Abstract
Background
Amyotrophic lateral sclerosis is a chronic neurodegenerative disease characterized by progressive paralysis due to degeneration of motor neurons by unknown causes. Recent evidence shows that Wnt signaling is involved in neurodegenerative processes, including Amyotrophic Lateral Sclerosis. However, to date, little is known regarding the expression of Wnt signaling components in this fatal condition. In the present study we used transgenic SOD1G93A mice to evaluate the expression of several Wnt signaling components, with special focus on Frizzled-5 cellular expression alteration along disease progression.
Findings
Based on previous studies demonstrating the expression of Wnts and their transcriptional regulation during Amyotrophic lateral sclerosis development, we have analyzed the mRNA expression of several Wnt signaling components in the spinal cord of SOD1G93A transgenic mice at different stages of the disease by using real time quantitative PCR analysis. Strikingly, one of the molecules that seemed not to be altered at mRNA level, Frizzled-5, showed a clear up-regulation at late stages in neurons, as evidenced by immunofluorescence assays. Moreover, increased Frizzled-5 appears to correlate with a decrease in NeuN signal in these cells, suggesting a correlation between neuronal affectation and the increased expression of this receptor.
Conclusions
Our data suggest the involvement of Wnt signaling pathways in the pathophysiology of Amyotrophic Lateral Sclerosis and, more specifically, the implication of Frizzled-5 receptor in the response of neuronal cells against neurodegeneration. Nevertheless, further experimental studies are needed to shed light on the specific role of Frizzled-5 and the emerging but increasing Wnt family of proteins research field as a potential target for this neuropathology.
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common and fatal form of motor neuron (MN) degenerative disease, with an annual incidence of 1–3 cases per 100,000 inhabitants [1]. The development of the pathology is characterized by progressive MN degeneration in the brain and spinal cord, leading to a deterioration of the neuromuscular function and as result weakness, atrophy, paralysis of skeletal muscles and eventually death from respiratory failure within 3 to 5 years from diagnosis [2–6]. The etiology of the disease is unknown in most ALS cases, which are described as sporadic (SALS), whereas a 10–20% of ALS cases are classified as familial (FALS) [1–5]. The first gene to be identified as being mutated in ALS was the cytosolic copper/zinc superoxide dismutase (Cu/Zn SOD, SOD1) [7, 8] and to date, over 150 different mutations in SOD1 have been discovered (http://alsod.iop.kcl.ac.uk/). Interestingly, both SALS and FALS are suggested to have common pathological hallmarks [4, 9]. The SOD1G93A transgenic mice is a valuable tool for ALS research, characterized by over-expression of the mutant human SOD1 and the development of an age-dependent degeneration of MNs, very similar to human ALS in terms of clinical and pathological features, including leading to progressive paralysis and death [10]. MN death seems to be driven by a convergence of damaging mechanisms, including glutamate excitotoxicity, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, defects in RNA processing, neurofilament accumulation, growth factor abnormalities, astroglia and/or microglia dysfunction, defects in axonal transport, metabolic alterations, accumulation of protein aggregates and immune imbalance [3, 6, 9, 11]. However, the exact cellular and molecular mechanisms responsible for MN degeneration in ALS are not completely understood, and to date, there is no cure for this neuropathology.
The Wnt family of proteins plays key roles during central nervous system (CNS) development and adult tissue homeostasis by regulating different cellular processes, such as migration, proliferation, differentiation, polarisation, axonal guidance, cell-cell adhesion and synapse physiology [12–16]. Furthermore, Wnt signaling has been involved in several neuropathologies during adulthood, including Alzheimer [17, 18], Parkinson [19] and Huntington [20] diseases, spinal cord injury (SCI) [21–26] and ALS [27–33]. Briefly, Wnt proteins can modulate, at least, three different signaling pathways. On one hand, in the canonical/β-catenin pathway, Wnt ligands interact with one of the 10 Frizzled (Fz) receptors and a low-density Lipoprotein Receptor-related Protein 5/6 (LRP5/6) co-receptor, leading to active β-catenin translocation to the nucleus and gene expression induction by the interaction with T-cell Factor/Lymphoid Enhancer Factor (TCF/LEF) family of DNA-binding proteins [34–38]. On the other hand, the non-canonical Wnt/Ca2+ and PCP pathways are activated by Fz receptors without LRP involvement, or by different non-conventional receptors, such as Ryk and Receptor Tyrosine Kinase-Like Orphan Receptor (ROR-1/2) [37, 39–41]. Moreover, there are different regulatory mechanisms for the Wnt-mediated signaling, including different extracellular antagonists, such as secreted Frizzled-Related Proteins (sFRPs), Dickkopf (Dkk) or Wnt inhibitory factor 1 (Wif-1) [42, 43].
Interestingly, recent results demonstrate the expression of different Wnt signaling components in the spinal cord of ALS transgenic mice, with variations in several gene expression levels during the progression of the disease [27–33]. To gain further understanding of involvement of Wnt mediated pathways in the pathogenesis of ALS, we analyzed the expression of several Wnt signaling components in the spinal cord of ALS transgenic mice at different stages by quantitative RT-PCR (RT-qPCR) and immunohistochemistry. Then, we determined the cellular expression pattern of Fz5 and its cellular protein expression changes at different stages of the pathology. Further experimental studies must be performed to determine the molecular mechanisms underlying the changes found and their role in the pathogenesis of ALS, which may lead to new strategies for treating neurodegenerative diseases through modulation of Wnt signaling pathways.
Materials and Methods
Animals
Experiments were performed in transgenic mice carrying the mutation G93A in SOD1 gene and in nontransgenic Wild-Type (WT) littermates as controls. Transgenic mice with the G93A human SOD1 mutation (B6SJL-Tg[SOD1-G93A]1Gur) were obtained from the Jackson Laboratory (Bar Harbor, ME), breaded at the animal facilities of the Universidad de Zaragoza (Spain) and maintained at the Universitat Autònoma de Barcelona (Spain). Hemizygote B6SJL SOD1G93A males were obtained by crossing with B6SJL females from the CBATEG (Barcelona, Spain). The offspring was identified by PCR amplification of DNA extracted from the tail tissue. Mice were kept in standard conditions of temperature (22 ± 2°C) and a 12:12 light:dark cycle with access to food and water ad libitum. All experimental procedures were approved by the Ethics Committee of the Universitat Autònoma de Barcelona. Studies were performed in 16 weeks old WT and 8, 12 and 16 weeks old SOD1G93A mice (n = 6 each), representing pre-symptomatic, clinical symptoms onset and end stage of the disease, respectively [44–48].
RNA isolation and RT-qPCR analysis
At each of the time points chosen for study, animals were terminally anesthetized with pentobarbital and perfused intracardially with heparinized saline to remove blood from the tissue. Lumbar spinal cords were harvested and the anterior horns were gently dissected under a microscope. Total mRNA was isolated by using the RNeasy Mini Kit (Qiagen). Complementary DNA (cDNA) synthesis from DNase-treated RNA (0.5 μg) and the relative quantifications were performed as previously described [23] using 50 ng of cDNA and specific primers validated previously [26]. β-actin (forward 5’-AAGTCCCTCACCCTCCCAAAAG-3’, reverse 5’- AAGCAATGCTGTCACCTTCCC-3’. GenBank accession number NM_007393.3) was quantified in a separate well as real-time reporter.
Immunohistochemistry
Similarly, three WT, and 8w, 12w and 16w old SOD1G93A mice were intracardially perfused with heparinized saline solution followed by 4% paraformaldehyde. The lumbar spinal cords were harvested and then post-fixed for 4h in 4% paraformaldehyde, cryoprotected by immersion in 30% sucrose for 48h, embedded in OCT frozen medium (Sakura). Ten series of 40μm transverse sections were cut using a cryotome (Leica) and collected serially in Olmos solution.
The cellular protein expression and localization of Fz1, Fz4 and Fz5 in both WT and transgenic mice spinal cord was performed by immunofluorescence assays following the same protocol detailed in a previous report [23]. The following primary antibodies produced in rabbit and obtained from Abcam were used: anti-Fz1 (1:50; ab71342), anti-Fz4 (1:5000; ab83042) and anti-Fz5 (1:100; ab75234), followed by the corresponding Dylight594 (1:500; Abcam, ab96897) anti-rabbit. All sections used were processed at the same time and following the same experimental protocol.
To determine the cellular Fz5 expression pattern, double immunofluorescence was performed by combining Fz5 staining with specific astrocytic (glial fibrillary acidic protein; GFAP), neuronal (neuronal nuclei; NeuN) and MN (choline acetyltransferase; ChAT) markers, and also with the mitochondrial SOD2 staining in MNs. The sections were processed as detailed above for Fz5 detection, and the following primary antibodies were used for the different cell types: mouse anti-GFAP (1:1000; Sigma, G3893), mouse anti-NeuN (1:250; Millipore, MAB377), mouse anti-SOD2 (1:100; Abcam, ab110300) and goat anti-ChAT (1:100; Millipore, AB144P). Subsequently, the corresponding Dylight488-linked anti-mouse (1:500; Abcam, ab96879) or Alexa488-linked anti-goat (1:1000; Life technologies, A11055) secondary antibody was used.
Finally, sections were analyzed using both the BX61 Motorized Research Microscope (Olympus) and a Leica TCS SP5 resonant scanner confocal microscope (Leica Microsystems). For single immunohistochemistry experiments, the sections processed without the primary antibody were used as controls. For double immunohistochemistry, in order to confirm lack of cross reactivity, sections were processed without the second primary antibody and used as controls. No non-specific staining was observed.
TUNEL Assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed to identify cells undergoing apoptosis. Using the In Situ Cell Death Detection Kit, TMR red from Roche (no. 12156792910), spinal cord sections were treated according to manufacturer’s instructions. For dual immunofluorescence, spinal cord sections were then exposed to Fz5 antibody as described above. As negative control, we omitted the TdT enzyme in the labeling reaction mixture, and, as a positive control, we pretreated sections with DNase I and induced TUNEL positivity in all cells.
Statistical analysis
All values are expressed as the mean ± SEM. Statistical comparisons were performed using one-way ANOVA, followed by Tukey’s post-hoc test to determine the individual differences between the means. In all cases, p < 0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism (version 6.01).
Results
The Wnt family mRNA expression is modulated by the progression of the ALS pathology
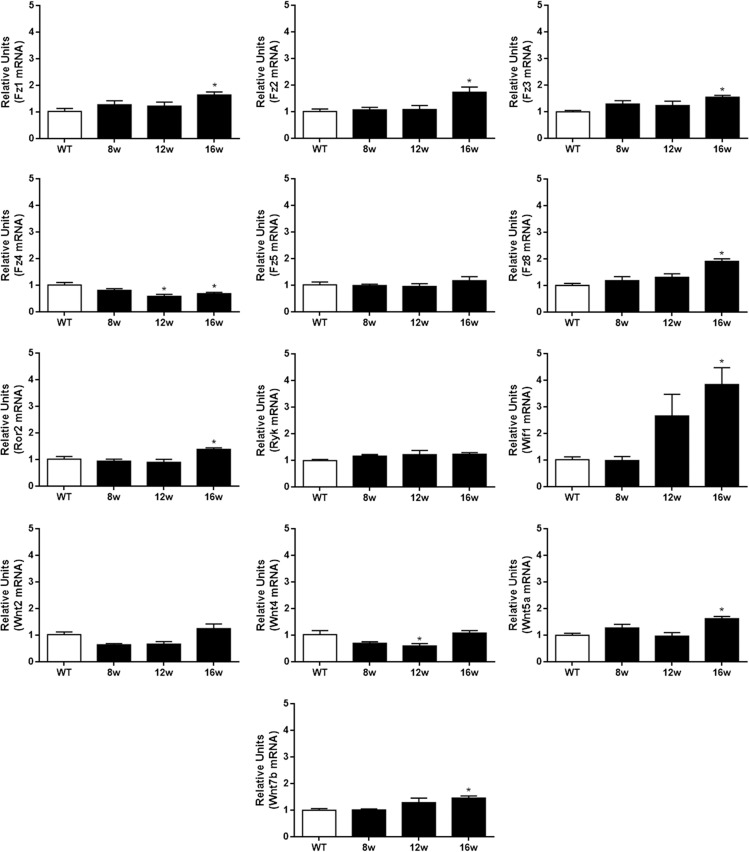
Based on previous findings on Wnt family expression in ALS [27–33], we first assessed the mRNA profile of a set of Wnt family members with reported changes of expression associated to the disease progression. We evaluated the mRNA expression of Wnt2, -4, -5a and -7b ligands; Fz1, -2, -3, -4, -5, -8, Ror2 and Ryk receptors; and the Wif1 secreted inhibitor in both WT and ALS transgenic mice. Between 8w and 12w, most of the molecules analyzed did not show significant changes in their expression levels, and only Wnt4 and Fz4 were significantly down-regulated at 12w (Fig 1). In contrast, the majority of significant changes were observed at the later stage of the pathology, at 16w. The mRNA expression of Wnt5a, Wnt7b, Fz1, Fz2, Fz3, Fz8, Ror2 and Wif1 was significantly up-regulated when compared to the levels detected in WT mice. Moreover, the mRNA expression of Fz4 was significantly down-regulated at this time point (Fig 1).
Fig 1. Temporal mRNA expression pattern of different Wnt family members in the spinal cord of both WT and ALS transgenic mice.
The temporal mRNA expression pattern of Fz1, Fz2, Fz3, Fz4, Fz5, Fz8, Ror2, Ryk, Wif1, Wnt2, Wnt4, Wnt5a and Wnt7b was quantified using real time quantitative PCR using specific primers, both in the WT and ALS transgenic mice spinal cord and at different stages of the disease. Differences were calculated by setting the expression values of the WT samples at 1 and normalising against ribosomal 18S rRNA. In all cases, the data are presented as the mean ± SEM; * p < 0.05 versus WT.
Protein expression of Fz1, Fz4 and Fz5 receptors and increased immunoreactivity of Fz5 in the spinal cord of ALS transgenic mice
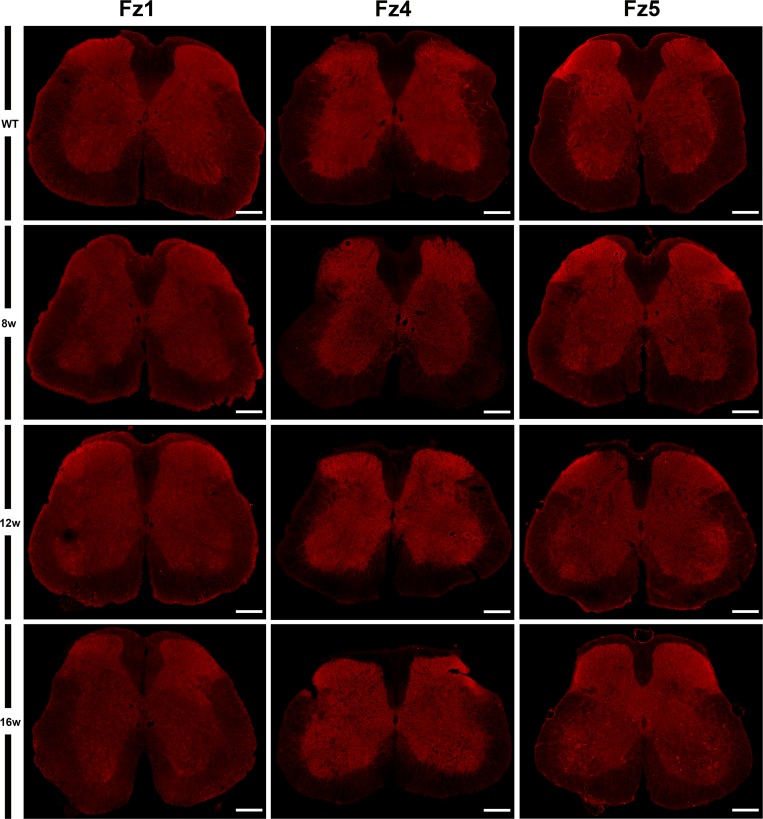
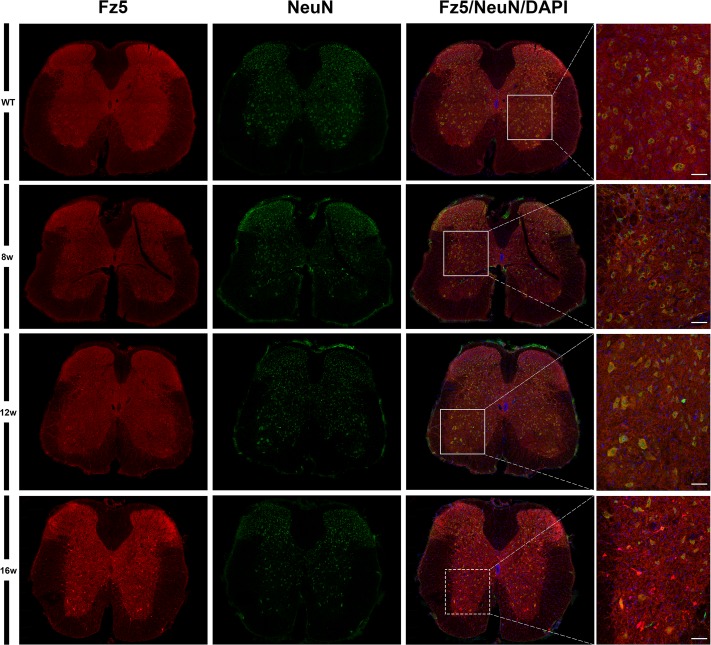
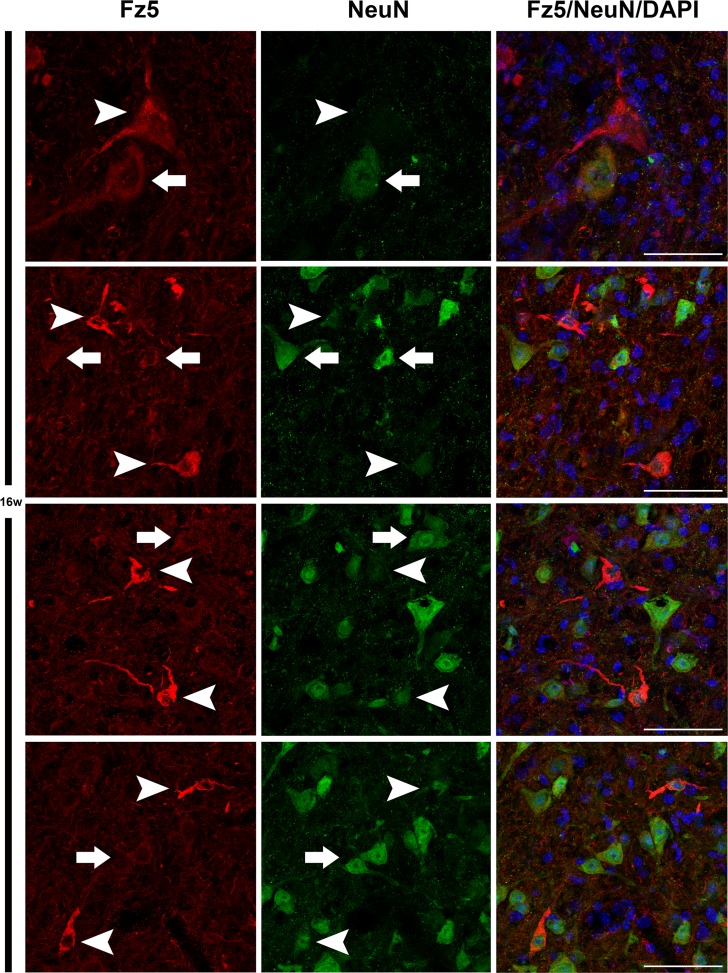
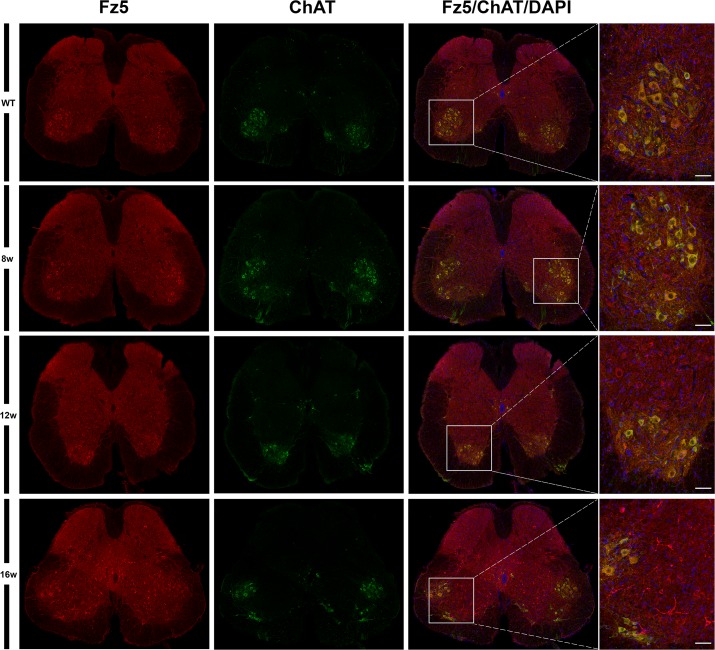
Next, using single immunohistochemistry, we investigated the protein expression of Fz1, Fz4 and Fz5, as representatives of an increase, a decrease and no change in mRNA expression levels. As previously reported by our group, Fz1 was expressed by neurons and oligodendrocytes while Fz4 was expressed by astrocytes [26] in the spinal cord of WT animals, a cell pattern and distribution that was unaltered in the transgenic mice. By contrast, Fz5 showed changes in its expression pattern during the progression of the disease (Fig 2), so the rest of experiments focused in this receptor. We found an increase in the immunoreactivity of Fz5 receptor in SOD1 mice spinal cord concomitant to disease progression, which seems to specifically affect neurons located in different layers of the spinal gray matter (Fig 3). Interestingly, this increase seemed to match with a decrease in NeuN immunoreactivity in these same cells (Fig 4). Indeed, Fz5 expression in the ventral horn of the spinal gray matter co-localized with the MN marker ChAT (Fig 5) of both WT and SOD1 transgenic mice at the different times analyzed. However, MNs were not among the cells with increased Fz5 immunoreactivity in the spinal cord of ALS transgenic mice.
Fig 2. Protein expression of Fz1, Fz4 and Fz5 receptors in the spinal cord of both WT and ALS transgenic mice.
This figure shows representative images obtained from the microscopic evaluation of sections processed by simple immunofluorescence to visualize Fz2, Fz4 and Fz5. Fz1 and Fz4 receptors do not undergo changes in their cellular expression pattern during disease progression. Instead, Fz5 immunoreactivity begins to increase at 12w in some cells, and clearly observed at 16w. The analysis was performed in WT and ALS mice spinal cords at 8w, 12w and 16w. Scale bars = 200 μm.
Fig 3. Neuronal location of Fz5 receptor in the spinal cord of both WT and ALS transgenic mice.
This figure shows representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Fz5 in neurons (NeuN). Fz5 was expressed in most of neurons at differents spinal cord laminae in WT mice and also at different neuropathological stages of ALS transgenic mice. The analysis was performed in WT and ALS mice spinal cords at 8w, 12w and 16w. The squares in the images showing the entire spinal cord sections represent the areas where the corresponding higher magnification micrographs were obtained. Scale bars = 50 μm.
Fig 4. Increased expression of Fz5 receptor matched with a decrease in NeuN immunoreactivity at 16w.
This figure shows representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Fz5 in neurons (NeuN). Cells with increased immunoreactivity for Fz5 showed low levels of NeuN staining (arrowheads). However, neurons with a baseline of Fz5 immunoreactivity exhibited normal NeuN expression (arrows). The analysis was performed in 16 ALS mice spinal cords. Scale bars = 50 μm.
Fig 5. Fz5 receptor was localized in motoneurons.
This figure shows representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Fz5 in MNs (Chat). Fz5 receptor was expressed by MNs immunopositive for Chat staining both WT and ALS transgenic mice. No changes were found regarding to any increase in Fz5 immunoreactivity associated with MNs. The analysis was performed in WT and ALS mice spinal cords at 8w, 12w and 16w. The squares in the images showing the entire spinal cord sections represent the areas where the corresponding higher magnification micrographs were obtained. Scale bars = 50 μm.
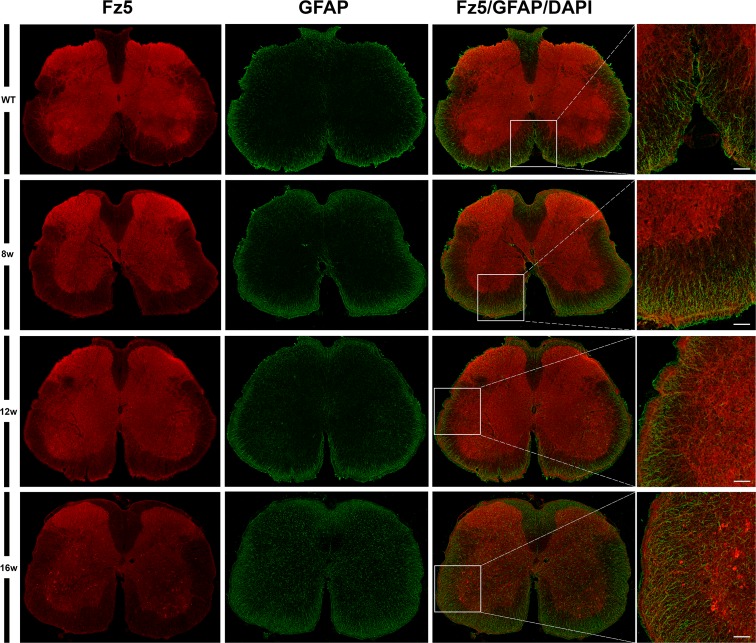
Fz5 was also located in astrocytes of both WT and SOD1 transgenic mice, mainly in the regions in close contact with the pial surface. However, we did not observe variations in protein expression of Fz5 in astrocytes, since immunoreactivity levels of the receptor do not seem to be altered at any of the stages evaluated for this condition, in contrast with the remarkable increase of GFAP expression or glial reactivity observed at advanced stages of the disease (Fig 6).
Fig 6. Fz5 receptor was localized in astrocytes.
This figure shows representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Fz5 in astrocytes (GFAP). Fz5 receptor was located in astrocytes both WT and ALS transgenic mice, mainly in contacting pial surface regions, but no changes were observed during disease progression. The analysis was performed in WT and ALS mice spinal cords at 8w, 12w and 16w. The squares in the images showing the entire spinal cord sections represent the areas where the corresponding higher magnification micrographs were obtained. Scale bars = 50 μm.
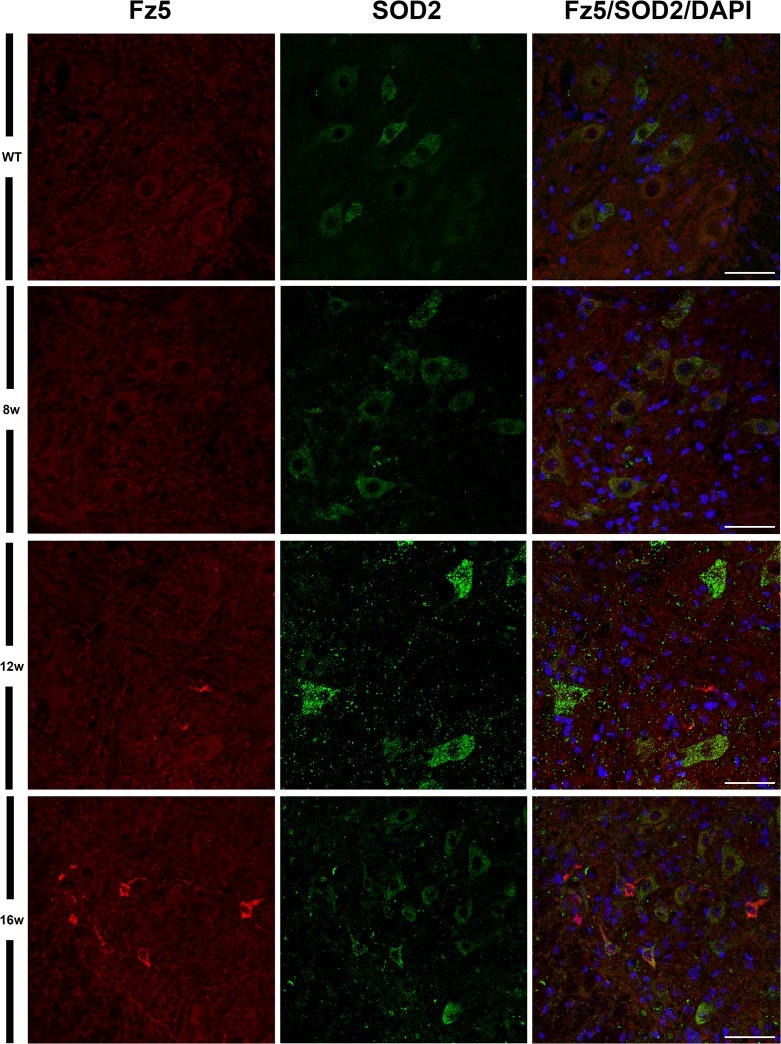
Although loss of MNs is the main characteristic of ALS, there is no conclusive evidence that apoptosis is involved [49, 50]. We performed TUNEL staining to look for evidences of nuclear DNA breakdown and identify cells undergoing apoptosis. Nevertheless, no TUNEL-positive neurons were identified in the spinal cords of transgenic mice (data not shown) at the different times analyzed. Furthermore, based on the literature that describes an increase of the SOD2 mitochondrial enzyme associated with neurodegenerative processes [51], we performed double immunohistochemistry assays to evaluate if there was any correlation between increased expression of Fz5 and SOD2 in these same cells. As expected, we found an important increase in the expression of SOD2 in correlation with the progression of the disease, however, we did not observe a clear correlation regarding co-localization with Fz5 (Fig 7).
Fig 7. Location of Fz5 receptor and the mitochondrial enzyme SOD2.
This figure shows representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Fz5 and mitochondrial enzyme SOD2. We did not observe a correlation between increased immunoreactivity of Fz5 and SOD2 in these neuronal cells, but an increase of SOD2 signal with the progression of the disease was observed. The analysis was performed in WT and ALS mice spinal cords at 8w, 12w and 16w. Scale bars = 50 μm.
Discussion
ALS is the most common form of MN disease characterized by both upper (corticospinal/corticobulbar) and lower (spinal/bulbar) MN degeneration [11]. To date, the only available treatment for ALS is riluzole, an antiglutamatergic agent that blocks the presynaptic release of glutamate, but this treatment only slows down the progression of the disease and gives the patient few months of extended life span [52, 53]. However, the causes of the disease and the mechanisms causing premature death of MN are not clear. Interestingly, recent studies have provided experimental evidences suggesting the involvement of the Wnt family of proteins in the progression of some neuropathologies [17–26], including ALS [27–33]. In this study, we found that several Wnt signaling components were differentially expressed at mRNA level in the spinal cord of SOD1G93A mice at different stages of the disease. In agreement with previous studies in the literature, we have identified an increase in mRNA expression of most of these molecules at late stages. Only Wnt4 at 12w and Fz4 at 12–16w showed significant decreases in the expression of their mRNA. Noteworthy, these results do not exactly match a previous study [29], but is important to point out that both time points for the different stages of the disease and the PCR technique (mice specific array for Wnt signaling pathway) differ from ours.
When we investigated the protein expression of Fz1, Fz4 and Fz5 as representatives of the different mRNA expression patterns, we found that Fz1 and Fz4 showed no changes in protein expression during the progression of the disease. Indeed, we have recently described the cellular expression pattern of Fz1 and Fz4 in spinal cord of healthy mice and after spinal cord injury [26], and in that study we neither found differences in the immunoreactivity of these receptors, although there were also variations on their levels of mRNA expression. Interestingly, the expression of Fz1 has been recently associated to neural progenitor cells and immature neurons in adult mice brain [54]. By contrast, Fz5 receptor, whose mRNA expression was not modified throughout symptoms progression, showed a clear alteration in its protein expression at cellular level. We found that Fz5 co-localized with MNs immunopositive for ChAT staining, and also with other neurons expressing the neuronal marker NeuN. Furthermore, from 12w and particularly at 16w, we observed a marked increase in the immunoreactivity of Fz5 in cells that co-express NeuN, and in others that appear to be losing the expression of this neuronal marker. In this regard, there is experimental evidence showing a decrease in NeuN immunoreactivity specifically in neurons that are undergoing a degenerative process [55–57]. Moreover, by using SOD2 immunoreactivity to label mitochondrial swelling [51], we investigated if those neurons showed this sign of damage, but we did not find a correlation in this sense. Neither was found these cells undergoing apoptosis since TUNEL assays failed to identify TUNEL-positive neurons in the spinal cords of transgenic mice at the different times analyzed (data not shown). This observation is in agreement with other studies which also did not found evidence of apoptosis [58–61], despite the existence of conflicting data regarding the activation of apoptotic pathways responsible for MN degeneration in ALS [49, 61, 62]. Therefore, the significance of the clear increase in Fz5 immunoreactivity associated to ALS progression is currently unknown.
The expression of Fz5 has been related with neuron survival and function. In this regard, a recent study described the necessary expression and activity of Fz5 receptor for neuronal survival in the parafascicular nucleus of the thalamus under physiological conditions, suggesting a role of this receptor in neural cell survival [63]. Other studies have described the participation of this receptor in synaptic physiology, being localized at both pre- and post-synaptic sites [64], and also in the establishment of neuronal morphogenesis and polarity in cultured hippocampal neurons [65]. Therefore, the observed increase in Fz5 immunoreactivity during the evolution of the pathology might be related with the survival and/or the synaptic maintenance of neuronal cells involved in the pathophysiology and the progression of the disease. In this regard, it is known that ALS involves degeneration of MNs, as mentioned above, but is also characterized by loss or changes in other types of neurons [66], and it has been described that abnormalities in the activity of interneurons that synapse onto MNs might lead to hyperexcitability and excitotoxicity, contributing to degeneration in ALS [51, 67, 68].
The pathophysiology of ALS also involves non-neuronal cells [11]. Accordingly, we have also investigated if there was any alteration in Fz5 immunoreactivity in astrocytes, since there are evidences in the literature showing the potential toxic role of astrocytes for MNs in ALS [1, 69–71]. We observed a clear up-regulation of GFAP concomitant with the evolution of the disease, but no changes in Fz5 immunoreactivity were detected. The expression of Fz5 seems to be restricted to those astrocytes located in close contact with the pial surface.
In summary, our findings match with other reports describing a differential expression of the Wnt signaling components in the spinal cord of SOD1G93A mice. In particular, this is the first report describing a disturbed Fz5 cellular distribution in good correlation with the progression of ALS, which might be indicative of a pathophysiological role in those neurons with increased levels of expression. Nevertheless, further studies are needed to characterize the individual role of this receptor in the spinal cord physiology and ALS pathophysiology, to eventually lead to the development of novel therapeutic strategies.
Acknowledgments
We thank Sandra Vazquez and Virginia Perez for their expert technical support.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants obtained from Fondo de Investigaciones Sanitarias (FIS) of the Instituto de Salud Carlos III (Grant PI12-2895 with Fondo Europeo de Desarrollo Regional [FEDER] cofunding) (URL: http://www.isciii.es), Grant TV3201428-10 of Fundació La Marato-TV3 (URL: http://www.tv3.cat/marato/), Grant 2014SGR-201 from the Generalitat de Catalunya (URL: http://www.idiapjgol.org/fitxers/descarrega_temp.php?id_fitxer=14940), TERCEL and CIBERNED funds from the Instituto de Salud Carlos III of Spain. (URL: http://www.isciii.es), and RM was recipient of a predoctoral fellowship from the Ministerio de Educación of Spain (URL: http://www.mineco.gob.es/portal/site/mineco/idi). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Valori CF, Brambilla L, Martorana F, Rossi D. The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014;71(2):287–97. Epub 2013/08/06. 10.1007/s00018-013-1429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim Biophys Acta. 2015;1852(4):679–84. Epub 2014/09/07. 10.1016/j.bbadis.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Moloney EB, de Winter F, Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front Neurosci. 2014;8:252 Epub 2014/09/02. 10.3389/fnins.2014.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 Suppl 1:S3–9. Epub 2009/02/05. 10.1002/ana.21543 [DOI] [PubMed] [Google Scholar]
- 5.McGoldrick P, Joyce PI, Fisher EM, Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832(9):1421–36. Epub 2013/03/26. 10.1016/j.bbadis.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, et al. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 2011;1398:113–25. Epub 2011/06/03. 10.1016/j.brainres.2011.04.049 [DOI] [PubMed] [Google Scholar]
- 7.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. Epub 1993/03/04. 10.1038/362059a0 [DOI] [PubMed] [Google Scholar]
- 8.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52(1):39–59. Epub 2006/10/04. 10.1016/j.neuron.2006.09.018 [DOI] [PubMed] [Google Scholar]
- 9.Brites D, Vaz AR. Microglia centered pathogenesis in ALS: insights in cell interconnectivity. Front Cell Neurosci. 2014;8:117 Epub 2014/06/07. 10.3389/fncel.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–5. Epub 1994/06/17 [DOI] [PubMed] [Google Scholar]
- 11.Mancuso R, Navarro X. Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Prog Neurobiol. 2015. Epub 2015/08/09. 10.1016/j.pneurobio.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129(9):2087–98. Epub 2002/04/18 [DOI] [PubMed] [Google Scholar]
- 13.Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62(10):1100–8. Epub 2005/06/02. 10.1007/s00018-005-4552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curinga G, Smith GM. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Exp Neurol. 2008;209(2):333–42. Epub 2007/08/21. 10.1016/j.expneurol.2007.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6(5):351–62. Epub 2005/04/16. 10.1038/nrn1665 [DOI] [PubMed] [Google Scholar]
- 16.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11(2):77–86. Epub 2009/12/17. 10.1038/nrn2755 [DOI] [PubMed] [Google Scholar]
- 17.De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8(2):195–208. Epub 2003/03/01. 10.1038/sj.mp.4001208 [DOI] [PubMed] [Google Scholar]
- 18.Inestrosa NC, Toledo EM. The role of Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol Neurodegener. 2008;3:9 Epub 2008/07/26. 10.1186/1750-1326-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011;41(2):508–27. Epub 2010/11/09. 10.1016/j.nbd.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington's disease. EMBO J. 2010;29(14):2433–45. Epub 2010/06/10. 10.1038/emboj.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28(33):8376–82. Epub 2008/08/15. 10.1523/JNEUROSCI.1939-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26(7):955–64. Epub 2009/05/29. 10.1089/neu.2008.0776 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6(11):e27000 Epub 2011/11/11. 10.1371/journal.pone.0027000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez P, Fernandez-Martos CM, Gonzalez-Fernandez C, Arenas E, Rodriguez FJ. Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One. 2012;7(12):e50793 Epub 2012/12/20. 10.1371/journal.pone.0050793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez P, Fernandez-Martos CM, Arenas E, Rodriguez FJ. The Ryk Receptor Is Expressed in Glial and Fibronectin-Expressing Cells after Spinal Cord Injury. J Neurotrauma. 2013;30(10):806–17. Epub 2013/01/17. 10.1089/neu.2012.2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Fernandez C, Fernandez-Martos CM, Shields S, Arenas E, Rodriguez FJ. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2013;31(6):565–81. Epub 2013/12/26. 10.1089/neu.2013.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto C, Cardenas P, Osses N, Henriquez JP. Characterization of Wnt/beta-catenin and BMP/Smad signaling pathways in an in vitro model of amyotrophic lateral sclerosis. Front Cell Neurosci. 2013;7:239 Epub 2013/12/19. 10.3389/fncel.2013.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Guan Y, Chen Y, Zhang C, Shi C, Zhou F, et al. Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int J Clin Exp Pathol. 2013;6(7):1245–60. Epub 2013/07/05 [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Guan Y, Wu X, Chen Y, Liu Z, Du H, et al. Wnt Signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem Res. 2013;38(9):1904–13. Epub 2013/06/21. 10.1007/s11064-013-1096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tury A, Tolentino K, Zou Y. Altered expression of atypical PKC and Ryk in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Dev Neurobiol. 2014;74(8):839–50. Epub 2013/10/15. 10.1002/dneu.22137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Guan Y, Chen Y, Li X, Zhang C, Yu L, et al. Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol Lett. 2013;35(8):1199–207. Epub 2013/04/05. 10.1007/s10529-013-1199-1 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, et al. Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res. 2012;34(4):390–9. Epub 2012/05/31. 10.1179/1743132812Y.0000000027 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, et al. Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;420(2):397–403. Epub 2012/03/20. 10.1016/j.bbrc.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Michaelidis TM, Lie DC. Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res. 2008;331(1):193–210. Epub 2007/09/11. 10.1007/s00441-007-0476-5 [DOI] [PubMed] [Google Scholar]
- 35.Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495(2):168–82. Epub 2000/02/05. [DOI] [PubMed] [Google Scholar]
- 36.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. Epub 2006/01/31. 10.1242/jcs.02826 [DOI] [PubMed] [Google Scholar]
- 37.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–77. Epub 2009/06/19. 10.1038/nrm2717 [DOI] [PubMed] [Google Scholar]
- 38.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51–9. Epub 2005/12/27. 10.1016/j.gde.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 39.Montcouquiol M, Crenshaw EB 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–86. Epub 2006/06/17. 10.1146/annurev.neuro.29.051605.112933 [DOI] [PubMed] [Google Scholar]
- 40.Fradkin LG, Dura JM, Noordermeer JN. Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends Neurosci. 2010;33(2):84–92. Epub 2009/12/17. 10.1016/j.tins.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 41.Minami Y, Oishi I, Endo M, Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn. 2010;239(1):1–15. Epub 2009/06/17. 10.1002/dvdy.21991 [DOI] [PubMed] [Google Scholar]
- 42.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–34. Epub 2003/05/31. 10.1242/jcs.00623 [DOI] [PubMed] [Google Scholar]
- 43.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–46. Epub 2008/03/07. 10.1242/jcs.026096 [DOI] [PubMed] [Google Scholar]
- 44.Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, et al. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6(4):349–62. Epub 1995/08/01. 10.1006/mcne.1995.1027 [DOI] [PubMed] [Google Scholar]
- 45.Mancuso R, Olivan S, Osta R, Navarro X. Evolution of gait abnormalities in SOD1(G93A) transgenic mice. Brain Res. 2011;1406:65–73. 10.1016/j.brainres.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 46.Mancuso R, Santos-Nogueira E, Osta R, Navarro X. Electrophysiological analysis of a murine model of motoneuron disease. Clin Neurophysiol. 2011;122(8):1660–70. 10.1016/j.clinph.2011.01.045 [DOI] [PubMed] [Google Scholar]
- 47.Mancuso R, Olivan S, Mancera P, Pasten-Zamorano A, Manzano R, Casas C, et al. Effect of genetic background on onset and disease progression in the SOD1-G93A model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(3):302–10. 10.3109/17482968.2012.662688 [DOI] [PubMed] [Google Scholar]
- 48.Mancuso R, Osta R, Navarro X. Presymptomatic electrophysiological tests predict clinical onset and survival in SOD1(G93A) ALS mice. Muscle Nerve. 2014;50(6):943–9. 10.1002/mus.24237 [DOI] [PubMed] [Google Scholar]
- 49.Sathasivam S, Shaw PJ. Apoptosis in amyotrophic lateral sclerosis—what is the evidence? Lancet Neurol. 2005;4(8):500–9. Epub 2005/07/22. 10.1016/S1474-4422(05)70142-3 [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki M, Esumi E, Nakano I. Is motoneuronal cell death in amyotrophic lateral sclerosis apoptosis? Neuropathology. 2005;25(4):381–7. Epub 2005/12/31 [DOI] [PubMed] [Google Scholar]
- 51.Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol. 2009;174(2):574–85. Epub 2009/01/01. 10.2353/ajpath.2009.080557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–91. Epub 1994/03/03. 10.1056/NEJM199403033300901 [DOI] [PubMed] [Google Scholar]
- 53.Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17(18):1942–199. Epub 2010/04/10. [DOI] [PubMed] [Google Scholar]
- 54.Mardones MD, Andaur GA, Varas-Godoy M, Henriquez JF, Salech F, Behrens MI, et al. Frizzled-1 receptor regulates adult hippocampal neurogenesis. Mol Brain. 2016;9(1):29 10.1186/s13041-016-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collombet JM, Masqueliez C, Four E, Burckhart MF, Bernabe D, Baubichon D, et al. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett. 2006;398(3):337–42. Epub 2006/02/14. 10.1016/j.neulet.2006.01.029 [DOI] [PubMed] [Google Scholar]
- 56.Davoli MA, Fourtounis J, Tam J, Xanthoudakis S, Nicholson D, Robertson GS, et al. Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience. 2002;115(1):125–36. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 57.Xu GP, Dave KR, Vivero R, Schmidt-Kastner R, Sick TJ, Perez-Pinzon MA. Improvement in neuronal survival after ischemic preconditioning in hippocampal slice cultures. Brain Res. 2002;952(2):153–8. Epub 2002/10/12. [DOI] [PubMed] [Google Scholar]
- 58.Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, et al. Lack of apoptosis in mice with ALS. Nat Med. 1999;5(9):966–7. Epub 1999/09/02. 10.1038/12381 [DOI] [PubMed] [Google Scholar]
- 59.Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M, et al. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191(1–2):25–33. Epub 2001/10/26. [DOI] [PubMed] [Google Scholar]
- 60.Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, Phani S, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81(5):1001–8. Epub 2014/02/11. 10.1016/j.neuron.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomik B, Adamek D, Pierzchalski P, Banares S, Duda A, Partyka D, et al. Does apoptosis occur in amyotrophic lateral sclerosis? TUNEL experience from human amyotrophic lateral sclerosis (ALS) tissues. Folia Neuropathol. 2005;43(2):75–80. Epub 2005/07/14. [PubMed] [Google Scholar]
- 62.Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol. 1999;58(5):459–71. Epub 1999/05/20 [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28(22):5641–53. Epub 2008/05/30. 10.1523/JNEUROSCI.1056-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137(13):2215–25. Epub 2010/06/10. 10.1242/dev.046722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slater PG, Ramirez VT, Gonzalez-Billault C, Varela-Nallar L, Inestrosa NC. Frizzled-5 receptor is involved in neuronal polarity and morphogenesis of hippocampal neurons. PLoS One. 2013;8(10):e78892 10.1371/journal.pone.0078892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephens B, Guiloff RJ, Navarrete R, Newman P, Nikhar N, Lewis P. Widespread loss of neuronal populations in the spinal ventral horn in sporadic motor neuron disease. A morphometric study. J Neurol Sci. 2006;244(1–2):41–58. Epub 2006/02/21. 10.1016/j.jns.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 67.McGown A, McDearmid JR, Panagiotaki N, Tong H, Al Mashhadi S, Redhead N, et al. Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model. Ann Neurol. 2013;73(2):246–58. Epub 2013/01/03. 10.1002/ana.23780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang M, Schuster JE, Fu R, Siddique T, Heckman CJ. Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2009;29(48):15031–8. Epub 2009/12/04. 10.1523/JNEUROSCI.0574-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vargas MR, Johnson JA. Astrogliosis in amyotrophic lateral sclerosis: role and therapeutic potential of astrocytes. Neurotherapeutics. 2010;7(4):471–81. Epub 2010/10/01. 10.1016/j.nurt.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papadeas ST, Kraig SE, O'Banion C, Lepore AC, Maragakis NJ. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc Natl Acad Sci U S A. 2011;108(43):17803–8. Epub 2011/10/05. 10.1073/pnas.1103141108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–22. Epub 2007/04/17. 10.1038/nn1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.