0.38) | 0.23 (0.15, 0.35) | <0.0001 | <0.0001 |
| V | 1 (ref) | 0.35 (0.23, 0.54) | 0.92 (0.62, 1.38) | 2.64 (1.72, 4.05) | <0.0001 | <0.0001 |
| Cr | 1 (ref) | 1.52 (1.02, 2.28) | 2.08 (1.39, 3.13) | 4.60 (2.98, 7.09) | <0.0001 | <0.0001 |
| Mn | 1 (ref) | 0.11 (0.06, 0.22) | 0.04 (0.02, 0.08) | 0.03 (0.02, 0.06) | <0.0001 | <0.0001 |
| Fe | 1 (ref) | 0.10 (0.05, 0.21) | 0.03 (0.01, 0.06) | 0.02 (0.01, 0.04) | <0.0001 | <0.0001 |
| Co | 1 (ref) | 1.18 (0.79, 1.77) | 1.20 (0.79, 1.84) | 0.90 (0.57, 1.42) | 0.6564 | 0.6564 |
| Ni | 1 (ref) | 0.04 (0.02, 0.08) | 0.03 (0.01, 0.06) | 0.02 (0.01, 0.05) | <0.0001 | <0.0001 |
| Cu | 1 (ref) | 1.27 (0.85, 1.92) | 1.49 (1.00, 2.23) | 2.17 (1.47, 3.19) | <0.0001 | <0.0001 |
| Zn | 1 (ref) | 0.45 (0.29, 0.69) | 0.21 (0.14, 0.33) | 0.19 (0.12, 0.29) | <0.0001 | <0.0001 |
| As | 1 (ref) | 1.61 (1.09, 2.38) | 1.61 (1.10, 2.38) | 1.78 (1.19, 2.65) | 0.0072 | 0.0088 |
| Se | 1 (ref) | 1.93 (1.24, 3.00) | 4.27 (2.70, 6.74) | 11.40 (6.82, 19.05) | <0.0001 | <0.0001 |
| Rb | 1 (ref) | 0.90 (0.62, 1.33) | 0.65 (0.44, 0.96) | 0.49 (0.33, 0.73) | 0.0002 | 0.0003 |
| Sr | 1 (ref) | 1.00 (0.67, 1.48) | 1.12 (0.75, 1.66) | 1.85 (1.23, 2.77) | 0.0022 | 0.0028 |
| Mo | 1 (ref) | 1.53 (1.02, 2.30) | 2.65 (1.76, 3.99) | 5.67 (3.63, 8.85) | <0.0001 | <0.0001 |
| Cd | 1 (ref) | 2.06 (1.37, 3.11) | 2.42 (1.56, 3.75) | 3.05 (1.94, 4.81) | <0.0001 | <0.0001 |
| Sn | 1 (ref) | 1.04 (0.70, 1.55) | 1.15 (0.77, 1.72) | 1.39 (0.94, 2.05) | 0.0831 | 0.0871 |
| Sb | 1 (ref) | 0.65 (0.43, 0.98) | 1.06 (0.71, 1.59) | 1.40 (0.94, 2.08) | 0.0144 | 0.0167 |
| Ba | 1 (ref) | 0.51 (0.34, 0.76) | 0.40 (0.27, 0.61) | 0.27 (0.18, 0.41) | <0.0001 | <0.0001 |
| W | 1 (ref) | 1.33 (0.90, 1.97) | 2.14 (1.43, 3.18) | 2.46 (1.65, 3.67) | <0.0001 | <0.0001 |
| Tl | 1 (ref) | 1.01 (0.68, 1.48) | 0.70 (0.47, 1.05) | 0.55 (0.37, 0.80) | 0.0004 | 0.0006 |
| Pb | 1 (ref) | 0.58 (0.38, 0.89) | 0.35 (0.22, 0.54) | 0.19 (0.12, 0.29) | <0.0001 | <0.0001 |
| U | 1 (ref) | 0.68 (0.46, 1.02) | 0.77 (0.52, 1.14) | 1.49 (1.00, 2.21) | 0.0237 | 0.0261 |
The model was adjusted by educational level, occupational dust exposure, family history of asthma, tobacco smoking, pet ownership, flower gardening, physical activity, and body mass index.
a p-value was adjusted by false discovery rate (FDR) for multiple testing.
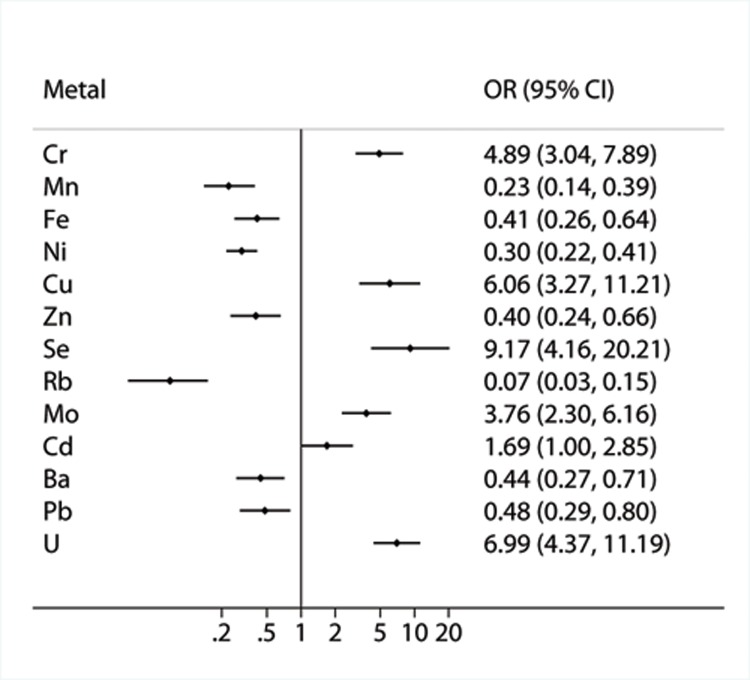
We further constructed multiple-metal model analyses including the above 19 metals using backward elimination (Fig 2). Six metals (Cd, Mo, Cr, Cu, U and Se) were significantly positively associated with the prevalence of asthma (OR (95%CI): 1.69 (1.00, 2.85) for Cd; 3.76 (2.30, 6.16) for Mo; 4.89 (3.04, 7.89) for Cr; 6.06 (3.27, 11.21) for Cu; 6.99 (4.37, 11.19) for U; 9.17 (4.16, 20.21) for Se). In contrast, elevated levels of seven metals (Pb, Ba, Fe, Zn, Ni, Mn and Rb) in urine were significantly negatively associated with the prevalence of asthma (OR (95%CI): 0.48 (0.29, 0.80) for Pb; 0.44 (0.27, 0.71) for Ba; 0.41(0.26, 0.64) for Fe; 0.40 (0.24, 0.66) for Zn; 0.30 (0.22, 0.41) for Ni; 0.23 (0.14, 0.39) for Mn; 0.07 (0.03, 0.15) for Rb).
Fig 2. Associations between urinary metals and asthma based on the multiple-metal models.
Metals were selected among 19 asthma-related metals from single-metal models by backward elimination in the multivariate logistic regression model (alpha = 0.05) with adjustment for age, gender, tobacco smoking, educational level, occupational dust exposure, family history of asthma, pet ownership, flower gardening, physical activity and body mass index. Abbreviation: OR: odds ratio; CI: confidential interval.
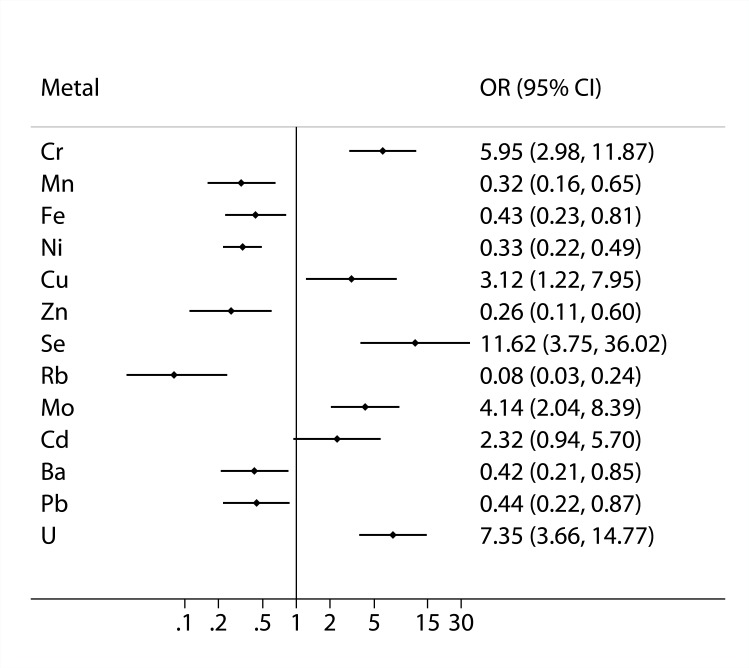
When we performed the same analyses in the newly diagnosed asthma and the matched controls, we found that asthma prevalence was positively associated with urinary Cr, Cu, Se, Mo, Cd and U, and negatively associated with urinary Mn, Fe, Ni, Zn, Rb, Ba and Pb, in the multiple-metal models (Fig 3). When we divided asthma patients by severity (intermittent and persistent) and compared their urinary metals with those in the matched controls (Table 5), we observed similar associations of asthma with most of 13 metals. However, no significant associations were observed between 3 metals (Cd, Ba and Pb) and asthma in the group of cases with intermittent asthma and matched controls (Cd: p = 0.1976; Ba: p = 0.0549; Pb: p = 0.2394). Likewise, we didn’t find association between Fe and asthma in the group of cases with persistent asthma and matched controls (p = 0.8825).
Fig 3. Associations between 13 metals in urine and asthma prevalence in the newly diagnosed asthma and matched controls based on the multiple-metal model.
The model was adjusted for age, gender, tobacco smoking, educational level, occupational dust exposure, family history of asthma, pet ownership, flower gardening, physical activity and body mass index. Abbreviation: OR: odds ratio; CI: confidential interval.
Table 5. Associations between asthma and 13 selected urinary metals in different subgroups on the multiple-metal models.
| Metal | Intermittent asthma and matched control | Persistent asthma and matched control | ||
|---|---|---|---|---|
| OR(95%CI) | p-value | OR(95%CI) | p-value | |
| Cr | 6.28 (2.61, 15.12) | <0.0001 | 5.34 (2.60, 10.93) | <0.0001 |
| Mn | 0.40 (0.18, 0.89) | 0.0247 | 0.11 (0.05, 0.25) | <0.0001 |
| Fe | 0.14 (0.06, 0.33) | <0.0001 | 0.95 (0.48, 1.89) | 0.8825 |
| Ni | 0.26 (0.15, 0.43) | <0.0001 | 0.25 (0.15, 0.43) | <0.0001 |
| Cu | 9.52 (3.34, 27.16) | <0.0001 | 4.55 (1.59, 13.01) | 0.0048 |
| Zn | 0.16 (0.06, 0.41) | 0.0001 | 0.53 (0.24, 1.15) | 0.1090 |
| Se | 19.28 (4.56, 81.52) | <0.0001 | 6.72 (2.10, 21.51) | 0.0013 |
| Rb | 0.06 (0.02, 0.24) | <0.0001 | 0.06 (0.02, 0.18) | <0.0001 |
| Mo | 5.98 (2.44, 14.68) | <0.0001 | 3.59 (1.68, 7.68) | 0.0010 |
| Cd | 1.82 (0.73, 4.54) | 0.1976 | 2.88 (1.05, 7.89) | 0.0394 |
| Ba | 0.46 (0.21, 1.02) | 0.0549 | 0.43 (0.21, 0.87) | 0.0190 |
| Pb | 0.58 (0.23, 1.44) | 0.2394 | 0.38 (0.17, 0.84) | 0.0159 |
| U | 6.56 (3.27, 13.17) | <0.0001 | 8.72 (4.11, 18.48) | <0.0001 |
Abbreviation: OR: odds ratio; CI: confidential interval.
The model was adjusted by educational level, occupational dust exposure, family history of asthma, tobacco smoking, pet ownership, flower gardening, physical activity, and body mass index.
Correlations between urinary metals and lung function
We further investigated the correlations between concentrations of 13 asthma-related metals and lung function with adjustment for age, gender, occupational dust exposure, family history of asthma, tobacco smoking, physical activity, and BMI (Table 6). We observed the significant positive correlations between FEV1 and 3 urinary metals (Mn, Fe and Ni), and significant negative correlation between FEV1 and Cd. Additionally, the ratio of FEV1/FVC was positively correlated with 7 urinary metals (Mn, Fe, Ni, Zn, Rb, Ba and Pb), and negatively correlated with 5 urinary metals (Cr, Cu, Se, Mo and Cd).
Table 6. Correlations between 13 selected urinary metals and lung function.
| Metal | FEV1(L) | FEV1/FVC (%) | ||
|---|---|---|---|---|
| r (95%CI) | p-value | r (95%CI) | p-value | |
| Cr | 0.01(-0.05, 0.07) | 0.7839 | -0.13(-0.18, -0.06) | <0.0001 |
| Mn | 0.07(0.01, 0.14) | 0.0165 | 0.26(0.20, 0.31) | <0.0001 |
| Fe | 0.08(0.02, 0.14) | 0.0095 | 0.31(0.26, 0.37) | <0.0001 |
| Ni | 0.08(0.02, 0.14) | 0.0146 | 0.25(0.19, 0.31) | <0.0001 |
| Cu | 0.01(-0.05, 0.07) | 0.714 | -0.09(-0.15, -0.03) | 0.0055 |
| Zn | 0.05(-0.01, 0.11) | 0.1244 | 0.16(0.10, 0.22) | <0.0001 |
| Se | -0.06(-0.12, 0.01) | 0.0644 | -0.18(-0.24, -0.12) | <0.0001 |
| Rb | -0.01(-0.07, 0.05) | 0.6767 | 0.12(0.06, 0.18) | 0.0002 |
| Mo | -0.04(-0.10, 0.03) | 0.2564 | -0.19(-0.25, -0.13) | <0.0001 |
| Cd | -0.10(-0.16, -0.03) | 0.0022 | -0.12(-0.18, -0.06) | <0.0001 |
| Ba | 0.03(-0.03, 0.09) | 0.2803 | 0.12(0.06, 0.18) | <0.0001 |
| Pb | -0.01(-0.07, 0.05) | 0.7126 | 0.16(0.10, 0.22) | <0.0001 |
| U | 0.01(-0.05, 0.07) | 0.7213 | -0.06(-0.12, 0.01) | 0.0681 |
Abbreviation: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Adjusted for age, gender, occupational dust exposure, family history of asthma, tobacco smoking, physical activity, and body mass index.
Discussion
Environmental factors were commonly considered to be associated with the incidence and prevalence of asthma. Up to now, the results investigating the association between metal elements and childhood asthma were inconsistent. The research on the effects of metal elements on adult-onset asthma is rare. In the present study, we observed that the prevalence of adult asthma was positively associated with increased urinary levels of Cr, Cu, Se, Mo, Cd, and U. In contrast, levels of seven metals (Mn, Fe, Ni, Zn, Rb, Ba and Pb) were negatively associated with adult asthma.
Some previous epidemiologic studies have reported associations of exposure to the known carcinogens Cr or Cd with an increased risk of asthma [31, 32]. In this study, we also observed a positive association of Cr with asthma. In vitro and in vivo, exposure to Cr (Ⅵ) could result in inflammation of lung tissue and release of inflammatory cytokines [33, 34]. Furthermore, inhalation of particulate forms of Cr (Ⅵ) may augment the severity of ongoing allergic asthma in mice [35]. Similarly, high serum Cd concentrations have been reported to contribute to increased oxidative stress and inflammation in the lung [36, 37].
Se is an essential micronutrient for human health, being a cofactor for enzymes with antioxidant activity that protect the organism from oxidative damage. Several investigations have showed the lower levels of Se were associated with recurrent wheezing and asthma [9]. However, other studies failed to find any association between Se levels and asthma [10]. A positive association of Se intake and bronchial responsiveness was observed in young Chilean adults [38], which was in consistent with our findings. The inconclusive results might be attributed to the different sites where the studies conducted. A multi-center study of plasma Se and asthma illustrated that a 10 μg/l increase in plasma Se was associated with a 52% decrease in risk of asthma in Lodz and a 35% increase in risk in Ghent, with a 68% increase in risk in Amsterdam [19]. Wuhan is located in Jianghan plain area which is abundant in Se. The urinary Se levels among the population in Wuhan were possibly higher. Moreover, animal models have suggested that any association between Se and AHR might not be linear [39]. Although increasing Se intake reduces oxidative stress, it may also boost immune responses. Se mainly induced inflammation and AHR at low or medium levels, while Se may have antioxidant effects while inducing inflammation at high levels, resulting in an overall reduction in asthma [39]. This dose-response manner may partly explain the inconsistent results of previous studies.
Only a few studies have shown that Mo, an essential element, is involved in lung injury. Molybdenum trioxide exposed workers had higher percentage counts of lymphocytes and neutrophils in bronchoalveolar lavage [40]. An animal study observed increased incidences of alveolar/bronchiolar adenoma or carcinoma (combined) in Mo-exposed mice [41]. In the present study, we found that the high Mo levels were positively associated with prevalence of asthma. Possible mechanisms may involve the effects of Mo on respiratory inflammatory stimuli and oxidative stress [41].
Zn is a trace element and plays a key role in the regulation of immune system. Fe is an essential element and performs several crucial functions. Cu is an integral part of many important enzymes, but chronic Cu-overload and/or excess exposure will initiate oxidative damage [42]. In the current study, Zn and Fe played protective roles against asthma, but Cu appeared to be a risk factor for asthma. Several previous studies have also reported lower concentrations of Zn, higher concentrations of Cu and a higher Cu/Zn ratio in the serum of asthma cases when compared with those of controls [12, 43, 44]. Interestingly, the increase of Cu/Zn ratio was thought to be more important than their separate increases or reductions [45]. In our study, we found that the OR of Cu/Zn reached 3.87 (3.07, 4.86) in the single-metal model which was higher than that of Cu. In addition, a nutritional supplement study showed that after receiving daily multiple nutrient supplements for two months, asthmatics had markedly reduced Cu and malondialdehyde levels, increased Zn levels and alleviation of syndromes [46]. It was postulated that an increase in serum Cu might lead to diminishment of serum Zn and could thus implicitly cause inflammation by decreasing the capability of the antioxidant system [12]. Similarly, Fe supplementation markedly decreased allergen-induced AHR, eosinophil infiltration, and production of pro-inflammatory cytokines [47]. Moreover, Zn exposure increased Fe uptake in respiratory epithelial cells and subsequently increased the expression of divalent metal transporter 1 and ferritin, which could diminish oxidative stress [48].
Mn is an essential element involved in antioxidant activities. In accordance with our results, Patel et al. found that symptomatic asthma in adults was associated with low dietary intake of Mn [49], and the lowest intakes of Mn were associated with a more than 5 times higher risk of bronchial reactivity [50]. Several studies also revealed that Mn-related antioxidants could mitigate the effects of oxidative stress in an asthma mice model, and a Mn -porphyrin compound could suppress both epithelial thickening and mucus accumulation in the epithelium [51, 52].
Due to its effects on promoting Th2 immune responses [53], Pb has been hypothesized to be a risk factor for asthma [54]. However, epidemiological evidence is controversial. Among African Americans, Joseph et al didn’t observe an effect of blood Pb levels on asthma risk [55]. In a retrospective cohort study, blood lead ≥10 μg/dL was not associated with asthma (adjusted OR = 0.91, 95% CI: 0.55, 1.48), nor was chronic lead exposure (adjusted OR = 0.95, 95% CI: 0.58, 1.55) [56]. In the present study, we even found a negative association between urinary Pb and asthma. Because Pb exposure and asthma share risk factors that are heavily influenced by socioeconomic status, it is difficult to obtain an unbiased estimate of true relationship between Pb exposure and prevalence of asthma, and levels of Pb in body may be less a predictor of asthma [55].
Some previous studies have reported adverse effects of U exposure on the respiratory system. To our knowledge, only one epidemiological study has observed a positive quantitative association between urinary U levels and asthma in adults [57], in agreement with our findings. In a birth-cohort study from New York City, a positive association was found between Ni and wheeze during the first 2 years of life [58]. Very recently, the prospective Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study found no association of Ni in particulate matter with incidence and prevalence of asthma in schoolchildren[11]. Even in our study, we observed negative association between urinary Ni and prevalence of asthma. In addition, our results suggested that levels of Ba and Rb were negatively associated with asthma, but the evidence is absent. Further investigations are needed to uncover underlying mechanisms.
Individuals exposed to a variety of metals, and we could not ignore the interaction between metals. After adjusting for other metals and confounding factors, there were some metals which were not associated with asthma prevalence in the multiple-metal models. As is a known lung, bladder, and skin carcinogen commonly found in drinking water [59]. Previous studies considered As as a risk factor for asthma. However, a cross-sectional study in the U.S. population demonstrated an insignificantly negative association between urinary As and prevalence of asthma [20]. In the current study, we only observed a positive association of urinary As with asthma in the single-metal model. The disputed results may be related to different body burdens of As in different regions [20]. The biological function of Sr in lung is unknown. An epidemiological study found that Sr bound to PM2.5 was associated with the reduction in FEF25-75 [60]. We only found a positive association of Sr with asthma in the single-metal model. This may be resulted from the effects of Sr on oxidative stress [61]. Epidemiological studies have reported associations between occupational asthma and metals in particulate matter, such as Al, V, Co, Sb, W and Tl [62–65]. However, the research in the general population is rare. In the present study, we did not find any associations between V, Co and asthma. In accordance with the result in the US adult population [57], we found a positive association between W and asthma prevalence in the single-metal model. Exposure to W could cause a marked inflammatory response in lung tissue and that the leukocyte exudates may invade alveolar areas of the lung [66]. As a continual variable, a positive association of borderline significance was observed for Sn in the single-metal model. But we did not observed a significant association of Sn with asthma, when dividing urinary concentrations of Sn into quartiles. The possible reason may be that the urinary concentrations of Sn were lower than LOQ in 26.50% of the samples and were replaced with half the LOQ.
The exact pathogenesis of asthma remains unclear, but airway inflammation and oxidative stress are believed to play crucial roles. They are also considered as the main pathways through which metals induce and influence the development of asthma. For instance, heavy metals, such as Cd and Cr, could induce airway inflammatory responses [35, 36], and lead to AHR and airway obstruction [67]. Activated immune cells undergo a respiratory burst with the generation of oxidants, such as reactive oxygen species, which are reported to sensitize airway muscles to acetylcholine-induced contraction [68], induce AHR [69], and increase mucus secretion and epithelial shedding [70]. Some metals like Se, Zn and Mn can also influence the oxidant/antioxidant balance, and further regulate inflammatory responses [71]. The association between inflammation and oxidative stress could set up a positive-feedback loop that exacerbates asthma.
Our study has several strengths. First, multiple logistic regression analysis allowed us to control for potential confounding factors which are supposed to be related with asthma. Second, we simultaneously determined a wide range of metals in urine to evaluate personal exposure from all sources, and established dose-response relationships between urinary metals and adult asthma. Third, newly diagnosed asthma and asthma of different severity were compared with the matched controls, and the associations of urinary metals with asthma were nearly the same. Several limitations in this study should be considered. First, one time urine samples were used for analysis, and might result in measurement error due to individual variability in short-term metal excretion and influenced the significance of the findings. However, it is not practical to collect 24-h urine samples in the large population study. Second, some asthma cases had been diagnosed several years previously. Therefore, they may have adjusted living habits following doctors’ recommendations, resulting in that incidence of smoking and flower gardening among asthma cases were not higher than those among the controls. Third, the limitation of hospital based case-control study is that we missed some asymptomatic cases because they don’t attend in the hospital. Forth, although we observed associations between urinary metal concentrations and adult asthma, the case-control study remains difficult to establish a causal relationship. Prospective studies are warranted to establish the causal relationships between metal exposure and asthma.
Conclusions
The findings of this study suggest that asthma prevalence in the Chinese adults was positively associated with urinary Cr, Cu, Se, Mo, Cd, and U, and negatively associated with urinary Mn, Fe, Ni, Zn, Rb, Ba and Pb. The results are partly in accordance with previous studies, and the mechanisms of some metals remain unknown. Given that exposure to metals differs by regions, as well as the possible error due to Serial measurements of a variety of metals, our preliminary findings need to be replicated in large populations in different regions.
Acknowledgments
We gratefully acknowledge all the staffs of Zhuan kou district diseases control and prevention, and the local community residents committees in Wuhan city.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Basic Research Program of China (2011CB503804). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Asthma 2013 [updated November 2013]. Available from: http://www.who.int/mediacentre/factsheets/fs307/en/.
- 2.Su N, Lin J, Liu G, Chen P, Zhou X, Wan H, et al. An epidemiological survey of current asthma control status in China. Zhonghua Nei Ke Za Zhi. 2014;53(8):601–6. [PubMed] [Google Scholar]
- 3.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49(2):171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SK, Zhang Q, Qiu Z, Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. Journal of thoracic disease. 2015;7(1):23–33. 10.3978/j.issn.2072-1439.2014.12.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malo JL. Occupational rhinitis and asthma due to metal salts. Allergy. 2005;60(2):138–9. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola JJ, Piipari R, Jaakkola MS. Occupation and asthma: a population-based incident case-control study. Am J Epidemiol. 2003;158(10):981–7. [DOI] [PubMed] [Google Scholar]
- 7.Wang TN, Lin MC, Wu CC, Leung SY, Huang MS, Chuang HY, et al. Risks of exposure to occupational asthmogens in atopic and nonatopic asthma: a case-control study in Taiwan. Am J Respir Crit Care Med. 2010;182(11):1369–76. 10.1164/rccm.200906-0969OC [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Nieto M, Quirce S, Carnes J, Sastre J. Occupational asthma due to chromium and nickel salts. Int Arch Occup Environ Health. 2006;79(6):483–6. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro MF, Rhoden CR, Amantea SL, Barbosa F Jr. Low concentrations of selenium and zinc in nails are associated with childhood asthma. Biol Trace Elem Res. 2011;144(1–3):244–52. 10.1007/s12011-011-9080-3 [DOI] [PubMed] [Google Scholar]
- 10.Thomson CD, Wickens K, Miller J, Ingham T, Lampshire P, Epton MJ, et al. Selenium status and allergic disease in a cohort of New Zealand children. Clin Exp Allergy. 2012;42(4):560–7. 10.1111/j.1365-2222.2012.03924.x [DOI] [PubMed] [Google Scholar]
- 11.Gehring U, Beelen R, Eeftens M, Hoek G, de Hoogh K, de Jongste JC, et al. Particulate matter composition and respiratory health: the PIAMA Birth Cohort study. Epidemiology. 2015;26(3):300–9. 10.1097/EDE.0000000000000264 [DOI] [PubMed] [Google Scholar]
- 12.Vural H, Uzun K, Uz E, Kocyigit A, Cigli A, Akyol O. Concentrations of copper, zinc and various elements in serum of patients with bronchial asthma. J Trace Elem Med Biol. 2000;14(2):88–91. [DOI] [PubMed] [Google Scholar]
- 13.Brigham EP, McCormack MC, Takemoto CM, Matsui EC. Iron status is associated with asthma and lung function in US women. PLoS One. 2015;10(2):e0117545 10.1371/journal.pone.0117545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan JC, Tan JH. Atmospheric heavy metals and Arsenic in China: Situation, sources and control policies. Atmos Environ. 2013;74:93–101. [Google Scholar]
- 15.Chuanhe Liu JH, Shang Yunxiao, Sun Jun. Comparison of asthma prevalence in children from 16 cities of China in 20 years. Chinese Journal of Practical Pediatrics. 2015;30(8):5. [Google Scholar]
- 16.Zeng X, Xu X, Zheng X, Reponen T, Chen A, Huo X. Heavy metals in PM and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environ Pollut. 2016;210:346–53. 10.1016/j.envpol.2016.01.025 [DOI] [PubMed] [Google Scholar]
- 17.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? European Respiratory Review. 2013;22(127):44–52. 10.1183/09059180.00007112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolders R, Koch HM, Moos RK, Cocker J, Jones K, Warren N, et al. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 1: metals. Toxicol Lett. 2014;231(2):249–60. 10.1016/j.toxlet.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 19.Burney P, Potts J, Makowska J, Kowalski M, Phillips J, Gnatiuc L, et al. A case-control study of the relation between plasma selenium and asthma in European populations: a GAL2EN project. Allergy. 2008;63(7):865–71. 10.1111/j.1398-9995.2008.01716.x [DOI] [PubMed] [Google Scholar]
- 20.Amster ED, Cho JI, Christiani D. Urine arsenic concentration and obstructive pulmonary disease in the U.S. population. J Toxicol Environ Health A. 2011;74(11):716–27. 10.1080/15287394.2011.556060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010;15(2):101–9. [PubMed] [Google Scholar]
- 22.Lei Yang YH, Zhou Xiaoqin, Huang Zhaoxuan. Investigation of childhood asthma morbidity rate and risk factors in Wuhan area. Chin J Appl Clin Prediatr. 2013;28(21):3. [Google Scholar]
- 23.Feng W, Huang X, Zhang C, Liu C, Cui X, Zhou Y, et al. The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: a population-based study in China. BMJ open. 2015;5(5):e007643 10.1136/bmjopen-2015-007643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–78. 10.1183/09031936.00138707 [DOI] [PubMed] [Google Scholar]
- 25.Toren K, Hermansson B- A. Incidence rate of adult-onset asthma in relation to age, sex, atopy and smoking: a Swedish population-based study of 15813 adults. The International Journal of Tuberculosis and Lung Disease. 1999;3(3):192–7. [PubMed] [Google Scholar]
- 26.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304–45. [DOI] [PubMed] [Google Scholar]
- 27.Heitland P, Koster HD. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin Chim Acta. 2006;365(1–2):310–8. [DOI] [PubMed] [Google Scholar]
- 28.Linsinger T. Comparison of measurement result with the certified value. European Reference Materials, July 2005. [Google Scholar]
- 29.Bland JM, Altman DG. The use of transformation when comparing two means. BMJ. 1996;312(7039):1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2(3):278–82. [Google Scholar]
- 31.Hannu T, Piipari R, Kasurinen H, Keskinen H, Tuppurainen M, Tuomi T. Occupational asthma due to manual metal-arc welding of special stainless steels. Eur Respir J. 2005;26(4):736–9. [DOI] [PubMed] [Google Scholar]
- 32.Willers S, Gerhardsson L, Lundh T. Environmental tobacco smoke (ETS) exposure in children with asthma-relation between lead and cadmium, and cotinine concentrations in urine. Respir Med. 2005;99(12):1521–7. [DOI] [PubMed] [Google Scholar]
- 33.Pascal LE, Tessier DM. Cytotoxicity of chromium and manganese to lung epithelial cells in vitro. Toxicol Lett. 2004;147(2):143–51. [DOI] [PubMed] [Google Scholar]
- 34.Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, et al. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ Health Perspect. 2009;117(12):1896–902. 10.1289/ehp.0900715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider BC, Constant SL, Patierno SR, Jurjus RA, Ceryak SM. Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol. 2012;259(1):38–44. 10.1016/j.taap.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh CM, Oh IH, Lee JK, Park YH, Choe BK, Yoon TY, et al. Blood cadmium levels are associated with a decline in lung function in males. Environ Res. 2014;132:119–25. 10.1016/j.envres.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 37.Rokadia H, Agarwal S. Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Chest. 2013;143(2):388–97. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Larsen V, Chinn S, Arts IC, Amigo H, Rona RJ. Atopy, wheeze and bronchial responsiveness in young Chilean adults. Do dietary antioxidants matter? Allergy. 2007;62(6):714–5. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann PR, Jourdan-Le Saux C, Hoffmann FW, Chang PS, Bollt O, He Q, et al. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179(5):3258–67. [DOI] [PubMed] [Google Scholar]
- 40.Ott HC, Prior C, Herold M, Riha M, Laufer G, Ott G. Respiratory symptoms and bronchoalveolar lavage abnormalities in molybdenum exposed workers. Wien Klin Wochenschr. 2004;116 Suppl 1:25–30. [PubMed] [Google Scholar]
- 41.Chan PC, Herbert RA, Roycroft JH, Haseman JK, Grumbein SL, Miller RA, et al. Lung tumor induction by inhalation exposure to molybdenum trioxide in rats and mice. Toxicol Sci. 1998;45(1):58–65. [DOI] [PubMed] [Google Scholar]
- 42.Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–63. [DOI] [PubMed] [Google Scholar]
- 43.Guo CH, Liu PJ, Hsia S, Chuang CJ, Chen PC. Role of certain trace minerals in oxidative stress, inflammation, CD4/CD8 lymphocyte ratios and lung function in asthmatic patients. Ann Clin Biochem. 2011;48(Pt 4):344–51. 10.1258/acb.2011.010266 [DOI] [PubMed] [Google Scholar]
- 44.el-Kholy MS, Gas Allah MA, el-Shimi S, el-Baz F, el-Tayeb H, Abdel-Hamid MS. Zinc and copper status in children with bronchial asthma and atopic dermatitis. J Egypt Public Health Assoc. 1990;65(5–6):657–68. [PubMed] [Google Scholar]
- 45.Uysalol M, Uysalol EP, Yilmaz Y, Parlakgul G, Ozden TA, Ertem HV, et al. Serum level of vitamin D and trace elements in children with recurrent wheezing: a cross-sectional study. BMC Pediatr. 2014;14:270 10.1186/1471-2431-14-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo CH, Liu PJ, Lin KP, Chen PC. Nutritional supplement therapy improves oxidative stress, immune response, pulmonary function, and quality of life in allergic asthma patients: an open-label pilot study. Altern Med Rev. 2012;17(1):42–56. [PubMed] [Google Scholar]
- 47.Hale LP, Kant EP, Greer PK, Foster WM. Iron supplementation decreases severity of allergic inflammation in murine lung. PLoS One. 2012;7(9):e45667 10.1371/journal.pone.0045667 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Deng Z, Dailey LA, Soukup J, Stonehuerner J, Richards JD, Callaghan KD, et al. Zinc transport by respiratory epithelial cells and interaction with iron homeostasis. Biometals. 2009;22(5):803–15. 10.1007/s10534-009-9227-2 [DOI] [PubMed] [Google Scholar]
- 49.Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw KT, et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61(5):388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soutar A, Seaton A, Brown K. Bronchial reactivity and dietary antioxidants. Thorax. 1997;52(2):166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terziev L, Dancheva V, Shopova V, Stavreva G. Antioxidant effect of MnTE-2-PyP on lung in asthma mice model. ScientificWorldJournal. 2012;2012:379360 10.1100/2012/379360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Y, Fujii M, Ishihara K, Akiba S, Yasui H, Nabe T. Effect of a peroxynitrite scavenger, a manganese-porphyrin compound on airway remodeling in a murine asthma. Biol Pharm Bull. 2013;36(5):850–5. [DOI] [PubMed] [Google Scholar]
- 53.Gao D, Mondal TK, Lawrence DA. Lead effects on development and function of bone marrow-derived dendritic cells promote Th2 immune responses. Toxicol Appl Pharmacol. 2007;222(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anetor JI, Adeniyi FA. Decreased immune status in Nigerian workers occupationally exposed to lead. Afr J Med Med Sci. 1998;27(3–4):169–72. [PubMed] [Google Scholar]
- 55.Joseph CL, Havstad S, Ownby DR, Peterson EL, Maliarik M, McCabe MJ Jr., et al. Blood lead level and risk of asthma. Environ Health Perspect. 2005;113(7):900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabito FA, Horter L, Langlois EC, Carlson JC, White LE, Schwartz K, et al. Blood lead and pediatric asthma. Epidemiology. 2013;24(3):474–6. 10.1097/EDE.0b013e31828c7673 [DOI] [PubMed] [Google Scholar]
- 57.Mendy A, Gasana J, Vieira ER. Urinary heavy metals and associated medical conditions in the US adult population. Int J Environ Health Res. 2012;22(2):105–18. 10.1080/09603123.2011.605877 [DOI] [PubMed] [Google Scholar]
- 58.Patel MM, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn JW, et al. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med. 2009;180(11):1107–13. 10.1164/rccm.200901-0122OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.International Agency for Research on Cancer. Some drinking-water disinfectants and contaminants, including arsenic: IARC; 2004. [PMC free article] [PubMed] [Google Scholar]
- 60.Cakmak S, Dales R, Kauri LM, Mahmud M, Van Ryswyk K, Vanos J, et al. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environ Pollut. 2014;189:208–14. 10.1016/j.envpol.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 61.Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect. 2010;118(4):579–83. 10.1289/ehp.0901077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kongerud J, Boe J, Soyseth V, Naalsund A, Magnus P. Aluminium potroom asthma: the Norwegian experience. European Respiratory Journal. 1994;7(1):165–72. [DOI] [PubMed] [Google Scholar]
- 63.Kim H, Heo Y, Oh S, Lee K, Lawrence D. Altered serum cytokine and immunoglobulin levels in the workers exposed to antimony. Human & experimental toxicology. 1999;18(10):607–13. [DOI] [PubMed] [Google Scholar]
- 64.Stefaniak AB, Day GA, Harvey CJ, Leonard SS, Schwegler-Berry DE, Chipera SJ, et al. Characteristics of dusts encountered during the production of cemented tungsten carbides. Industrial health. 2007;45(6):793–803. [DOI] [PubMed] [Google Scholar]
- 65.Hunter D, Milton R, Perry KM. Asthma caused by the complex salts of platinum. British journal of industrial medicine. 1945;2(2):92. [Google Scholar]
- 66.Peao MN, Aguas AP, de Sa CM, Grande NR. Inflammatory response of the lung to tungsten particles: an experimental study in mice submitted to intratracheal instillation of a calcium tungstate powder. Lung. 1993;171(4):187–201. [DOI] [PubMed] [Google Scholar]
- 67.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. [DOI] [PubMed] [Google Scholar]
- 68.Katsumata U, Miura M, Ichinose M, Kimura K, Takahashi T, Inoue H, et al. Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. Am Rev Respir Dis. 1990;141(5 Pt 1):1158–61. [DOI] [PubMed] [Google Scholar]
- 69.Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol. 2005;289(1):C207–16. [DOI] [PubMed] [Google Scholar]
- 70.Nabe T, Ikedo A, Hosokawa F, Kishima M, Fujii M, Mizutani N, et al. Regulatory role of antigen-induced interleukin-10, produced by CD4(+) T cells, in airway neutrophilia in a murine model for asthma. Eur J Pharmacol. 2012;677(1–3):154–62. 10.1016/j.ejphar.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 71.Shanmugasundaram KR, Kumar SS, Rajajee S. Excessive free radical generation in the blood of children suffering from asthma. Clin Chim Acta. 2001;305(1–2):107–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.