Abstract
Objective
In a population with type 2 diabetes mellitus (T2DM), we examined associations of short-term air pollutant exposures with pulmonary inflammation, measured as fraction of exhaled pulmonary nitric oxide (FeNO).
Methods
Sixty-nine Boston Metropolitan residents with T2DM completed up to 5 bi-weekly visits with 321 offline FeNO measurements. We measured ambient concentrations of particle mass, number and components at our stationary central site. Ambient concentrations of gaseous air pollutants were obtained from state monitors. We used linear models with fixed effects for participants, adjusting for 24-hour mean temperature, 24-hour mean water vapor pressure, season, and scrubbed room NO the day of the visit, to estimate associations between FeNO and interquartile range increases in exposure.
Results
Interquartile increases in the 6-hour averages of black carbon (BC) (0.5 μg/m3) and particle number (PN) (1,000 particles/cm3) were associated with increases in FeNO of 3.84% (95% CI 0.60% to 7.18%) and 9.86 % (95% CI 3.59% to 16.52%), respectively. We also found significant associations of increases in FeNO with increases in 24-hour moving averages of BC, PN and nitrogen oxides (NOx).
Conclusion
Recent studies have focused on FeNO as a marker for eosinophilic pulmonary inflammation in asthmatic populations. This study adds support to the relevance of FeNO as a marker for pulmonary inflammation in diabetic populations, whose underlying chronic inflammatory status is likely to be related to innate immunity and proinflammatory adipokines.
Keywords: air pollution, fraction of exhaled nitric oxide, diabetes mellitus, epidemiology, particles
INTRODUCTION
Higher concentrations of particulate air pollution are associated with increased pulmonary and cardiovascular morbidity and mortality. (Brook et al. 2004; Pope et al. 2004; Schwartz et al. 1996) Controlled human and animal exposure studies suggest that adverse clinical effects of pollution may be mediated through pulmonary and systemic inflammatory responses that can be initiated by an influx of pro-inflammatory cells, (Gurgueira et al. 2002) induction of reactive oxygen species (ROS) (Gurgueira et al. 2002) and production of pro-inflammatory cytokines in the lung. (Vogel et al. 2005) (Ghio et al. 2000)
Nitric oxide (NO) is a labile short-lived molecule and has been recognized to play important roles in changes in cardiopulmonary disease, neurotransmission, (Zanzinger 1999) immune defense, (De Groote and Fang 1995) and inflammation. (Bogdan 2001) In recent years, the fraction of exhaled nitric oxide (FeNO) has been served as a less invasive volatile marker of sub-clinical airway inflammatory responses in the studies of allergic diseases, (Adamkiewicz et al. 2004; Adar et al. 2007) and often correlates with sputum eosinophilic counts. (Dupont et al. 2003; Jones et al. 2001) For example, in people with asthma, elevated FeNO has been associated with eosinophilia and corticosteroid responsiveness (Dweik et al. 2011). Other studies have found FeNO to be elevated in subjects with chronic obstructive pulmonary disease (COPD), (Maziak et al. 1998) bronchiectasis (Kharitonov et al. 1995) and interstitial lung disease. (Kharitonov and Barnes 2001) However, the relation of elevated FeNO to non-allergic / noneosinophilic inflammation is less well-understood. Few studies in recent years examined the effect of air pollution of FeNO levels in non-asthmatic populations. Van Amsterdam et al showed that morning hour ambient pollution was associated with elevated FeNO levels in healthy subjects, (Van Amsterdam et al. 1999) Adamkiewicz et al also found that increased FeNO measures with increased particulate pollution in an elderly population in Steubenville, Ohio. (Adamkiewicz et al. 2004) In two separate air pollution studies of diesel exhaust, Adar et al found strong associations between microenvironmental particulate exposure with FeNO during a diesel powered bus trip, (Adar et al. 2007) and Barath et al showed that FeNO level increased upon exposure to diesel exhaust in healthy human subjects. (Barath et al. 2013) Nonetheless, the use of FeNO in studies of environmental pollution induced inflammation is still in a preliminary stage.
Type 2 diabetes mellitus (T2DM) is associated with chronic, non-allergic (innate / adipokine) systemic inflammation, (Shore 2008) which may increase susceptibility to the acute inflammatory (Dubowsky et al. 2006) and adverse clinical effects of pollution. (Zanobetti and Schwartz 2002) While some repeated measure community-based studies have shown increased levels of FeNO with increased exposure to particles, (Adamkiewicz et al. 2004; Adar et al. 2007) little is known about FeNO responses to pollutants in people with T2DM. In a repeated measures study of 321 observations on seventy type 2 diabetes mellitus (T2DM) patients, we investigated associations between levels of FeNO and particulate air pollution, fine particulate mass (particles with an aerodynamic diameter of <2.5μm PM2.5), black carbon (BC), organic carbon (OC), sulfate particles (SO 2-4), and particle number (PN), as well as gaseous components including nitrogen oxides (NOx) and ozone (O3).
METHODS
Study Panel
Seventy adults with T2DM who lived in the metropolitan Boston (MA) area participated in a repeated measures study of pollution effects on inflammatory and vascular outcomes during the period August 2006 through July 2010. A detailed protocol description has been published previously. (Hoffmann et al. 2012) In brief, initial screening included fasting blood work, blood pressure and assessment of socio-demographic characteristics, health status, medical history, medication and life style, with up to 5 biweekly clinical examinations per participant. Exclusion criteria included factors that might introduce pollutant exposure errors (e.g., second hand smoking at home, living >25 km away from the central monitoring site) or interpretation of primary inflammatory and vascular outcomes (e.g., solid organ transplant, active autoimmune disease, dementia, diabetes type 1, renal failure, seizure disorder or stroke, sleep apnea. (Hoffmann et al. 2012) Participants fasted for 12 hours prior to each of their screening and follow-up assessments, all of which were conducted in the morning. FeNO was measured during each follow up visit.
Air Pollution and Meteorology
Ambient concentrations of fine particle mass (with aerodynamic diameter <2.5 μm, PM2.5), particle components including BC, OC, SO42-, as well as PN were measured hourly at a central monitoring site (Harvard Supersite) in Boston, MA. Hourly gas exposure concentrations of NOx calculated as (NO2 + NO) and O3 were obtained from the Massachusetts Department of Environmental Protection's Greater Boston monitoring sites. All hourly pollutants were summarized in 6-hour (3 AM to 9 AM) and 24-hour intervals (9 AM to 9 AM), and as cumulative averaged pollution exposures up to the previous 7 days. All moving averages of pollutant concentrations required having 75% or more of the hourly data.
We also measured particle concentrations at home and during the trip to the clinic. Five days prior to each clinic visit samplers were placed in the study participant's home. On the day of the visit the participant brought in the pollution sampler (still in operation) to the clinic. A custom-made Harvard sampling system was used to collect fine particles (PM2.5) on Teflon filters for the determination of mass concentration by gravimetric analysis and BC by reflectance. The sampling system also included a SidePak (TSI, Inc.) which provided continuous measurements of particle mass concentration for the entire sampling period.
Weather parameters of hourly temperature and dew point temperature were obtained from the National Weather Service Station at Logan Airport (East Boston) located approximately 12 km from the examination site.
Absolute humidity was computed as:
Absolute humidity = 6.1078*10((7.5*dewpoint)/237.7 + dewpoint))
Offline Exhaled NO Measurements
The concentration of NO in exhaled breath was measured with the Deadspace Discard Bag Collection and Sampling Kit (BSK 01400) in accordance with the American Thoracic Society guidelines for offline measurements (American Thoracic and European Respiratory 2005). The clinic where FeNO was measured was located next to a busy A3 urban roadway. To avoid contamination of breath sample with ambient inspired NO, prior to exhaling from total lung capacity, participants inhaled to total lung capacity through an NO scrubber placed in-line with the system, and then exhaled immediately. The scrubber contained charcoal to remove ambient or room NO. A divert valve permitted discarding the first portion of the exhalation (dead-space gas), to capture lower airway NO while discarding NO from ambient or nasal sources. The remainder of the exhalation was diverted into a Mylar bag, maintaining a constant oropharyngeal pressure of 5 cm H2O for a flow of 50 mL/second. Subjects were coached by trained technicians throughout each maneuver. Three sequential exhaled breath samples for each subject were collected during each follow up visit. In addition, to assess the efficacy of the charcoal scrubber in removing ambient or room NO during the patient maneuver, at each visit 2 types of room air samples were obtained—one grab sample without use of the charcoal scrubber, and one grab sample obtained through the charcoal scrubber. Mylar balloons were transported in refrigerated coolers (5°C) from the clinic to HSPH Environmental Chemistry laboratory and were analyzed using the Thermo Electron Model 42i Chemiluminescence Analyzer (Thermo Electron Corporation, Franklin, MA) within 24 hours. Finally, the mean of FeNO, room NO, and scrubbed room NO concentrations for each subject at each visit were calculated.
Statistical Analysis
Primary exposure metrics included averaging 6 and 24-hour exposure periods for pollution prior to and including up to 9 AM the day of the FeNO measurement. Fixed effect models with subject specific intercepts were used to control for both measured and unmeasured time invariant characteristics. Ambient temperature, absolute humidity, season and scrubbed room NO levels were modeled as time-varying covariates. Moreover, because some particles were produced by the same photochemical reactions that produced O3, particles and O3 may confound the effect of each other. In separate analysis, we additionally adjusted for 24-hour moving average of O3 concentrations in final models for fine particles (particle mass, number and component), and final models for gases additionally were controlled for 24-hour moving average of PM2.5 concentrations.
Seasonal effect was modeled using as Fourier series terms (i.e., cos(2π*doy/365.25) and sin(2π*doy/365.25), doy = date of year). Temperature and absolute humidity were expressed as the current day average. We log-transformed the outcome variable to approximate a normal distribution of this right-skewed outcome, and results were expressed as percent changes in FeNO. Model fit was assessed by AIC values. AIC assesses the goodness of fit of the model, while penalizing on the number of covariates.
In the sensitivity analyses, we also used moving averages up to 7 days to further examine the relative role of different exposure windows on FeNO Effect estimates were expressed as percent changes in FeNO per interquartile range increase (IQR) in the relevant exposures. We also compared our analysis without and with adjustment for scrubbed room NO concentrations. All statistical analysis was performed using the Proc GLM procedure in SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
A total of 321 FeNO samples were collected and of the 70 participants, 69 had at least 1 FeNO measurement. We present baseline subject characteristics in Table 1. The study population was predominantly white, with an age range from 44.9 to 85.5, of which 52% were female participants. The mean of FeNO concentration was 21.4 ppb, with a 10-90 percentile range of 11.6 to 30.0 ppb. Most participants had long history of diabetes (mean 9.9 years), and 77% had a diagnosis of hypertension. Of the 69 participants with FeNO measurements, four had a COPD diagnosis, four had an asthma diagnosis and one had both.
Table 1.
Population characteristics (N = 69 participants)
| Characteristic | mean (range) |
|---|---|
| Follow up visits per subject | 4.8 (1-5) |
| Age, years | 64.2 (44.9-85.5) |
| BMI (kg/m2) | 31.1 (20.5-57.0) |
| FeNO* (ppb) (10-90 percentile range) | 21.4 (11.6-30.0) |
| Years of diabetes† | 10.0 (1 – 38) |
| n (%) | |
| Race | |
| White | 57 (83%) |
| Black | 7 (10%) |
| Other | 5 (7%) |
| Female | 36 (52%) |
| Medical history | |
| Asthma† | 5(8%) |
| COPD‡ | 5 (7%) |
| MI§ | 4 (6%) |
| Hypertension | 53 (77%) |
| Medication | |
| Beta blocker | 25 (36%) |
| Calcium blocker | 15 (22%) |
FeNO, fractional exhaled nitric oxide, based on 321 observations.
n = 66
n = 67
n = 68
In Table 2 we present summary statistics and correlations amongst ambient PM2.5 mass, PM2.5 constituents (BC, OC, SO42- and PN), ambient gases, ambient temperature, NO in the clinic room, as well as scrubbed room NO and indoor / trip to clinic PM2.5 and BC measurements. PM2.5 and SO42- were highly correlated (r = 0.80). PN was inversely correlated with PM2.5, SO42- and temperature. This is consistent with previously published observations that during high pollution days, ultrafine particles tend to coagulate more quickly to the accumulation mode which is the major contributor to PM2.5 mass; (Zhu 2002) and at lower temperatures condensation favors particle formation. (Sioutas et al. 2005) O3 was positively correlated with temperature, likely because tropospheric O3 is a secondary pollutant which is formed from its precursors NOx and volatile organic compounds via photochemical reactions favored by high temperature conditions. NO concentrations in the clinic room had a median of 25.11 ppb with an IQR of 14.25 to 45.03 ppb, consistent with the reality that clinic rooms were located near a busy A3 road in a major medical area in central Boston; after passing through the scrubber, NO in the clinic room was reduced to a median concentration of 4.34 ppb and an IQR of 2.97 to 7.80 ppb. This suggests that the scrubber was effective at removing NO in the room / breathing area, although it did not remove it completely.
Table 2.
Summary statistics and Spearman correlation of 24 hour moving average ambient air pollution concentrations and indoor NO measurements
| Exposure | Summary statistics |
Spearman correlation coefficient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ambient 24 hr averages‡ | N | 25th | 50th | 75th | PM2.5 | BC | OC | SO42− | PN | NOx | O3 | Temp |
| PM2.5 (μg/m3) | 296 | 5.22 | 7.22 | 9.75 | 1 | 0.59† | 0.53† | 0.80† | −0.20† | 0.22† | 0.22† | 0.35† |
| BC (μg/m3) | 302 | 0.40 | 0.53 | 0.74 | 1 | 0.48† | 0.50† | −0.00006 | 0.52† | −0.13* | 0.28† | |
| OC (μg/m3) | 259 | 2.07 | 2.88 | 3.85 | 1 | 0.52† | −0.13* | 0.26† | 0.20† | 0.24† | ||
| SO42− (μg/m3) | 222 | 0.95 | 1.65 | 2.55 | 1 | −0.34† | 0.18† | 0.27† | 0.32† | |||
| PN (1,000 particle/cm3) | 292 | 9.05 | 12.50 | 17.32 | 1 | 0.53† | −0.41† | −0.78† | ||||
| NOx (ppb) | 314 | 18.30 | 22.89 | 29.49 | 1 | −0.44† | −0.35† | |||||
| O3 (ppb) | 314 | 20.50 | 26.76 | 32.57 | 1 | 0.37† | ||||||
| Temperature [°C] | 314 | 7.31 | 14.66 | 20.93 | 1 | |||||||
| Indoor / clinic measurements | N | 25th | 50th | 75th | PM2.5 | BC | OC | SO42− | PN | NOx | O3 | Temp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Room NO§ (ppb) | 321 | 14.25 | 25.11 | 45.03 | −0.07 | 0.20† | 0.008 | −0.03 | 0.26† | 0.43† | −0.53† | −0.13* |
| Room NO through scrubber§ (ppb) | 320 | 2.97 | 4.34 | 7.80 | −0.01 | 0.17† | 0.12 | 0.02 | 0.09 | 0.28† | −0.35† | 0.03 |
| 24hr SidePak PM2.5 (μg/m3) | 298 | 4.40 | 6.41 | 9.54 | 0.39† | 0.30† | 0.17† | 0.39† | −0.13* | 0.03 | 0.07 | 0.19† |
| 5 day integrated PM2.5 (μg/m ) | 311 | 4.88 | 7.10 | 10.40 | 0.22† | 0.25† | 0.03 | 0.21† | −0.05 | 0.09 | −0.08 | 0.19† |
| 5 day integrated BC (μg/m3) | 309 | 0.55 | 0.70 | 0.84 | 0.14* | 0.20† | 0.06 | 0.16* | 0.15* | 0.25† | −0.14* | −0.09 |
p<0.05
p<0.01.
Ambient 24 hour moving averages were calculated from 9AM to 9AM.
Single samples collected from the clinic room coincident with each breath sampling.
Table 3 shows the percent increases in FeNO scaled by IQR increases in mean pollutant concentrations for exposures during the previous 6 and 24-hours. To control for residual room NO that may have been inhaled through the scrubber (and then exhaled) during the maneuver, we based our analyses on models adjusting for room NO measured through the scrubber. Elevated concentrations of BC, PN and NOx were most consistently associated with increased FeNO. For example, IQR increases in ambient BC and PN in the previous 6 hours were associated with FeNO increases of 3.84% (95% CI 0.60% to 7.18%) and 9.86% (95% CI 3.59% to 16.52%), respectively. Significant associations were also observed for BC, PN and NOx for the 24-hour moving averages (Table 3). In contrast, an IQR increase in the mean O3 concentrations in the previous 24-hour was associated with a decrease in FeNO of 4.80% (−8.70% to −0.73%). Because some particles are produced by the same photochemical reactions that produced O3, we selectively evaluated two-pollutant models in secondary analyses, adjusting for 24-hour moving average of O3 concentrations in models for particles, and adjusting for 24-hour moving average of PM2.5 concentrations in models for gases (Table 3). Estimates for SO 2-4 associations with FeNO were larger and stronger after additional adjustment for O3. The diabetic subjects with COPD or asthma did not have greater pollution effects on FeNO than the diabetic subjects without these respiratory diagnoses (results not shown).
Table 3.
Estimated change of FeNO related to increase in the 6-hour and 24-hour mean of ambient air pollutants.
| Pollutant | IQR exposure metric | Estimated change in FeNO (ppb) per pollutant IQR (95% CI) | ||
|---|---|---|---|---|
| Single pollutant model | Particles adjusted for 24hr O3† | Gases adjusted for 24hr PM2.5‡ | ||
| PM2.5 | ||||
| 6-hour mean | 5.8 μg/m3 | 1.60 (−1.15 to 4.42) | 2.43 (−0.37 to 5.31) | - |
| 24-hour mean | 4.5 μg/m3 | 0.50 (−1.94 to 3.00) | 1.31 (−1.20 to 3.89) | - |
| BC | ||||
| 6-hour mean | 0.5 μg/m3 | 3.84 (0.60 to 7.18) | 3.45 (0.22 to 6.78) | - |
| 24-hour mean | 0.3 μg/m3 | 3.00 (0.06 to 6.02) | 2.81 (−0.11 to 5.81) | - |
| OC | ||||
| 6-hour mean | 1.6 μg/m3 | 2.07 (−1.81 to 6.11) | 2.39 (−1.53 to 6.47) | - |
| 24-hour mean | 1.8 μg/m3 | −0.63 (−3.34 to 2.16) | −0.29 (−3.09 to 2.59) | - |
| SO42− | ||||
| 6-hour mean | 2.0 μg/m3 | 2.17 (−0.76 to 5.18) | 3.61 (0.48 to 6.83) | - |
| 24-hour mean | 1.6 μg/m3 | 1.58 (−1.50 to 4.77) | 3.24 (−0.10 to 6.70) | - |
| PN | ||||
| 6-hour mean | 10.1[103 particle/cm ] | 9.86 (3.59 to 16.52) | 9.12 (2.86 to 15.77) | - |
| 24-hour mean | 8.3 [103 particle/cm3] | 9.39 (2.41 to 16.85) | 8.57 (1.62 to 16.00) | - |
| NOx | ||||
| 6-hour mean | 17.8 ppb | 1.86 (−0.63 to 4.41) | - | 2.31 (−0.35 to 5.04) |
| 24-hour mean | 11.2 ppb | 3.31 (0.41 to 6.29) | - | 3.90 (0.73 to 7.17) |
| O3 | ||||
| 6-hour mean | 13.8 ppb | −2.10 (−6.56 to 2.57) | - | −2.56 (−7.25 to 2.36) |
| 24-hour mean | 12.1 ppb | −4.80 (−8.70 to 0.73 | - | −5.80 (−10.17 to 1.22) |
Each model is adjusted for subject, 24-hour mean temperature, 24-hour mean water vapor pressure, season, and scrubbed room NO.
Fine particles were additionally adjusted for 24-hour moving average of O3 concentrations.
Gases were additionally adjusted for 24-hour moving average of PM2.5 concentrations.
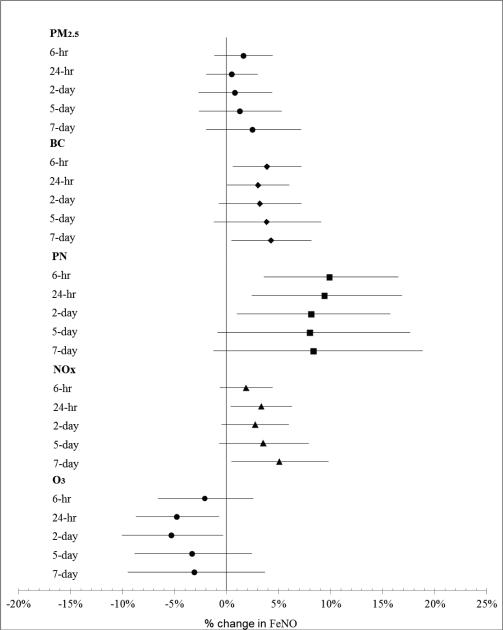
While the most consistent associations of pollutants with FeNO occurred with cumulative averages of pollution levels in the previous 24 to 48-hours, for NOx, PN and BC, cumulative averaged pollution levels up to 7 days were also associated with increased FeNO (Fig. 1). The 5-day-averaged central site PM2.5 or BC measured at the home / trip to clinic period were not significantly associated with FeNO levels with estimated changes in FeNO of (0.6%, 95% CI −2.30% to 3.59%) for an IQR increase in PM2.5 and (−0.34%, 95% CI −2.48% to 1.86%) for an IQR increase in BC respectively.
Fig. 1.
Association of FeNO with particles and gases at different moving averages. Estimates are expressed as changes per interquartile increase in pollutant concentrations. Error bars indicate 95% confidence interval. Each model is adjusted for subject, 24-hour mean temperature, 24-hour mean water vapor pressure, season, and scrubbed room NO.
DISCUSSION
Traffic-related particulate pollutants were associated with increased FeNO measurements in this study of individuals with T2DM. Influential pollutants included BC, whose sources are both regional and local traffic, and PN which others have found correlates with ultrafine particle levels, and with fresh emissions from motor vehicles. (Thurston and Laird 1985) Exposure to NOx, which has automobile as well as stationary combustion sources and includes primary pollutants (e.g., NO) from vehicular combustion processes, was also significantly associated with elevated FeNO measurements.
Associations of PN exposure with FeNO produced the most robust effect estimates (Table 3), which were generally 2-3 times higher than for other pollutants. This provides supportive evidence suggesting that ultrafine particles may be responsible for a significant proportion of pollution effects, and that in our study, PN is a better surrogate for local traffic exposures. Relatively little is known about the spatial variation of ultrafine particles in Boston, making the relationship between personal exposures and central site measures hard to characterize. Ultrafine particles coagulate very fast and their counts drop fast as moving away from traffic, perhaps explaining why we found wider confidence intervals for PN exposure effect estimates. The fact that we found strong associations of PN with our outcome, despite potentially higher local heterogeneity and potential exposure misclassification, supports our conclusion that PN may be specifically contributing to increased airway inflammation in our study participants.
After adjustment for ozone, higher FeNO was also associated with 6-hour averages of SO4, suggesting that pollution effects were not exclusively related to traffic, and that regional sources of pollution like power plants might also be contributing to the pollution-related pulmonary inflammation.
Most of the cumulative traffic pollution effects on FeNO levels occurred within a 2-day window, with peak associations with 6 to 24-hour averages of pollution. While far from fully understood, the rise in FeNO may result primarily from up-regulation of inducible form of NO synthase (iNOS) in response to inflammatory cytokine stimulation by air pollutants. Tarantini et al showed hypomethylation at the promoter region of iNOS gene after exposure to particulate matter, where iNOS gene expression was suppressed by DNA methylation. (Tarantini et al. 2009)
In contrast to the bleachery workers’ study, where Olin et al found elevated concentrations of FeNO after exposure to high peaks of O3 repeatedly, (Olin et al. 2004) we found negative associations of O3 with FeNO. The negative association between O3 and FeNO that we observed may, in part, be a function of its negative correlation with ambient fine particles and NOx (Table 2), rather than representing an actual physiologic effect. In addition, since the clinic examination was conducted near a busy A3 road, traffic related pollutants may appear to have greater impact on FeNO than other pollutants.
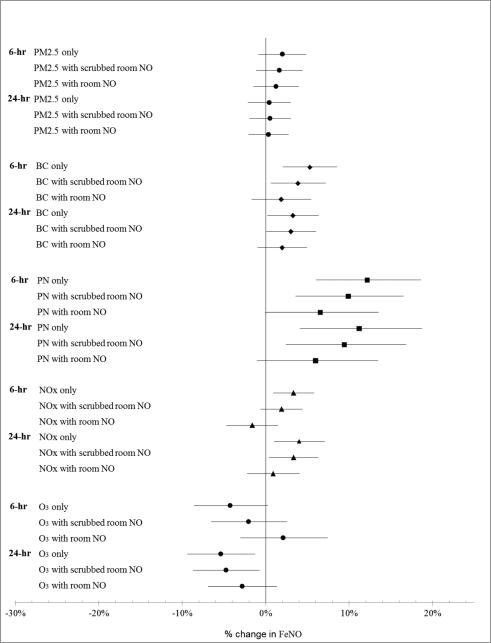
To eliminate potential contamination of patient samples from ambient inspired NO, participants breathed in through a NO scrubber, and the first portion of the exhalation (dead-space gas) was discarded. In addition, in our primary analyses we adjusted for the level of room NO collected through the scrubber that the patient used for the FeNO maneuver. By doing so, we further ensured that the exhaled NO we measured came from participants’ lungs, rather than from NO in the study room. Adjusting for unscrubbed room NO further reduced associations of pollution with our outcomes (Fig. 2), but we believe that this is “over-adjustment”. The unscrubbed grab sample of room NO (mean 34.55 ppb, standard deviation 29.03 ppb), which was variably correlated with other pollutants (Table 2), was often as high as or higher than 24-hour averaged NO measured at the EPA sites (mean 10.52 ppb, standard deviation 7.61 ppb, r = 0.53), likely not only because it represented a shorter averaging period during peak traffic, but also because it more accurately represented local traffic pollutant exposures encountered by the participant that may have led to FeNO production in the airways. Room NO was strongly predictive of FeNO (7.66% increase in FeNO per IQR increase in room NO; 95% CI 4.69% to 10.71%) in a model not adjusting for other pollutants. Many offline FeNO studies do not measure or take into account residual NO that may be inhaled despite scrubbing in clinical exam rooms where local NO from traffic is high; we suggest that assessment of the efficacy of the scrubber in high NO / high traffic settings may help in interpreting study findings. A Southern California Children's Health Study paper suggests that taking into account time to analysis or refrigeration until measurement could reduce measurement error in offline FeNO measurement. (Linn et al. 2004) In our case, however, our samples were refrigerated at 5°C until the time of measurement and there was little variability in the lag between time of sample collection, which always occurred in the morning, and time of offline FeNO measurement. The median time between collection and measurement was 6.85 hours (25th percentile, 5.70 hours; 75th percentile 8.37 hours). We performed further sensitivity analyses that confirmed that taking into account time to offline measurement of bagged specimens, did not improve (or significantly change) the magnitude or precision of our effect estimates (results not shown).
Fig. 2.
Estimates association of 6-hour and 24-hour mean pollution with pollutant alone, adjusted for scrubbed room NO, and adjusted for room NO, respectively. Estimates are expressed as changes per interquartile increase in pollutant concentrations. Error bars indicate 95% confidence interval. Each model is adjusted for subject, 24-hour mean temperature, 24-hour mean water vapor pressure, and season.
We addressed the potential misclassification of exposure measured at the central site through the home / trip to clinic particle monitoring. However, because the peak associations of PM2.5 and BC were observed within 6 to 24-hours of the clinic visit, our 5-day integrated home / trip to clinic PM2.5 and BC measurements were not associated with FeNO. The effect estimates were similar to those of 5-day means of PM2.5 and BC measured at the central site.
FeNO has been described as classically representing allergic and eosinophilic airway inflammation in asthmatic patients, (Dweik et al. 2011) Ambient air pollution has been associated with increased FeNO in children with asthma in a number of epidemiologic studies. (Delfino et al. 2006; Liu et al. 2009; Mar et al. 2005) COPD is classically described as involving chronic neutrophilic rather than eosinophilic inflammation, yet we demonstrated FeNO responses specifically in a small subgroup with COPD within a panel study of elders from Steubenville, OH. (Adamkiewicz et al. 2004) In this Boston study of people with diabetes we found no effect modification either by asthma or COPD diagnosis, though sample size limited power to detect effect modification by diagnosis.
The underlying chronic inflammation in people with T2DM is well-known to involve non-IgE mediated pathways with pro-inflammatory innate and adipokine-related cytokines. (Gold 2008) While one 2009 review suggested use of FeNO as well as other biomarkers in evaluation of inflammation in people with T2DM, (Maniscalco et al. 2009) we found only two small case reports in the literature, both focused on medication effects on inflammation. (Sexton et al. 2014; Yalcin et al. 2014) Neither report, one on anti-IgE (2 subjects) (Yalcin et al. 2014) and the other on metformin (17 participants) administration, (Sexton et al. 2014) demonstrate changes in FeNO with medication administration, but these studies are limited and their design and study population differ from that of our study. In a St. Louis panel study of elders we showed that diabetes, obesity and hypertension increased vulnerability to the systemic inflammatory effects of air pollution. (Dubowsky et al. 2006) Our study suggests that people with T2DM, who have underlying non allergic inflammation, are vulnerable to the pulmonary inflammatory effects of pollution, and that FeNO can be a useful marker of this response.
ACKNOWLEDGEMENTS
This study is funded by NIEHS PO1 ES-09825, NIEHS R21 ES-020194-01, US EPA RD-83241601, US EPA RD-83479801. The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors are grateful to all participants of the study and the staff responsible for data collection.
Footnotes
CONFLICT OF INTEREST
None of the authors has any actual or potential competing financial interests.
REFERENCES
- Adamkiewicz G, et al. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204–209. doi: 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip Environmental health perspectives. 2007;115:507–512. doi: 10.1289/ehp.9386. doi:10.1289/ehp.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. 2005 doi: 10.1164/rccm.200406-710ST. 2005. [DOI] [PubMed] [Google Scholar]
- American journal of respiratory and critical care medicine. 171:912–930. doi: 10.1164/rccm.200406-710ST. doi:10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Barath S, Mills NL, Adelroth E, Olin AC, Blomberg A. Diesel exhaust but not ozone increases fraction of exhaled nitric oxide in a randomized controlled experimental exposure study of healthy human subjects. Environmental health : a global access science source. 2013;12:36. doi: 10.1186/1476-069X-12-36. doi:10.1186/1476-069X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. doi:10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Brook RD, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. doi:10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- De Groote MA, Fang FC. NO inhibitions: antimicrobial properties of nitric oxide. Clin Infect Dis. 1995;21(Suppl 2):S162–165. doi: 10.1093/clinids/21.supplement_2.s162. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environmental health perspectives. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environmental health perspectives. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest. 2003;123:751–756. doi: 10.1378/chest.123.3.751. [DOI] [PubMed] [Google Scholar]
- Dweik RA, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American journal of respiratory and critical care medicine. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. doi:10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. American journal of respiratory and critical care medicine. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. doi:10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Gold DR. Vulnerability to cardiovascular effects of air pollution in people with diabetes Current diabetes reports. 2008;8:333–335. doi: 10.1007/s11892-008-0058-2. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environmental health perspectives. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environmental health perspectives. 2012;120:241–246. doi: 10.1289/ehp.1103647. doi:10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. American journal of respiratory and critical care medicine. 2001;164:738–743. doi: 10.1164/ajrccm.164.5.2012125. doi:10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Barnes PJ. Exhaled markers of inflammation. Curr Opin Allergy Clin Immunol. 2001;1:217–224. doi: 10.1097/01.all.0000011017.58506.f1. [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Wells AU, O'Connor BJ, Cole PJ, Hansell DM, Logan-Sinclair RB, Barnes PJ. Elevated levels of exhaled nitric oxide in bronchiectasis. Am J Respir Crit Care Med. 1995;151:1889–1893. doi: 10.1164/ajrccm.151.6.7767536. doi:10.1164/ajrccm.151.6.7767536. [DOI] [PubMed] [Google Scholar]
- Linn WS, Avila M, Gong H., Jr. Exhaled nitric oxide: sources of error in offline measurement. Archives of environmental health. 2004;59:385–391. doi: 10.3200/AEOH.59.8.385-391. doi:10.3200/AEOH.59.8.385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children Environmental health perspectives. 2009;117:668–674. doi: 10.1289/ehp11813. doi:10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco M, Palladino F, Mormile M, Sofia M. Exhaled nitric oxide and other major exhaled compounds for the diagnosis of metabolic diseases. Expert opinion on medical diagnostics. 2009;3:547–556. doi: 10.1517/17530050903104072. doi:10.1517/17530050903104072. [DOI] [PubMed] [Google Scholar]
- Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environmental health perspectives. 2005;113:1791–1794. doi: 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Loukides S, Culpitt S, Sullivan P, Kharitonov SA, Barnes PJ. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:998–1002. doi: 10.1164/ajrccm.157.3.97-05009. doi:10.1164/ajrccm.157.3.97-05009. [DOI] [PubMed] [Google Scholar]
- Olin AC, Andersson E, Andersson M, Granung G, Hagberg S, Toren K. Prevalence of asthma and exhaled nitric oxide are increased in bleachery workers exposed to ozone. The European respiratory journal. 2004;23:87–92. doi: 10.1183/09031936.03.00044402. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. doi:10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46:927–939. [PubMed] [Google Scholar]
- Sexton P, Metcalf P, Kolbe J. Respiratory effects of insulin sensitisation with metformin: a prospective observational study. Copd. 2014;11:133–142. doi: 10.3109/15412555.2013.808614. doi:10.3109/15412555.2013.808614. [DOI] [PubMed] [Google Scholar]
- Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. quiz 1094-1085 doi:10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environmental health perspectives. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini L, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environmental health perspectives. 2009;117:217–222. doi: 10.1289/ehp.11898. doi:10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston GD, Laird NM. Tracing aerosol pollution. Science. 1985;227:1406–1407. doi: 10.1126/science.227.4693.1406. doi:10.1126/science.227.4693.1406. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam JG, Verlaan BP, Van Loveren H, Elzakker BG, Vos SG, Opperhuizen A, Steerenberg PA. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Archives of environmental health. 1999;54:331–335. doi: 10.1080/00039899909602496. doi:10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environmental health perspectives. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin AD, Gorczynski RM, Cilli A, Strauss L. Omalizumab (anti-IgE) therapy increases blood glucose levels in severe persistent allergic asthma patients with diabetes mellitus: 18 month follow-up. Clinical laboratory. 2014;60:1561–1564. doi: 10.7754/clin.lab.2013.130302. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–592. doi: 10.1097/00001648-200209000-00016. doi:10.1097/01.EDE.0000020321.67963.7B. [DOI] [PubMed] [Google Scholar]
- Zanzinger J. Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc Res. 1999;43:639–649. doi: 10.1016/s0008-6363(99)00085-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hinders WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air & Waste Manage Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]