Abstract
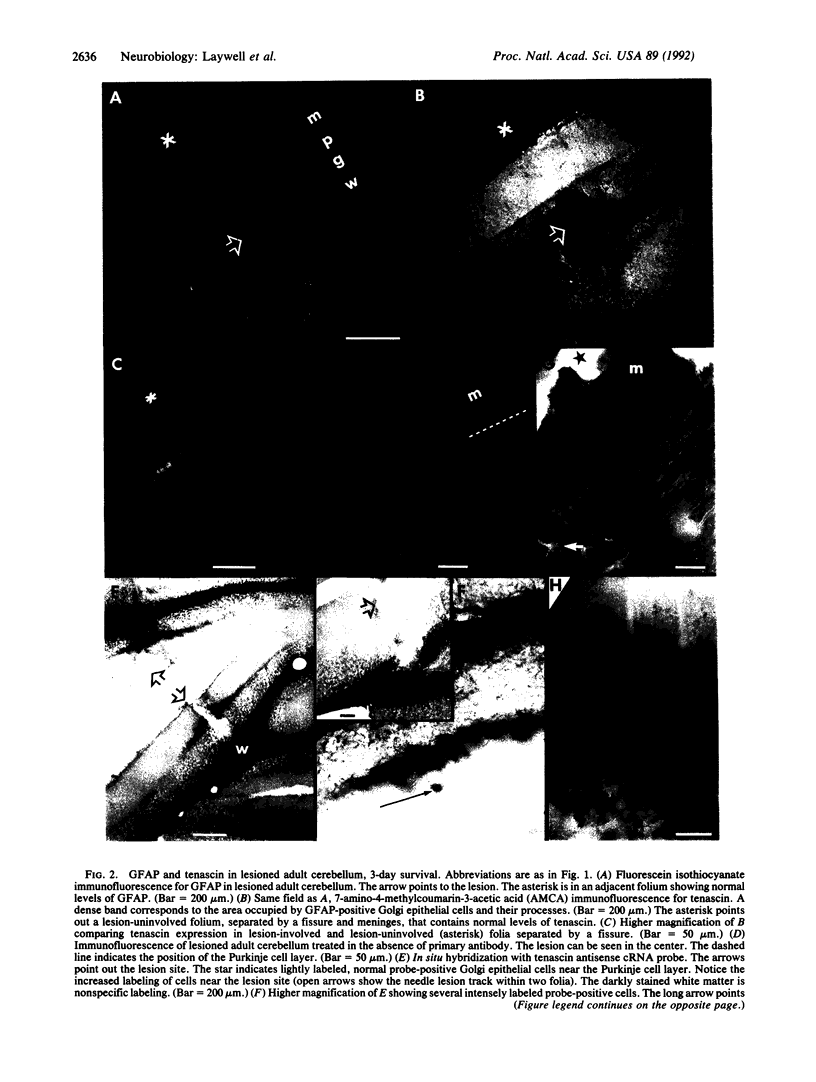
Tenascin is an extracellular matrix molecule synthesized and released by young astrocytes during embryonic and early postnatal development of the nervous system, and it is concentrated in boundaries around emerging functional neuronal units. In the adult nervous system, tenascin can be detected only in very low levels. Distinct spatial and temporal distributions of tenascin during developmental events suggest a role in the guidance and/or segregation of neurons and their processes within incipient functional patterns. We show here, using in situ hybridization and immunocytochemistry, that stab wounds of the adult mouse cerebellar and cerebral cortices result in an enhanced expression of tenascin in a discrete region around the lesion site that is associated with a subset of glial fibrillary acidic protein-positive astrocytes. Tenascin up-regulation in the lesioned adult brain may be directly involved in failed regeneration or indirectly involved through its interactions with other glycoconjugates that either inhibit or facilitate neurite growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbin G., Katz D. M., Chamak B., Glowinski J., Prochiantz A. Brain astrocytes express region-specific surface glycoproteins in culture. Glia. 1988;1(1):96–103. doi: 10.1002/glia.440010111. [DOI] [PubMed] [Google Scholar]
- Cooper N. G., Steindler D. A. Monoclonal antibody to glial fibrillary acidic protein reveals a parcellation of individual barrels in the early postnatal mouse somatosensory cortex. Brain Res. 1986 Aug 20;380(2):341–348. doi: 10.1016/0006-8993(86)90232-5. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Grumet M., Thiery J. P., Edelman G. M. Site-restricted expression of cytotactin during development of the chicken embryo. J Cell Biol. 1986 May;102(5):1917–1930. doi: 10.1083/jcb.102.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Hoffman S., Tan S. S., Edelman G. M. Cytotactin and its proteoglycan ligand mark structural and functional boundaries in somatosensory cortex of the early postnatal mouse. Dev Biol. 1989 Dec;136(2):381–392. doi: 10.1016/0012-1606(89)90264-9. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Prieto A. L., Hoffman S., Jones F. S., Friedlander D. R. Expression of adhesion molecules and the establishment of boundaries during embryonic and neural development. Exp Neurol. 1990 Jul;109(1):6–18. doi: 10.1016/s0014-4886(05)80004-4. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Faissner A., Kruse J., Chiquet-Ehrismann R., Mackie E. The high-molecular-weight J1 glycoproteins are immunochemically related to tenascin. Differentiation. 1988;37(2):104–114. doi: 10.1111/j.1432-0436.1988.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Faissner A., Kruse J. J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron. 1990 Nov;5(5):627–637. doi: 10.1016/0896-6273(90)90217-4. [DOI] [PubMed] [Google Scholar]
- Finne J., Bitter-Suermann D., Goridis C., Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987 Jun 15;138(12):4402–4407. [PubMed] [Google Scholar]
- Giulian D., Young D. G., Woodward J., Brown D. C., Lachman L. B. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988 Feb;8(2):709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Crossin K. L., Edelman G. M. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8075–8079. doi: 10.1073/pnas.82.23.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W., Chiquet-Ehrismann R., Tucker R. P. The effect of tenascin and embryonic basal lamina on the behavior and morphology of neural crest cells in vitro. Dev Biol. 1989 Mar;132(1):14–25. doi: 10.1016/0012-1606(89)90200-5. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Kruse J., Keilhauer G., Faissner A., Timpl R., Schachner M. The J1 glycoprotein--a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature. 1985 Jul 11;316(6024):146–148. doi: 10.1038/316146a0. [DOI] [PubMed] [Google Scholar]
- Lochter A., Vaughan L., Kaplony A., Prochiantz A., Schachner M., Faissner A. J1/tenascin in substrate-bound and soluble form displays contrary effects on neurite outgrowth. J Cell Biol. 1991 Jun;113(5):1159–1171. doi: 10.1083/jcb.113.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R., Schachner M., Faissner A. Enhanced expression of the extracellular matrix molecule J1/tenascin in the regenerating adult mouse sciatic nerve. J Neurocytol. 1990 Aug;19(4):601–616. doi: 10.1007/BF01257247. [DOI] [PubMed] [Google Scholar]
- Pesheva P., Spiess E., Schachner M. J1-160 and J1-180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol. 1989 Oct;109(4 Pt 1):1765–1778. doi: 10.1083/jcb.109.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Tomaselli K. J. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow D. M., Steindler D. A., Silver J. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev Biol. 1990 Apr;138(2):359–376. doi: 10.1016/0012-1606(90)90203-u. [DOI] [PubMed] [Google Scholar]
- Steindler D. A., Cooper N. G., Faissner A., Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol. 1989 Jan;131(1):243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- Steindler D. A., O'Brien T. F., Laywell E., Harrington K., Faissner A., Schachner M. Boundaries during normal and abnormal brain development: in vivo and in vitro studies of glia and glycoconjugates. Exp Neurol. 1990 Jul;109(1):35–56. doi: 10.1016/s0014-4886(05)80007-x. [DOI] [PubMed] [Google Scholar]