Abstract
Multidirectional interactions between the nervous and immune systems have been documented in homeostasis and pathologies ranging from multiple sclerosis to autism, and from leukemia to acute and chronic inflammation. Recent studies have addressed this crosstalk using cell-specific targeting, novel sequencing, imaging and analytical tools, shedding light on unappreciated mechanisms of neuro-immune regulation. This review focuses on neuro-immune interactions at barrier surfaces, mostly the gut, but also including the skin and the airways, areas densely populated by neurons and immune cells that constantly sense and adapt to tissue-specific environmental challenges.
The nervous system and immune system are the main body sensory interfaces that perceive, integrate and respond to environmental challenges. Both systems have the capacity to recall earlier challenges and events, mounting memory responses that anticipate and efficiently adapt to ever changing conditions. Nervous and immune cell functions rely on cell-to-cell contacts and on soluble molecules that act on proximal or distant target cells. These communication molecules include cytokines, chemokines, neuropeptides and neurotrophins (Ordovas-Montanes et al., 2015). Neurotransmitters and their receptors are expressed by immune cells, and neurons can sense and influence immune pathways (Kioussis and Pachnis, 2009), putting forward the intriguing hypothesis that functional neuro-immune interactions play an important role in tissue physiology. In line with this idea, the similarities between the nervous and the immune systems give rise to the concept that these two systems may be evolutionary related through a common ancestral precursor or by independent evolutionary ancestors with the co-option of distinct genetic traits from each other (Arendt, 2008). From an evolutionary angle, it is also likely that the concerted action of the immune and nervous systems may have ensured improved tissue, organ and organismic integrity in health and disease.
Neuro-immune interactions during hematopoiesis
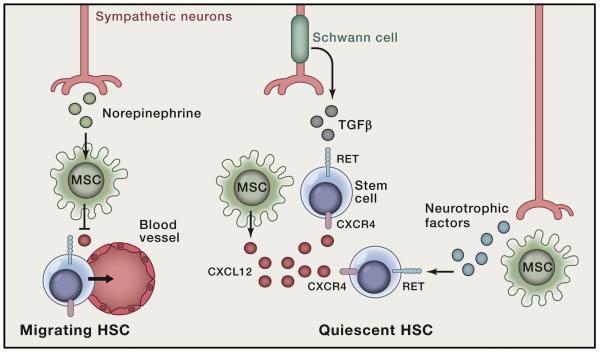
Neuro-immune interactions can be traced back to the earliest steps of the immune system ontogeny. Immune cells are generated through hematopoiesis, a developmentally regulated cascade that gives rise to all blood cell lineages from quiescent hematopoietic stem cells (HSC). In adult life this process occurs in the bone marrow where sympathetic nerves are highly abundant and neuronal components contribute to the HSC niche (Isern et al., 2014; Katayama et al., 2006; Yamazaki et al., 2011). Sympathetic neuron-derived norepinephrine was shown to increase HSC mobilization into the blood, via regulation of CXCL12 expression in bone marrow mesenchymal stem cells (Katayama et al., 2006; Mendez-Ferrer et al., 2010) (Figure 1). These cellular and molecular signaling axes are further regulated by circadian rhythms and are of high relevance in the context of psychological stress (Heidt et al., 2014; Mendez-Ferrer et al., 2008). Importantly, sympathetic neuropathy was shown to regulate myeloproliferative neoplasms, further highlighting the importance of nervous/mesenchymal/HSC interactions in health and disease (Arranz et al., 2014). Schwann cells, which ensheath bone marrow nerves, were also shown to control HSC quiescence through activation of latent transforming growth factor β (TGF-β) (Yamazaki et al., 2011), and neuron-derived catecholamines can directly control HSC mobilization (Spiegel et al., 2007) (Figure 1).
Figure 1. Neuroregulators control hematopoietic stem cells (HSC).
Neuron satellite Schwann cells, induce activation of latent TGF-β that ensures HSC quiescence. In turn, neurotrophic factors directly activate HSC via the tyrosine kinase receptor RET leading to improved HSC survival. Sympathetic nervous fibers produce norepinephrine that downregulates CXCL12 expression in mesenchymal stem cells (MSC). Reduced CXCL12 expression by MSC prompts HSC egress from the niche.
The anatomical and functional interactions between autonomic nerves and HSC gave rise to the concept that neurons and HSC may be co-regulated through similar signals. In agreement, the neurotrophic factor receptor RET was shown to drive HSC survival, expansion and function (Fonseca-Pereira et al., 2014). Hematopoietic-intrinsic ablation of Ret led to impaired HSC survival, reduced HSC numbers and loss of stress response and reconstitution potential (Fonseca-Pereira et al., 2014). RET signals provide mouse and human HSC with Bcl2 and Bcl2l1 survival cues, downstream of p38/MAP kinase and CREB activation (Fonseca-Pereira et al., 2014) (Figure 1). Thus, it is possible that neuronal activity might be regulated by blood progenitors through neurotrophic factor consumption in the HSC environment.
The largest lymphoid tissue meets the second brain
In addition to the crosstalk between sympathetic neurons and HSC, other striking parallels were established between the development of the enteric nervous system (ENS) and lymphoid organogenesis in the intestine. Notably, the neurotrophic factor receptor RET was shown to be critical to both Peyer’s patches and nervous system development in the intestine (Patel et al., 2012; Schuchardt et al., 1994; Veiga-Fernandes et al., 2007). Moreover, the development of lymphoid tissue inducer cells, an immune cell subset responsible for secondary lymphoid organ formation, strictly depends on cell-autonomous retinoic acid signals (van de Pavert et al., 2014) that could be provided by adjacent neurons (van de Pavert et al., 2009). While most anatomical constituents of the ENS form during embryogenesis, a post-natal neurogenesis and biogenesis wave occurs shortly after birth, which coincides with microbiota colonization, contact with dietary antigens and development of fully mature enteric immune structures. Is therefore tempting to speculate that enteric neural networks might be shaped by microbial and dietary signals-induced immune milieu.
In general, tissue-associated neurons are classified as intrinsic (cell bodies that lie within the tissue) versus extrinsic (cell bodies that lie outside of the tissue, including the sympathetic and the parasympathetic autonomic nervous system) (Furness et al., 1999). In adulthood the intestine harbors the largest lymphoid cell compartment in the body, but it also contains what has been coined as the “second brain”, a neuronal network with as many neurons as the spinal cord (Gershon, 2010). Enteric-associated neurons (EAN) include an extensive and diffuse network of millions of sensory neurons, interneurons, and motor neurons, which control a variety of functions within the gastrointestinal (GI) tract (Mayer, 2011) (Figure 2). The intrinsic neural ganglia within the intestine (also known as the enteric nervous system) are organized in several plexuses throughout the intestinal wall: the myenteric (or Auerbach's plexus, between the circular and longitudinal muscle layer) and submucosal (or Meissner's plexus, in the submucosa) plexuses. Both areas are relatively poor in terms of diversity of immune cells, although macrophages accumulate in large numbers (Bogunovic et al., 2009) and mast cells are reported to surround nerve terminals (Schemann and Camilleri, 2013). Although EAN express a diverse set of neurotransmitters, the exact physiological relevance of many of these molecules in the gut remains unclear.
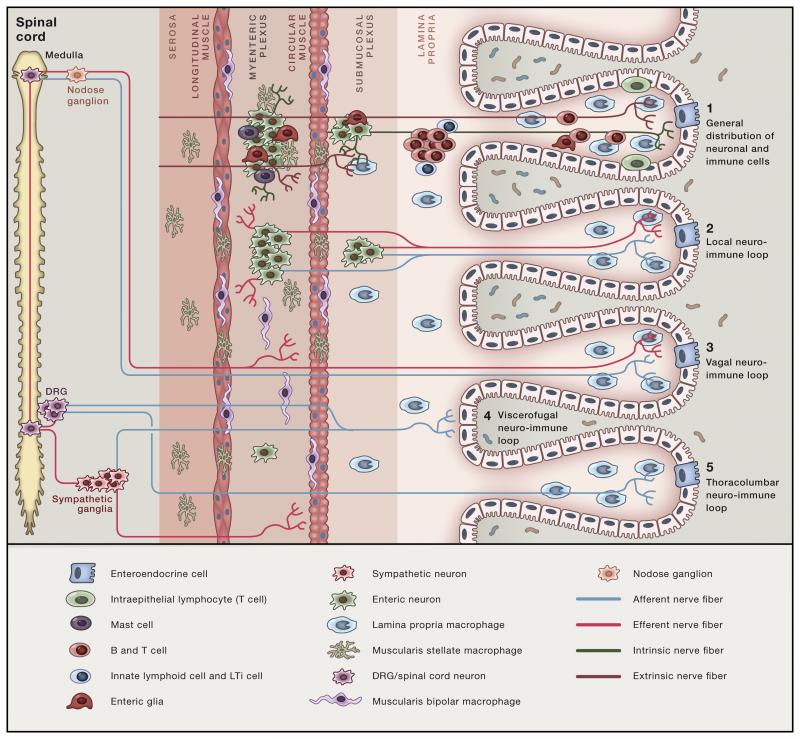
Figure 2. Neuro-immune circuits in the intestine.
(1) Most of immune cells accumulate in the lamina propria of the mucosa regions while neuronal cell bodies are restricted to the submucosal and myenteric plexuses, regions populated by distinct muscularis macrophages and mucosal mast cells. Extrinsic and intrinsic neuronal processes innervate all intestinal layers. Initial sensing of luminal perturbations may be mediated by intestinal epithelial cells (IECs), innate immune cells or directly by neurons. Among several possible downstream neuro-immune circuits, four are depicted: 2- Short local neuro-immune circuit between lamina propria and muscularis immune cells and the (intrinsic and extrinsic) nervous system. 3- Vagal neuro-immune loop with afferent and efferent fibers originating within the medulla oblongata. 4- Long-range enteric-associated neuron-immune loop, with intestinofugal fibers projecting to the spinal cord or ganglion (a) or afferent and efferent fibers originating in the DRG and spinal cord, respectively (b). Red processes: afferent. Light blue processes: efferent.
The mucosal layer also harbors nerve and glial cell networks known as the mucosal plexus (Nijhuis et al., 2010; Van Landeghem et al., 2011), including nerve endings that are potentially in contact with mucosal immune cells, highly concentrated in this layer. In contrast to enteric neurons that are found within enteric ganglia, enteric glial cells, which outnumber enteric neurons (Furness, 2008), are also found in interganglionic regions, within the smooth muscle layers and the intestinal lamina propria. Enteric glial cells express several markers including p75, Sox-10, GFAP and S100β (Boesmans et al., 2015) and using flow cytometry analysis glial cells fall into a population of CD45negTer119negCD31negCD49bpos (Joseph et al., 2011) (Fig.2).
Earlier observations highlighted the functional importance of glial cells in mucosal protection. It was found that in the inflamed ileum of Crohn’s disease patients, glial cells express MHC-II, suggesting a possible role for these cells in neuro-immune interactions (Geboes et al., 1992; Koretz et al., 1987). Additionally, ablation of enteric glial cells led to fulminant jejunoileitis with consequent loss of gut integrity but through a mechanism that still remains elusive (Bush et al., 1998; Cornet et al., 2001). While a complex and dense network of glial cells is present in the mucosal layer (Liu et al., 2013; Neunlist et al., 2013; Wedel et al., 1999), where most enteric immune cells locate, very little is known on the relevance of glial-immune cell interactions in the intestinal tract.
In addition to the enteric nervous system, the central nervous system communicates with the intestine through the extrinsic innervation and the hypothalamic pituitary adrenal axis (HPA), forming what has been coined the “gut-brain axis”. The vagus represents the main extrinsic parasympathetic nerve connecting the brainstem to the gut. Vagal preganglionic fibers emerge from motor neurons in the dorsal nucleus of the vagus nerve and establish connections with postganglionic neurons in the intestinal myenteric plexus of the mid gut (Figure 2). Interestingly, while the proximal colon is innervated by the vagus nerve, the distal colon parasympathetic innervation originates from sacral spinal nerves (Willemze et al., 2015). Altogether, these inputs modulate several aspects of intestinal physiology through stimulation of the HPA axis and secretion of neuropeptides. Adding to the intricate nature of these neuronal networks, different neuropeptides often co-localize in the same neurons and regulate similar functions, activating similar or identical pathways (Shepherd et al., 2005).
Revisiting neuro-immune interactions
Major pitfalls in the study of neuro-immune interactions include the use of static imaging analysis, which provides poor insight into spatio-temporal dynamics, the lack of deep-tissue imaging technologies and limited in vitro approaches. Moreover, lack of specific driver lines targeting discrete immune cell subsets and local or peripheral neurons has hindered faster progress in the understanding of neuro-immune interactions. Nevertheless, over the last decade some of these technical obstacles have been partly surmounted, shedding light on novel aspects of barrier tissue physiology (Table 1).
Table 1.
Examples of available tools for “neuro-immune 2.0”
| Category | Name | Application | Limitation | REFs |
|---|---|---|---|---|
| Cell-sorting independent transcriptomics |
BacTRAP | Translating mRNA profiling (GFP-tagging) |
RNAses and additional enzymes in mucosal surfaces |
(Schmidt et al., 2013) |
| RiboTag | Translating mRNA profiling (HA-tagging) |
RNAses and additional enzymes in mucosal surfaces; HA tagging requires additional staining for cell visualization |
1, (Sanz et al., 2009) | |
| Imaging tools | CLARITY, CUBIC, Scale, iDISCO and others |
Improved tissue clearing techniques associated with novel cell labeling tools and modern microscopy. |
Generally time- consuming; reduced preservation of fluorescence reporters; variable results regarding preservation of tissue architecture and specific cellular localization (reviewed by (Richardson and Lichtman, 2015) |
(Chung et al., 2013; Erturk et al., 2012; Gabanyi et al., 2016; Renier et al., 2014; Tainaka et al., 2014; Tomer et al., 2015; Tomer et al., 2014; Zhang et al., 2014) |
| Intravital multi- photon microscopy |
Visualize neuro-immune cell dynamics and activation patterns in vivo |
Anesthesia and manipulation may interfere with firing patterns; difficult access of several mucosal sites |
(Gabanyi et al., 2016; Koenigsknecht-Talboo et al., 2008; Kong et al., 2015; Zariwala et al., 2012) |
|
| In vivo manipulation tools |
DREADD | Stimulate or inhibit neurons upon exposure to synthetic GPCRs |
Requires neuronal-type (and location) specificity to avoid off-target (e.g. CNS) effects |
(Alexander et al., 2009) |
| Optogenetics, | Optical control of neuronal activation or inhibition |
Difficult access of specific barrier sites |
(Emiliani et al., 2015; Prakash et al., 2012) | |
| In vitro tools | iPS- or stem cell-based tissues |
iPS can be differentiated towards functional immune or neuronal cells |
Limited recapitulation of physiological interactions |
(Fattahi et al., 2016; Sato et al., 2009; Spence et al., 2011) |
Isolation of immune cells by flow cytometry followed by gene expression or protein analysis throughout development allowed a detailed classification of immune cell lineages and subsets. However, in-depth characterization of specific neuronal subtypes at barrier surfaces has been hampered by the difficulty of isolating intact neurons for functional or gene expression analysis. In the past few years, the development of translating ribosome, affinity purification-based profiling techniques, helped mapping and better defining circuitry and the identity of CNS neurons (Schmidt et al., 2013). Strains containing modified tagged ribosomal constructs, such as BacTRAP and RiboTag, associated with lineage-specific Cre-based approaches, have allowed further expansion of sorting-independent analyses of cell populations through affinity-tagged purification, enabling the identification of actively translated ribosome-bound mRNAs in cells from intact tissues (Sanz et al., 2009), including peripheral neurons populating barrier surfaces, such as the intestine (Gabanyi et al., 2016).
Advances in intravital multiphoton microscopy (IVM) allowed simultaneous neuronal and immune cell visualization in vivo with minimal manipulation (Koenigsknecht-Talboo et al., 2008; Kong et al., 2015). Recent studies merged novel imaging and functional assays in a cell-specific manner in vivo, thus associating in vivo imaging of neuron cell dynamics, activity and synaptic tracing in health and disease. These technologies, including mouse strains carrying genetically-encoded Cre-dependent calcium indicator (GCaMPs) (Zariwala et al., 2012) can now be employed to define the patterns of neuronal firing in the steady state barrier surfaces and upon microbial stimuli (Gabanyi et al., 2016). Nevertheless, how additional mucosal aggressions may affect neuronal firing remains to be determined.
Additionally, groundbreaking improvements in tissue-clearing techniques, associated with high-resolution light sheet microscopy have allowed 3-dimensional (3D) analysis of cellular networks in intact tissues (Chung et al., 2013; Erturk et al., 2012; Renier et al., 2014; Tainaka et al., 2014; Tomer et al., 2014). In particular, the combination of enhanced techniques to improve tissue optical transparency while preserving the fluorescence of gene reporters (or associating it with immune-labeling tools) will allow the visualization of neuro-immune circuits at cellular-resolution (reviewed by (Richardson and Lichtman, 2015)). For instance, the CLARITY technique allowed the identification of a bilaterally connected neuronal system with dendrites that embrace the dorsal columns in the mouse spinal cord (Zhang et al., 2014). Additionally, immunolabeling-enabled three-dimensional imaging of solvent-cleared organs (iDISCO) (Renier et al., 2014) revealed a deep-tissue, 3D view of EAN distribution within the intact intestinal tissue and provided insights into neuronal networks innervating areas where macrophages are abundant (Gabanyi et al., 2016). Furthermore, recent faster scanning technology, made possible by extended depth of view of light sheet microscopy, will likely bring whole tissue imaging even further into subcellular resolution of larger mammalian tissues, with the exciting possibility to simultaneously analyze Ca2+ signaling, thus allowing the analysis of structure and function of deep tissue neurons (Tomer et al., 2015).
Another important tool developed to influence neuronal activation is the DREADD technology, a chemical-genetic approach to enhance or decrease neuronal firing potential noninvasively through a G protein-coupled receptor (GPCR) (Alexander et al., 2009). Conditional intersectional genetics permits the selective manipulation of designer receptors exclusively activated by designer drugs (DREADDs). Notably, activation of the expression of the synthetic receptor Gq-coupled stimulatory human M3 muscarinic DREADD (hM3Dq) or inhibitory human M4 muscarinic DREADD (hM4Di), which can be temporally-controlled by the timing of clozapine-N-oxide (CNO) administration. An alternative to DREADD systems, currently widely used to modulate neuronal activity in vivo is optogenetics (Prakash et al., 2012). By introducing microbial opsin genes into neurons to regulate firing potential, current optogenetic approaches include both gain- and loss-of-function strategies, based on a diverse set of mouse strains or on in situ-delivery of adeno-associated virus (AAV) expressing Channelrhodopsin-2 (ChR2) (activating), archaerhodopsin (Arch) (inhibitory) or additional probes to manipulate neuronal activation with a millisecond precision (Emiliani et al., 2015). Although these approaches were utilized for manipulation of neurons at barrier sites, efforts to modulate neuro-immune interactions by light have been limited. Nevertheless, current optogenetic toolbox can provide sophisticated, cell-specific and spatially precise intervention strategies for barrier sites.
Finally, recent progress in derivation of mammalian pluripotent stem (PS) cells into differentiated tissue cells (Takahashi and Yamanaka, 2006) has opened yet another window of opportunity for studies interrogating cellular and molecular mechanisms of neuro-immune interactions. Specific culture conditions allowed iPS cell differentiation towards functional enteric neurons capable of rescuing mice that would otherwise develop Hirschsprung’s disease or congenital intestinal aganglionosis (Fattahi et al., 2016), which is caused by defects in enteric innervation. Interestingly, severe enteric inflammation is frequently observed in Hirschsprung’s disease patients (Harrington et al., 2005), but it remains to define whether these are due to intrinsic immune defects or secondary to enteric neuron-motility dysfunction. Recent advances in this technology include 3D tissue organoids with an intricate array of cell types (Sato et al., 2009; Spence et al., 2011), raising the possibility that in the near future iPS-derived EANs, as well as immune, muscle, neuron and glia cells will allow complex in vitro organoid setups. For instance, neurons isolated from the myenteric plexus of adult mice could give rise to neurospheres that might be possibly used in combination with epithelial mini-gut organoids (Grundmann et al., 2015; Sato et al., 2009). The multi-tissue organoids will be extremely useful in deciphering functional aspects of central and peripheral neurons and how these cells respond to environment cues or modulate neighboring cells, and vice-versa.
Neuro-immune sensing at barrier surfaces
Sensing is a property routinely ascribed to both immune and nervous systems, although it implies structurally different processes such as immune recognition of pathogens versus nociceptive neurons sensing a potentially damaging perturbation. In the communication between neural and immune cells, complementary types of sensing may take place at barrier surfaces. Immune cells, particularly innate immune cells, utilize several different mechanisms of sensing infectious agents and other potentially pathogenic perturbations, including pathogen recognition receptors, which sense pathogen-associated molecular patterns (PAMPs), such as LPS and CpG, but they also sense molecules associated with sterile inflammation and tissue damage, also known as damage-associated molecular patterns (DAMPs), such as HMGB1 and heat-shock proteins (Iwasaki and Medzhitov, 2015). Several antigen-presenting cells, such as skin Langerhans cells and intestinal CX3CR1 mononuclear phagocytes and CD103+ epithelial dendritic cells are located at the interface between the body and the antigen-rich environmental and can serve as initial sensors of epithelial insults (Farache et al., 2013; Iwasaki and Medzhitov, 2015; Mazzini et al., 2014). Furthermore, epithelial cells, also considered part of innate immunity, are known to express multiple PAMPs and other immune-receptors (Iwasaki and Medzhitov, 2015). Epithelial cells can sense microorganisms as well as stress or damaged tissues and communicate to neighboring cells, which include neurons or neuronal processes. Among epithelial cells, enteroendocrine or enterochromaffin cells (ECs) are also able to produce several hormones, neurotransmitters and peptides, including Glucagon-related peptides (GLPs), peptide YY, gastric inhibitory polypeptide (GIP) and the majority of the body’s serotonin (Gershon, 2010). The production of secreted molecules by ECs largely influences immune cells located in the epithelial and lamina propria compartments. ECs also affect fibers innervating the mucosal regions, activating a circuit that modulates gut motility (Bulbring and Crema, 1959) as well as CNS function. Conversely, pulmonary neuroendocrine cells (PNECs), directly exposed to large quantities of airborne antigens, were recently shown to trigger lung immune cell activation via secretion of neuropeptides (Branchfield et al., 2016). PNECs, as well as eosinophils induced during allergic responses co-localize with airway innervation (Costello et al., 1997; Kuo and Krasnow, 2015). Thus, it is likely that PNECs and eosinophils influence neuronal sensing during airway challenges, analogous to what is thought to operate in the intestine. In line with this idea, it was found that eosinophil-derived granule proteins stimulate vagal pulmonary C-fibers and release of substance P and CGRP can promote eosinophil chemotaxis (Lee et al., 2001; Numao and Agrawal, 1992).
In contrast to the airway and intestinal mucosa, much less is known of neuro-immune sensing circuits in the genital tract. Studies on herpes simplex virus (HSV), a neurotropic virus that infects the majority of human population, have shown that after skin or mucosal epithelial cell infection, HSV invades sensory neurons and follows retrograde axonal transport to reach the sensory ganglia, persisting as latent infection with occasional reactivation, which leads to anterograde transport to mucosal sites and inflammation (Shin and Iwasaki, 2013). Cytosolic HSV DNA sensing by epithelial cells initiates an immune cascade involved in resistance to neuronal invasion and damage (Royer and Carr, 2015). Nevertheless, the afferent and efferent components of this circuit remain to be determined.
The secretory function of specialized epithelial cells appears to be largely influenced by the particular barrier environment. For instance, spore-forming bacteria-derived metabolites, observed in the steady state intestine, were shown to promote serotonin synthesis by colonic ECs (Yano et al., 2015). Additionally, a recent study that performed human microbiota transplant into germ-free mice concluded that dietary composition influences enteric neuronal activity and gut motility via the modulation of the microbiota, although a role of ECs in this process was not examined (Dey et al., 2015). High-resolution microscopy and ECs reporter strains revealed a direct contact between cytoplasmic projections (neuropods) of intestinal ECs and neurons innervating the small intestine and colon (Bohorquez et al., 2015). Another example of this communication was described in the skin, where the epithelial cell-derived cytokine thymic stromal lymphopoietin (TSLP) mediates the activation of skin nociceptive afferent sensory neurons via the transient receptor potential cation channel, subfamily A, member 1 (TRPA-1), promoting itch (Wilson et al., 2013). The constitutive expression of TSLP receptors in sensory neurons, associated with the upregulation of this cytokine during the course of allergic responses (Wilson et al., 2013), reinforces the role of epithelial cells in communicating environmental perturbations to surrounding neurons. Therefore, in addition to function as biosensors to nutrients and bacterial metabolites, epithelial cells can mediate a cascade of functional responses including chemosensing, regulation of motility and food intake, and energy homeostasis (Furness et al., 2013).
In addition, direct neuronal sensing of environmental alarms has been proposed at barrier sites in mammals. In the airways, nociceptive neurons can directly sense bacterial-derived N-formylated peptides or toxins, inducing pain, a process thought to negatively regulate inflammation (Chiu et al., 2013). Expression of Toll-like receptors (TLRs) by sensory neurons has also been shown (Liu et al., 2010). In addition to the TSLP pathway described above, activation of TLR7 in nociceptive neurons in the skin was also shown to induce itch, although it appeared dispensable for neuropathic pain, which required both TLR7 and TRPA1-derived signals (Liu et al., 2010; Park et al., 2014). Enteric neurons were proposed to express PRRs, including TLR2 or TLR4, which could directly or indirectly aid the activation of nociceptive-associated ion channels (Anitha et al., 2012; Barajon et al., 2009; Meseguer et al., 2014). Similarly, enteric glial cells were also shown to express TLR2, TLR3, TLR4 and TLR7 (Barajon et al., 2009; Brun et al., 2013; Brun et al., 2015; Rumio et al., 2006). However, studies utilizing cell-specific transcriptomics tools and precise, lineage-specific intersectional genetics are still required to further define these sensing circuits, and how they influence inflammatory or neuronal cells at local and distal sites. Nevertheless, direct neuronal sensing of environmental alarm signals has been reported in worms, which suggest that this pathway is evolutionary conserved. TOL-1, the sole TLR-related molecule found in C. elegans, was found to be involved in CO2 sensing by chemosensory neurons (Brandt and Ringstad, 2015). Of relevance, detection of microbial respiration by sensory neurons in worms results in pathogen avoidance behavior (Brandt and Ringstad, 2015; Pujol et al., 2001), another parallel with the mammalian system, discussed below.
Neuro-immune interactions in health and disease
Multidirectional functional consequences of neuro-immune interactions have been appreciated for decades (Besedovsky et al., 1983; Stead et al., 1987), with emphasis given to stress-mediated alterations in immune responses and, conversely, cytokine-mediated alterations in CNS including animal behavior and brain activity (Matsunaga et al., 2011; Serrats et al., 2010). As an example, efferent vagal nerve signals were shown to attenuate macrophage activity leading to anti-inflammatory responses in the intestine (de Jonge et al., 2005; Rosas-Ballina et al., 2011; Wang et al., 2003). More recently, additional reports have incorporated the barrier surfaces as a source for perturbations observed in the CNS, adding complexity to these earlier findings.
The gut microbiota was proposed to play an important role in autism spectrum disorders (ASD). Colonization with indigenous species of the gut microbiota partially rescued defects in anxiety-like and sensorimotor behaviors in offspring of mice subjected to the maternal-induced activation (MIA) model, in which synthetic dsRNA [poly(I:C)] administration to pregnant mice results in ASD symptoms in the progeny (Hsiao et al., 2013). More recently, MIA offspring behavioral defects were linked to elevated levels of the maternal cytokine IL-17A in the fetal brain, which correlated with abnormal cortical development and ASD-like behavioral abnormalities, including defects in ultrasonic vocalization responses, social interactions and repetitive/perseverative behaviors (Choi et al., 2016). Therefore, cytokines produced in response to gut microbes can reach the CNS, affecting brain development and animal behavior.
Intestinal glial cell development relies on a mature microbiota (Kabouridis et al., 2015) and glial cells have been reported to sense microbial products through TLR molecules (Barajon et al., 2009; Brun et al., 2013; Brun et al., 2015; Rumio et al., 2006). Activation of glial cells by pathogens leads to S-100b activation and induction of NO synthase (iNOS) (Sharkey, 2015). Interestingly, S-100b regulation of iNOS activity has been shown in ulcerative colitis and celiac disease (Cirillo et al., 2009; Cirillo et al., 2011; Esposito et al., 2007), and increased iNOS activity may lead to disrupted barrier function (Xiao et al., 2011). Altogether, these data indicate that the intestinal neuroglia may sense microbial products and pathogenic insults, but whether and how glial-derived signals modulate immune cell functions in the intestine remains elusive.
Sensing of immunogenic stimuli, including helminthic infection, noxious xenobiotics, venoms and irritants, during allergic responses at barrier sites can also lead to local and systemic behavior responses (discussed in (Palm et al., 2012)). Noradrenergic neurotransmitter nerves can directly inhibit NKT cell function, which contributes to systemic immunosuppression (Wong et al., 2011). Nociceptive neurons initiate “protective reflexes”, which during airway and gastrointestinal allergic responses are associated with coughing and bronchoconstriction or nausea, diarrhea and vomiting, respectively. The TH2 cytokine IL-5 was shown to activate lung nociceptive neurons, which, in turn, secrete vasoactive intestinal peptide (VIP) to activate innate-lymphoid cells and lymphocytes associated with the development of airway allergic responses (Talbot et al., 2015). Similarly, airway parasympathetic neuron-derived eotaxin induces eosinophil migration towards airway nerves in a CCR3-dependent manner (Fryer et al., 2006). Reciprocally, depletion or inhibition of nociceptive neurons in the airways prevents the development of airway allergic responses (Talbot et al., 2015). Sensory neurons were also shown to modulate innate and adaptive immunity in several other pathologies, including allergy, psoriasis, rheumatoid arthritis and colitis (Caceres et al., 2009; Engel et al., 2011; Levine et al., 1984; Ostrowski et al., 2011). An interesting, yet relatively unexplored phenomenon, is food aversion induced by the oral exposure to an allergen, which results in a IgE-dependent activation of specific brain regions related to anxiety and avoidance behavior (Basso et al., 2003; Cara et al., 1994). Avoidance behavior can also be induced upon airway exposure to antigens, albeit in this case the aversion is directed towards the environment containing the antigen (Costa-Pinto et al., 2005). In both cases, release of soluble factors upon antigen contact to IgE-bound mast cells appears to mediate local activation of C-sensitive fibers (Basso et al., 2003; Costa-Pinto et al., 2005).
The association between mast cells and neurons has been described in the skin and mucosal sites, particularly in the intestinal submucosal and myenteric plexuses (Stead et al., 1989; Stead et al., 1987; van Diest et al., 2012). The privileged location of mast cells is coupled to their capacity to quickly release pre-formed and de novo synthesized mediators such as histamine, serotonin, TNF-α and tryptase, known to modulate both inflammatory responses and neuronal activation (van Diest et al., 2012). Furthermore, the mast cell’s ability to respond to environmental cues derived of immune cells, such as bound IgE-mediated degranulation, as well of neurons, such as to substance P and corticotropin releasing factor (CRH), strongly suggests a key role for mast cells in the communication between the nervous and immune systems (van Diest et al., 2012). Whether signals from mast cells utilize vagal innervation or dorsal root ganglia to communicate enteric signals to the CNS is not entirely clear. Additionally, the exact cellular and molecular mechanisms of this circuitry remain to be determined. Nevertheless, the fact that some of the molecules involved in mast cell-neuron interaction are co-opted to sense environmental alarms, such as noxious substances, capsaicin and lipids (found both in allergens and in parasites), suggest a conserved mechanism of neuro-immune interaction to regulate behavior. Indeed, an analogous mechanism of avoidance to prevent the ingestion of pathogenic substances occurs in worms, which utilize a direct neuronal chemosensory mechanism to detect bacterial metabolites or carbon dioxide, inducing avoidance (Brandt and Ringstad, 2015; Meisel et al., 2014). Conversely, non-neuronal serotonergic responses, induced during cellular stress is sufficient to trigger neuronal-dependent avoidance in worms (Melo and Ruvkun, 2012). These parallel observations in worms and mammals suggest that microbes or noxious agents that disrupt core cellular functions can trigger a remarkably conserved surveillance mechanism that synchronizes various inputs to accordingly modulate organism’ behavior (Palm et al., 2012).
In addition to mast cell-neuron structural proximity and functional interactions, recent studies suggest that barrier tissue resident macrophages also establish bi-directional functional interactions with neurons. Macrophages located at the muscularis (MMs) region of the intestine directly regulate the activity of enteric neurons and peristalsis via secretion of BMP-2 in a microbiota-dependent manner (Muller et al., 2014). Conversely, IVM recordings suggested that stellate-shaped MMs, which surround ganglia and neuronal cell bodies, are able to sense neuronal signals (Gabanyi et al., 2016). Indeed, microbiota colonization correlated with the expression of CSF-1 by enteric neurons juxtaposed to CSF-1R+ MMs (Balmer et al., 2014). Additionally, activation of extrinsic sympathetic ganglia by enteric bacterial infection was shown to modulate adrenergic β2R+ MMs polarization towards tissue-protective gene expression (Gabanyi et al., 2016). Although neither the sensing circuit of these pathways, nor its downstream consequences are yet elucidated, the proximity of macrophages expressing a tissue-protective program to neurons may have important implications for infection- or inflammation-induced tissue damage disease tolerance (Medzhitov et al., 2012), perhaps representing an analogous activity to that described for microglia in the CNS: protecting closely associated neuronal processes (Davalos et al., 2005; El Khoury et al., 2007; Nimmerjahn et al., 2005; Wang et al., 2015). Whether this pathway is represented at additional barrier surfaces and, like mast cells, whether tissue macrophages play a role in modulating distal CNS activity or behavior remains to be determined.
Perspectives
While It has been clearly established that lymphocytes compete with their peers for limited resources (Freitas and Rocha, 2000), it remains unclear if neuro-immune interactions might be also co-regulated by competition for commonly used resources. As an example, HSC have been shown to be direct and indirect targets of neuroregulatory molecules (Fonseca-Pereira et al., 2014; Isern et al., 2014; Katayama et al., 2006; Yamazaki et al., 2011); whether consumption of neuromediators and neurotrophins in the HSC environment shape neuronal or immune cell fates remains to be explored. Similarly, neurons and immune cells at barrier surfaces might share common niches where competition for specific resources may shape each cell type fate.
Dissection of the micro-anatomical confinement of enteric glia revealed a dense network of mucosal glial cells that largely outnumbers neurons (Liu et al., 2013; Neunlist et al., 2013; Wedel et al., 1999). The mucosal lamina propria also harbors large numbers of lymphocytes, notably innate lymphoid cells and adaptive B and T cells. In addition, the increasing evidence demonstrating micro-environmental sensing properties of glial cells puts forward the hypothesis of functional glial-immune cell interactions at the intestinal mucosa. Similarly, how intricate and closely associated networks of neuronal, immune and glia cells integrate endogenous and exogenous perturbations during physiology and disease conditions, regulating each other’s activity, constitutes a yet unresolved question.
The emergent evidence for micro-anatomical and functional neuro-immune units has not been fully complemented by mechanistic and physiological and pathological insights. Recent progress in iPS technology and epithelial and glial tissue engineering will most certainly generate complex “multi-tissue” organoid setups allowing for the study of mechanistical aspects of neuro/glial/immune interactions. Despite the notable technological and conceptual advances in discrete neuro-immune interactions, a deeper understanding of the physiology of system interactions at organism-level is still lacking. In this regard, tissue-clearing and novel imaging techniques may pave the way for future understanding of organismic neuro-immune circuits (Chung et al., 2013; Erturk et al., 2012; Renier et al., 2014; Tainaka et al., 2014; Tomer et al., 2014). Nevertheless, it is towards the establishment of tissue homeostasis following injuries, including infection, obesity, dietary insults or xenobiotic aggressions.
In conclusion, over the past decade we have witnessed the transforming power of new tools in deciphering neuro-immune physiology. Tissue-specific genetic targeting, novel sequencing approaches and groundbreaking imaging tools revealed unappreciated functional and mechanistic consequences of neuro-immune interactions. The technology step-change of “neuro-immune 2.0” has just started and we can certainly anticipate that new tools will allow for the discovery of new anatomical and functional bases for neuro-immune units at an organismic level, in health and disease.
Acknowledgements
We are indebted to members of our laboratories, particularly D. Esterhazy, D. Hoytema, Paul Muller (Mucida lab) and S. Ibiza, B. García-Cassani (Veiga-Fernandes lab) for discussions, help with the figures, and critical reading and editing of the manuscript. We thank Rui M. Costa for helpful discussions. D.M. and H.V.-F. are supported by a Kenneth Rainin Foundation Awards. H.V.-F. is also supported by EMBO (1648); ERC (647274), EU; Chron’s and Colitis Foundation of America, US; and FCT, Portugal.
Footnotes
The authors have no conflicting financial interests.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. e1004. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D. The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet. 2008;9:868–882. doi: 10.1038/nrg2416. [DOI] [PubMed] [Google Scholar]
- Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntion S, Tzeng YS, Lai DM, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6:237ra266. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AS, Pinto FA, Russo M, Britto LR, de Sa-Rocha LC, Palermo Neto J. Neural correlates of IgE-mediated food allergy. J Neuroimmunol. 2003;140:69–77. doi: 10.1016/s0165-5728(03)00166-8. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Da Prada M, Burri R, Honegger C. The immune response evokes changes in brain noradrenergic neurons. Science. 1983;221:564–566. doi: 10.1126/science.6867729. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015 doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016 doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt JP, Ringstad N. Toll-like Receptor Signaling Promotes Development and Function of Sensory Neurons Required for a C. elegans Pathogen-Avoidance Behavior. Curr Biol. 2015;25:2228–2237. doi: 10.1016/j.cub.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323–1333. doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palu G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Bulbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol. 1959;146:29–53. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cara DC, Conde AA, Vaz NM. Immunological induction of flavor aversion in mice. Braz J Med Biol Res. 1994;27:1331–1341. [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science. 2016 doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Cali G, D'Armiento FP, Rocco A, Nardone G, et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil. 2009;21:1209–e1112. doi: 10.1111/j.1365-2982.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil. 2011;23:e372–382. doi: 10.1111/j.1365-2982.2011.01748.x. [DOI] [PubMed] [Google Scholar]
- Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci U S A. 2001;98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pinto FA, Basso AS, Britto LR, Malucelli BE, Russo M. Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav Immun. 2005;19:52–60. doi: 10.1016/j.bbi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273:L93–103. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, Ahmed T, Gordon JI. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Emiliani V, Cohen AE, Deisseroth K, Hausser M. All-Optical Interrogation of Neural Circuits. J Neurosci. 2015;35:13917–13926. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc. 2012;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- Esposito G, Cirillo C, Sarnelli G, De Filippis D, D'Armiento FP, Rocco A, Nardone G, Petruzzelli R, Grosso M, Izzo P, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–925. doi: 10.1053/j.gastro.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, Zeltner N, Mica Y, El-Nachef W, Zhao H, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016 doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Pereira D, Arroz-Madeira S, Rodrigues-Campos M, Barbosa IA, Domingues RG, Bento T, Almeida AR, Ribeiro H, Potocnik AJ, Enomoto H, et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature. 2014;514:98–101. doi: 10.1038/nature13498. [DOI] [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. doi: 10.1172/JCI25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl 1):32–38. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922–928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K, Rutgeerts P, Ectors N, Mebis J, Penninckx F, Vantrappen G, Desmet VJ. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992;103:439–447. doi: 10.1016/0016-5085(92)90832-j. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446–456. doi: 10.1016/j.tins.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Grundmann D, Klotz M, Rabe H, Glanemann M, Schafer KH. Isolation of high-purity myenteric plexus from adult human and mouse gastrointestinal tract. Sci Rep. 2015;5:9226. doi: 10.1038/srep09226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, He S, Quintana E, Kim YG, Nunez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kioussis D, Pachnis V. Immune and nervous systems: more than just a superficial similarity? Immunity. 2009;31:705–710. doi: 10.1016/j.immuni.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Tang J, Little JP, Yu Y, Lammermann T, Lin CP, Germain RN, Cui M. Continuous volumetric imaging via an optical phase-locked ultrasound lens. Nat Methods. 2015;12:759–762. doi: 10.1038/nmeth.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz K, Momburg F, Otto HF, Moller P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am J Pathol. 1987;129:493–502. [PMC free article] [PubMed] [Google Scholar]
- Kuo CS, Krasnow MA. Formation of a Neurosensory Organ by Epithelial Cell Slithering. Cell. 2015;163:394–405. doi: 10.1016/j.cell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol (1985) 2001;91:1318–1326. doi: 10.1152/jappl.2001.91.3.1318. [DOI] [PubMed] [Google Scholar]
- Levine JD, Clark R, Devor M, Helms C, Moskowitz MA, Basbaum AI. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984;226:547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, Pasricha PJ, Tang SC. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol Motil. 2013;25:e324–338. doi: 10.1111/nmo.12115. [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Hokari R, Ueda T, Kurihara C, Hozumi H, Higashiyama M, Okada Y, Watanabe C, Komoto S, Nakamura M, et al. Physiological stress exacerbates murine colitis by enhancing proinflammatory cytokine expression that is dependent on IL-18. Am J Physiol Gastrointest Liver Physiol. 2011;301:G555–564. doi: 10.1152/ajpgi.00482.2010. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014;159:267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149:452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90–100. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- Nijhuis LE, Olivier BJ, de Jonge WJ. Neurogenic regulation of dendritic cells in the intestine. Biochem Pharmacol. 2010;80:2002–2008. doi: 10.1016/j.bcp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149:3309–3315. [PubMed] [Google Scholar]
- Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015;36:578–604. doi: 10.1016/j.it.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, Ji RR. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Harker N, Moreira-Santos L, Ferreira M, Alden K, Timmis J, Foster K, Garefalaki A, Pachnis P, Andrews P, et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci Signal. 2012;5:ra55. doi: 10.1126/scisignal.2002734. [DOI] [PubMed] [Google Scholar]
- Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Link EM, Liu LX, Kurz CL, Alloing G, Tan MW, Ray KP, Solari R, Johnson CD, Ewbank JJ. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr Biol. 2001;11:809–821. doi: 10.1016/s0960-9822(01)00241-x. [DOI] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Richardson DS, Lichtman JW. Clarifying Tissue Clearing. Cell. 2015;162:246–257. doi: 10.1016/j.cell.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer DJ, Carr DJ. A STING-dependent innate-sensing pathway mediates resistance to corneal HSV-1 infection via upregulation of the antiviral effector tetherin. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, Akira S, Uematsu S, Bignami P, Ceriani V, et al. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol. 2006;208:47–54. doi: 10.1002/jcp.20632. [DOI] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schemann M, Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013;144:698–704. e694. doi: 10.1053/j.gastro.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Kus L, Gong S, Heintz N. BAC transgenic mice and the GENSAT database of engineered mouse strains. Cold Spring Harb Protoc. 2013;2013 doi: 10.1101/pdb.top073692. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125:918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Beresford LJ, Bell EB, Miyan JA. Mobilisation of specific T cells from lymph nodes in contact sensitivity requires substance P. J Neuroimmunol. 2005;164:115–123. doi: 10.1016/j.jneuroim.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. Generating protective immunity against genital herpes. Trends Immunol. 2013;34:487–494. doi: 10.1016/j.it.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987;84:2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainaka K, Kubota SI, Suyama TQ, Susaki EA, Perrin D, Ukai-Tadenuma M, Ukai H, Ueda HR. Whole-body imaging with single-cell resolution by tissue decolorization. Cell. 2014;159:911–924. doi: 10.1016/j.cell.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Lovett-Barron M, Kauvar I, Andalman A, Burns VM, Sankaran S, Grosenick L, Broxton M, Yang S, Deisseroth K. SPED Light Sheet Microscopy: Fast Mapping of Biological System Structure and Function. Cell. 2015;163:1796–1806. doi: 10.1016/j.cell.2015.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc. 2014;9:1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012;1822:74–84. doi: 10.1016/j.bbadis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Chevalier J, Mahe MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976–987. doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, Barlow A, Pachnis V, Kioussis D. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446:547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kuhnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181:327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- Willemze RA, Luyer MD, Buurman WA, de Jonge WJ. Neural reflex pathways in intestinal inflammation: hypotheses to viable therapy. Nat Rev Gastroenterol Hepatol. 2015;12:353–362. doi: 10.1038/nrgastro.2015.56. [DOI] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci. 2011;46:527–534. doi: 10.1016/j.mcn.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MD, Tortoriello G, Hsueh B, Tomer R, Ye L, Mitsios N, Borgius L, Grant G, Kiehn O, Watanabe M, et al. Neuronal calcium-binding proteins 1/2 localize to dorsal root ganglia and excitatory spinal neurons and are regulated by nerve injury. Proc Natl Acad Sci U S A. 2014;111:E1149–1158. doi: 10.1073/pnas.1402318111. [DOI] [PMC free article] [PubMed] [Google Scholar]