Abstract
The prevalence of CKD has increased considerably over the past 2 decades. The rising rates of CKD have been attributed to known comorbidities such as diabetes, hypertension, and obesity; however, recent research has begun to explore the degree to which social, economic, and psychological factors have implications for the prevalence and progression of CKD, especially among high-risk populations such as African Americans. It has been suggested that stress can have implications for CKD, but this area of research has been largely unexplored. One contributing factor associated with the paucity of research on CKD is that many of the social, psychological, and environmental stressors cannot be recreated or simulated in a laboratory setting. Social science has established that stress can have implications for health, and we believe that stress is an important determinant of the development and progression of CKD. We draw heavily from the social scientific and social epidemiologic literature to present an intersectional conceptual frame specifying how stress can have implications for kidney disease, its progression, and its complications through multiple stressors and pathways.
Keywords: Kidney disease, Psychological stress, Social determinants of health, Minority health, Socioeconomic factors
CKD is fast becoming a global health problem. The prevalence of CKD remains high, whereas the incidence of ESRD or kidney failure continues to increase.1 If current trends continue, the global implications will be immense because the social and financial costs of care for ESRD patients are considerable. Current estimates for the United States indicate that the cost of ESRD exceeded $42 billion in 2009, more than doubling the cost in 2000.2 These trends indicate that kidney disease represents a serious threat to the world’s physical and financial health.
Results from a recent study reports that nearly 6 of every 10 Americans will experience moderate kidney disease in their lifetime3; however, the burden of kidney disease is not distributed equally across the population.4–7 The prevalence of ESRD for African Americans, for example, quadruples the corresponding prevalence for whites.2,8,9 The disparities in ESRD, CKD, and their complications (ie, anemia, bone disease, cardiovascular disease and death) have been linked to differing rates of kidney function decline.10 It has been suggested that the accelerated rates of disease progression among populations such as African Americans and the poor can be attributed to the high prevalence rates of metabolic conditions known to impair kidney function (ie, diabetes, hypertension). But, the presence of these comorbidities does not entirely explain accelerated kidney disease progression among those most at risk.7
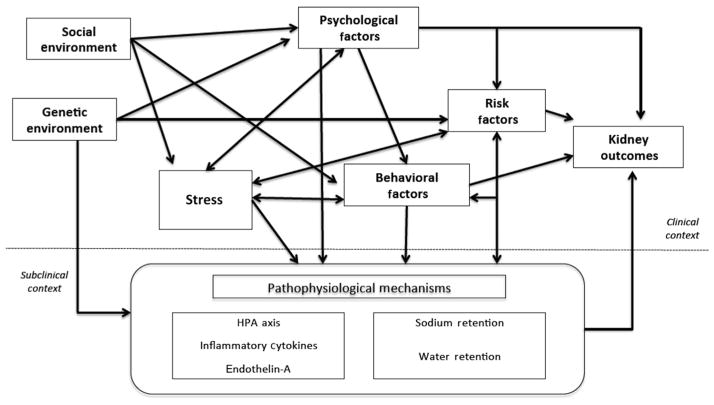
A growing segment of nephrologists has begun to examine sociologic and psychological factors potentially contributing to the incidence and progression of CKD and its complications.9,11–19 Stress is a factor that has been studied extensively by social and behavioral scientists; however, it has remained largely unexplored in the nephrology community. As such, we draw from the social science and social epidemiologic literature to illustrate how stress can have implications for CKD initiation, progression, and complications. Figure 1 depicts a heuristic model of the multiple pathways through which stress can have implications for CKD, its progression to ESRD, and premature mortality.
Figure 1.
Heuristic model of the association between environmental factors, stress, psychological factors, behavioral factors, CKD risk factors, and CKD progression and complications.
Stress: A Brief Overview
Stress refers to an environmental, social, or internal demand that results in a psychological, physiological, or behavioral response.20–22 These factors or stressors can lead to a state of physiological or emotional arousal that can affect physical and psychological health. Nearly 60 years ago, Selye23 observed that long-term exposure to noxious stressors were associated with tissue damage and disease in laboratory animals. Work examining stress in humans was bolstered considerably by a study by Holmes and Rahe,20 suggesting that an accumulation of major life eventswas related to illness. Literally, thousands of studies emerged from this early work, and nearly all have been founded on the premise that the accumulation of stressors tax or exceed the adaptive capacity of individual to a point where psychological or physiological responses to the stress condition can place them at risk for illness, injury, or disease.
This line of research has presented evidence, suggesting that stress can have implications for the development and progression of chronic diseases such as CKD. Stress has been shown to be associated with CKD risk factors such as hypertension, diabetes, or obesity.13,24–27 Scientists agree that stress can have implications for CKD and health outcomes; however, research examining the relationship between stress and kidney disease has been limited. One factor contributing to the scarcity of research in this area is that stress is a multidimensional concept that can be operationalized in multiple ways.13
Stress in the empirical literature has 3 forms: major life events, chronic strains, and daily hassles.21 Stressors categorized as major life events are occurrences that require considerable behavioral modification over a relatively short period of time (eg, death of a loved one, divorce, loss of a job). Chronic strains are persistent stressors that call for behavioral adjustment over a prolonged period of time (eg, poverty, disability). Daily hassles are stressors over the course of a day. These stressors (eg, traffic problems, interactions with rude people) are often regarded as minor; however, they can elicit physiological, psychological, and/or behavioral responses. Studies focusing on the impact of major life events or chronic strain represent the bulk of the literature examining the relationship between stress and health; however, a growing line of research has begun to examine the impact of daily hassles on health behaviors and outcomes. Much of the literature on stress and coping builds on interactional or transactional models of stress that highlight the social and cultural context of stress and coping, with each suggesting that perceptions of stressors are the primary determinants of behavior and health status.28–30
Stress, Pathophysiology, and Kidney Disease
Results from this extensive body of research have shown that stress can have an adverse effect on illness and disease, directly through physiological effects and indirectly through behaviors and practices that have implications for health.31,32 No study to date has developed a comprehensive stress model specifying the biologic pathways between stressors and the development and progression of CKD because most social, psychological, and environmental stressors cannot be recreated or simulated in a laboratory setting.33 Nonetheless, results from recent studies examining the relationship between acute stress and CKD risks factors suggest some biologic pathways through which stress may be associated with CKD and its complications.
CLINICAL SUMMARY.
Stress can have implications for kidney disease through multiple pathways.
Intersectionality is an approach that can be useful for identifying how stress has implications for race-, gender-, and age-related disparities in kidney disease development and progression.
Many studies examining the relationship between stress and pathophysiology have focused on outcomes such as blood pressure, heart rate, and vascular reactivity.34–41 Results from this line of research indicate that both blood pressure and heart rate increase and vascular reactivity decreases with most models of acute stress. These relationships between stress and pathophysiology are thought to be associated with alterations in the sympathetic/autonomic nervous system activity, the hypothalamic-pituitary-adrenal axis, inflammatory cytokines, and endothelin-A.42,43 These alterations suggest that pathologic link between stress, hypertension, and CKD is possible as kidney sympathetic nerves innervate all segments of the kidney, and neural mechanisms regulate sodium and water retention.44
It also has been suggested that stress may be linked to CKD via diabetes and insulin resistance. Environmental stressors have been found to be associated with the development of insulin resistance, metabolic syndrome, obesity, and ultimately type 2 diabetes.45,46 The biologic link is thought to involve alterations in the neuroendocrine system including the hypothalamic-pituitary-adrenal axis (increased glucocorticoid and other stress hormones) in addition to sympathetic nervous system factors and inflammatory cytokines.39,47
Stress also is thought to have implications in utero. The “Barker hypothesis” posits that disruption of the fetal environment or undernutrition translates into pathology. 48 Cell division and subsequent fetal growth are influenced by products of the hypothalamic-pituitary-adrenal axis and neuroendocrine hormones. Undernutrition brought about by stress can slow cell division in a manner that adversely affects the number of cells in particular organs and, ultimately, fetal growth. These and other alterations in the fetal environment are thought to contribute to low birth weight, a factor that has been associated with metabolic syndrome, type 2 diabetes, and CKD in adult life.49
Recent studies have also examined the extent to which genes are associated with CKD and ESRD in African Americans and other at risk groups. Genetic variants on the MYH9-APOL1 region on chromosome 22 have been found to account for the excessive risk of kidney disease among African Americans.50–60 Recent research has shown that African Americans with the APOL1 G1 and G2 risk alleles are more likely to develop CKD and progress faster to ESRD than their counterparts with 0 or 1 risk allele.51 These genetic variants are rare in white populations, suggesting that racial disparities in kidney disease may include a genetic component. Additional research is required to determine how other CKD risk factors and gene-environment interactions have implications for individuals with an apparent genetic risk for the development of kidney disease.
In summary, stress appears to enhance sympathetic nervous system activity, increase glucocorticoid secretion, and potentially increase levels of inflammatory cytokines. These factors contribute to higher prevalence of hypertension, diabetes, and vascular disease—all major risk factors for CKD. The physiological effects may be experienced in utero, exerting early influences that may further heighten the adult risk for CKD. In patients with CKD, the levels of another hormone, renalase, that metabolizes products of the sympathetic nervous system are lower.61,62 Therefore, it is plausible that chronic stressors result in unchecked increased sympathetic nervous system activity once CKD develops which sets in motion a vicious cycle.
Social Determinants, Stress, and Kidney Disease
Scientists agree that kidney disease risk can vary by population, and this variance is reflected in the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation used to estimate the glomerular filtration rate (GFR), a value indicating how well the kidneys are functioning. Specifically, the CKD-EPI formula uses serum creatinine levels along with patient characteristics (age, gender, and race) to calculate GFR [GFR = 141 × min (Scr/κ, 1)α× max(Scr/κ, 1)−1.209 × 0.993Age × (1.018 if female) × (1.1.59 if African American)].63 Social science literature has established that stress is associated with each of the individual characteristics included in the CKD-EPI formula. As such, stress can have implications for kidney disease and its progression to kidney failure through multiple stressors and pathways.
Stress and Age
Kidney disease has been thought to be a function of aging because kidney function decreases as individuals get older. However, the relationship between age and kidney disease appears to vary by group because African Americans experience kidney failure at younger ages and have greater incidence rates of ESRD at each decade of life compared with any other racial/ethnic group.9,10 It has been asserted that the progression of aging can vary by race, gender, socioeconomic status, and/or other sociodemographic characteristics, and some researchers suggest that stress can be attributed, in part, to the early onset of health conditions associated with old age, but the relationship between stress, aging, and health conditions has not been examined extensively. One explanation for the limited research in this area is that both aging and stress can be dynamic and the mechanisms associated with each can change as individuals get older. A life course approach offers a potential fruitful line of research, an optimal opportunity to identify critical points in the life course where stress has the most adverse effects and to understand the impact that the duration of stress has on health outcomes including CKD and ESRD.64,65 The life course approach incorporates 3 primary conceptual models (ie, critical period models, pathway models, and accumulation models) to account for temporal ordering of exposure variables and their inter-relationships.66 Cumulative disadvantage theory and weathering hypothesis are 2 theories associated with the life course approach that are useful for situating stress as a determinant of kidney disease.
Cumulative disadvantage theory highlights how early social and economic advantage or disadvantage shape the health outcomes of socially defined groups over time.67–71 Early life experiences set in motion a chain of risk or protective factors for short- and long-term outcomes. Some people have little or no stress in their life, and this advantage may compound over time to produce health benefits, whereas others have endured and continue to endure stress because of social or environmental factors. These social, environmental, and/or economic disadvantages also accumulate over time and may be potentially associated with CKD or ESRD.72
The weathering hypothesis states that the health status of African Americans begins to decline prematurely in early adulthood.73 The earlier health declines are a result of long-term and compound exposure to unfavorable social and environmental conditions.73 The weathering hypothesis provides a framework to assist in advancing our understanding of how stress contributes to disparities in the initiation of health conditions such as CKD or ESRD by elucidating how social environmental stressors can adversely affect health over time.74,75 Often, minorities experience life differently in the United States because of a large amount of social and structural inequalities that limit social, economic, and health-promoting opportunities that create stress, which can contribute to early onset of age-related diseases such as CKD and premature mortality.13,31,76,77
Stress and Gender
Gender disparities in kidney disease have been noted in nephrology literature as men have been shown to have a higher incidence and prevalence of ESRD.78 Men tend to have an earlier onset of ESRD, thereby starting dialysis at younger ages.79 A small line of research has attempted to identify biologic sources of men’s elevated risk for kidney disease and kidney failure, but the results have not been definitive.80,81 An emphasis on biologic differences suggests the nephrology community has focused primarily on sex differences rather than gender differences. Although “sex” and “gender” have been used interchangeably in a significant segment of the health science literature, these terms refer to different mechanisms and processes. Sex differences involve rigid classification (ie, male, female) by reproductive organs and their function and secondary sex characteristics. Gender distinctions involve categories (ie, masculine, feminine) based on psychological and behavioral outcomes that are shaped by the surrounding cultural and social environment. Stress can be linked to gender disparities in kidney disease because stressors arise from one’s social location and can have implications for health behaviors. Gender identities, roles, and relations are diverse and mediate health behaviors and practices82,83; thus, it is critical to examine how genders intersect with other identities and experiences to accurately explain health behaviors and health outcomes. An important source of stress is how people define themselves and the gendered roles they play in their families and communities.84–88
Health behaviors are used in daily interactions to help people negotiate social power and social status, and these same health practices can either undermine or promote health.85 Enacting gender through the interpretation and choice of coping behavior can both privilege and harm population groups.89 In addition to examining the types and sources of stress that affect health, it is critical to consider how people respond to and cope with stress.90,91
The methods chosen to cope with stressors are largely dictated by the nature of the problem. Modifiable and controllable stressors often are addressed with more problem-focused coping behaviors, whereas chronic, uncontrollable, and seemingly immutable situations often elicit more emotion-focused responses.90 The resources at peoples’ disposal shape their coping responses.91 Self-constructs and identity are important mediators of the association between stressful experiences and responses to stressors. These intrapersonal factors have important implications for health. Symbolic interactionism emphasizes the intrapersonal meaning construction process that shapes the coping process.91 This meaning is dynamic and evolves over time.
Some of the ways that men and women try to demonstrate their gender identity may change over the life course as the fundamental meaning of masculinity and femininity and the salience of different aspects of gender change over the life course. It is critical to consider how gendered notions, stressors, and strains change over time, particularly in relation to pressures to engage in key health behaviors that adversely affect CKD.83,92,93
Stress and Race
General interest in health disparities has raised the profile of research examining racial differences in the incidence, prevalence, and progression of kidney disease. The risks for CKD and ESRD among African Americans are considerably greater than other racial/ethnic groups.2,5,6,8,9,14,15,94,95 Explanations for racial disparities in CKD or ESRD have been primarily biological as they have been attributed to high levels of diabetes, hypertension, and obesity among African Americans.13 Health scientists recognize that the primary risk factors for CKD and ESRD are largely preventable and a growing segment of the nephrology community have begun to consider how factors such as stress contribute to the excess risks for kidney disease among African Americans.
Social scientists have highlighted a number of social environmental stressors encountered by African Americans and other marginalized racial groups. Recent epidemiologic research has shown that living in racially segregated, unstable, and poverty-stricken areas have consequences for the general health and well-being of residents.96–99 Economic deprivation has been a central component in epidemiologic studies including African Americans, and results have indicated that socioeconomic factors at the individual and community levels can have implications for kidney disease.6,16,18,19,95,100
It has been suggested that the primary stressors for African Americans are economic101,102; however, race-related stress can involve other factors such as racial discrimination, institutionalized racism, and prejudice.77,103 It has been nearly 40 years since the end of the civil rights movement, yet African Americans continue to report encounters with individuals and institutions that they believe to be racially motivated.104–108 Repeated exposures to interactions perceived to be discriminatory or racist, such as unfair treatment on the job, in the housing market, or at public events can elicit a physiological response, regardless of the intentions of others involved or the accuracy of the perception.76,109–111 Further, institutional racism, redlining, and race-based residential segregation are associated with African Americans being more likely than their white counterparts to live areas plagued by substandard educational resources, inadequate housing, family disruption, general disorder, pollution, and violence.112–114 Recent studies have shown that being exposed to discrimination and racism are related to CKD risk factors such as elevated blood pressure,110,115 and exposure to these stressful experiences adversely affects CKD progression and complications.
Stress, Intersectionality, and Kidney Disease: An Intriguing Avenue of Inquiry
Scientists agree that stress can have implications for health outcomes like kidney disease. However, the relationship between stress and chronic diseases like CKD has not been pursued extensively and few efforts to explain these relationships can explain how age, gender, and race pattern health outcomes. One factor contributing to the paucity of research in this area is that stress is a factor that has been conceptualized and operationalized in manner with limited utility for explaining racial disparities in CKD. Psychological research often presents stress as an acontextual psychological construct with universal mechanisms and pathways28,89; yet, characteristics (eg, race, ethnicity, life stage) that are socially meaningful in their societal context85,116,117 shape what aspects of life are deemed stressful. Stress appraisals have traditionally been based on subjective perceptions of threat, controllability, change, and other characteristics, but critiques of this approach argue that these measures conflate appraisals of events with reactions to these events.91 It has been critical to distinguish the social patterns of inequality from the cultural structure of shared meanings of stressful experiences.118 The social origins of meaning, particularly how meanings of stressful experiences are constructed over time, are essential to understand how stress has implications for disease risk. How people negotiate structural and cultural constraints is essential to explaining the mechanisms and pathways that connect stress and chronic diseases like CKD.13,91,118 Although it is certainly shaped by intrapersonal factors, meaning is a social product of the connotations that stimuli have for individuals and interpersonal relationships. Thus, it is not simply the objective social arrangements that act as stressors, but the subjective interpretation of these stressors that shape physiological and behavioral responses that can affect kidney disease risk, progression, and mortality.91,118
There may be systematic social causes of stress and psychological and physiological aspects of strain that vary by socially meaningful characteristics and social determinants of health.119 Stressors that arise from one’s unique position in social systems with unequal distributions of resources, opportunities, life chances, power, privilege, and prestige are best examined through an intersectional lens.120 Social status can shape how people construct meaning118 and provide access to social, political, and economic resources that can be used to cope with stressors.121–123 Understanding group differences in health outcomes such as kidney disease includes considering how social determinants of health intersect to shape people’s lives and experiences.13,123–125 Poverty, poor educational opportunities, underemployment and unemployment, incarceration, and social and racial discrimination all vary by socially defined characteristics and also influence the capacity of individuals to achieve and maintain good health.123,125–127 Intersectionality is a perspective that examines how socially defined and socially meaningful characteristics are inextricably intertwined, 84,117,128 thereby enabling scientists to consider multiple pathways through which stress has implications for disparities in the development and progression of kidney disease.
Over the past few years, the number of studies examining the relationship between nonbiologic factors and kidney disease has grown as a larger segment of the nephrology community has been interested in identifying and addressing modifiable risk factors.129 The study of factors, such as stress, is relatively new; however, we believe that this line of research can provide unique insights into the development and progression of CKD and ESRD. Methodologic development, however, is one area in need of considerable attention. Many of the factors discussed here, including stress, are measured with indices that are limited. Stress and environmental measurement development are critical for the development of testable intersectional models that specify why and how determinants of health combine to increase risk and the conditions through which environmental stressors “get under the skin.” Psychonephrology is a promising line of research because it has the potential for the development of biopsychosocial models through which seeming incommensurable relationships can be tested. Biopsychosocial model development can encourage researchers to give greater attention to the complexities associated with the multiple environments in which individuals are embedded. As such, future studies can explore pathways through which gene and social environmental factors interact to have implications for CKD development and progression. Empirical models emerging from this line of work may require scientists to use statistical methodology that accounts for multiple levels and unobserved factors simultaneously. Genetic predisposition along with economic, social, and psychological factors, including stress, can interact to have a detrimental impact on the health of individuals. As such, it may be necessary to use techniques such as structural equation and/or latent class models that enable scientists to observe how multiple stressors and pathways can have implications for the development of kidney disease and progression to kidney failure.
The results from this line of work can have implications for clinical or policy-oriented segments of the nephrology community. Identifying how stress affects group-specific processes associated with kidney disease would enable health care providers and public health officials to develop culturally and context-specific interventions to help reduce disparities in CKD development and progression in the short term and eliminate them in the long term. Stress has emerged as a critical determinant of health and a central barrier to the effective management of the risk and progression of illnesses associated with CKD (eg, diabetes, hypertension).130–133 Increasingly, stress management is being included in state-of-the-art interventions to prevent, manage, and reduce the risk of illnesses associated with CKD.134–138 Future research should similarly incorporate stress management in CKD prevention, management, and treatment interventions, and it will be important for these studies to incorporate an intersectional lens to most effectively understand and address how stress and other determinants of CKD are patterned.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Harwood L, Wilson B, Sontrop J. Sociodemographic differences in stressful experience and coping amongst adults with chronic kidney disease. J Adv Nurs. 2011;67(8):1779–1789. doi: 10.1111/j.1365-2648.2010.05605.x. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 3.Grams ME, Coresh J, Segev DL, Chow EKH. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook ED, Patel SR. Diabetic nephropathy in African-American patients. Curr Diab Rep. 2004;4(6):455–461. doi: 10.1007/s11892-004-0056-y. [DOI] [PubMed] [Google Scholar]
- 5.Norris K, Nissenson A. Racial disparities in chronic kidney disease: tragedy, opportunity, or both? Clin J Am Soc Nephrol. 2008;3(2):314–316. doi: 10.2215/CJN.00370108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19(7):1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 7.Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int. 2003;64(2):763–772. doi: 10.1046/j.1523-1755.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 8.Martins D, Tareen N, Norris K. The epidemiology of end-stage renal disease among African Americans. Am J Med Sci. 2002;323(2):65–71. doi: 10.1097/00000441-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Tareen N, Zadshir A, Martins D, Pan D, Nicholas S, Norris K. Chronic kidney disease in African American and Mexican American populations. Kidney Int. 2005;68(suppl 97):S137–S140. doi: 10.1111/j.1523-1755.2005.09723.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsu C-Y, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 11.Bruce MA, Beech BM, Crook ED, et al. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2010;55(6):1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce MA, Beech BM, Edwards CL, et al. Weight status and high blood pressure among low-income African American men. Am J Mens Health. 2011;5(3):255–260. doi: 10.1177/1557988310385447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce MA, Beech BM, Sims M, et al. Social environmental stressors, psychological factors, and kidney disease. J Investig Med. 2009;57(4):583–589. doi: 10.231/JIM.0b013e31819dbb91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crews DC, Pfaff T, Powe NR. Socioeconomic factors and racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33(5):468–475. doi: 10.1016/j.semnephrol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Merkin SS, Coresh J, Roux Diez AV, Taylor HA, Powe NR. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46(2):203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Plantinga LC. Socio-economic impact in CKD. Nephrol Ther. 2013;9:1–7. doi: 10.1016/j.nephro.2012.07.361. [DOI] [PubMed] [Google Scholar]
- 18.Shoham DA, Vupputuri S, Diez Roux AV, et al. Kidney disease in life-course socioeconomic context: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2007;49(2):217–226. doi: 10.1053/j.ajkd.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19(2):356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 21.Thoits PA. Stress, coping, and social support processes: where are we? What next? J Health Soc Behav. 1995;(Spec no):53–79. [PubMed] [Google Scholar]
- 22.Cohen S, Kessler RC, Gordon LU. Personality characteristics as moderators of the relationship between stress and disorder. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring Stress: A Guide for Health and Social Scientists. New York, NY: Oxford University Press; 1995. pp. 3–26. [Google Scholar]
- 23.Selye H. The Stess of Life. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- 24.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 25.Fremont A, Bird C. Social and psychological factors, physiological processes, and physical health. In: Bird C, Conrad P, Fremont A, editors. Handbook of Medical Sociology. Upper Saddle River, NJ: Prentice Hall; 2000. pp. 334–352. [Google Scholar]
- 26.Theorell T, Kareseck RA. Current issues in relating psychosocial job strain and cardiovascular disease research. J Occup Health Psychol. 1996;1(1):9–26. doi: 10.1037//1076-8998.1.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Tsurugano S, Nakao M, Takeuchi T, Nomura K, Yano E. Job stress strengthens the link between metabolic risk factors and renal dysfunction in adult men. Tohoku J Exp Med. 2012;226(2):101–108. doi: 10.1620/tjem.226.101. [DOI] [PubMed] [Google Scholar]
- 28.Skinner EA, Edge K, Altman J, Sherwood H. Searching for the structure of coping: a review and critique of category systems for classifying ways of coping. Psychol Bull. 2003;129(2):216–269. doi: 10.1037/0033-2909.129.2.216. [DOI] [PubMed] [Google Scholar]
- 29.Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. J Pers Soc Psychol. 1986;50(3):571–579. doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- 30.Folkman S. Personal control and stress and coping processes: a theoretical analysis. J Pers Soc Psychol. 1984;46(4):839–852. doi: 10.1037//0022-3514.46.4.839. [DOI] [PubMed] [Google Scholar]
- 31.Williams DR. The health of men: structured inequalities and opportunities. Am J Public Health. 2003;93(5):724–731. doi: 10.2105/ajph.93.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenzel L, Glantz K, Lerman C. Stress, coping, and health behavior. In: Glantz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education. 3. San Francisco, CA: John Wiley & Sons; 2002. pp. 201–239. [Google Scholar]
- 33.Glynn LM, Christenfeld N, Gerin W. Recreating cardiovascular responses with rumination: the effects of a delay between harassment and its recall. Int J Psychophysiol. 2007;66(2):135–140. doi: 10.1016/j.ijpsycho.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Clark R, Benkert RA, Flack JM. Large arterial elasticity varies as a function of gender and racism-related vigilance in black youth. J Adolesc Health. 2006;39(4):562–569. doi: 10.1016/j.jadohealth.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Harris CW, Edwards JL, Baruch A, et al. Effects of mental stress on brachial artery flow-mediated vasodilation in healthy normal individuals. Am Heart J. 2000;139(3):405–411. doi: 10.1016/s0002-8703(00)90083-8. [DOI] [PubMed] [Google Scholar]
- 36.Kop WJ, Verdino RJ, Gottdiener JS, O’Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events (1) J Am Coll Cardiol. 2001;38(3):742–749. doi: 10.1016/s0735-1097(01)01451-6. [DOI] [PubMed] [Google Scholar]
- 37.Kovach JA, Nearing BD, Verrier RL. Angerlike behavioral state potentiates myocardial ischemia-induced T-wave alternans in canines. J Am Coll Cardiol. 2001;37(6):1719–1725. doi: 10.1016/s0735-1097(01)01196-2. [DOI] [PubMed] [Google Scholar]
- 38.Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press. 2002;11(1):22–27. doi: 10.1080/080370502753543927. [DOI] [PubMed] [Google Scholar]
- 39.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Williams JE, Nieto FJ, Sanford CP, Couper DJ, Tyroler HA. The association between trait anger and incident stroke risk: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2002;30(1):13–19. doi: 10.1161/hs0102.101625. [DOI] [PubMed] [Google Scholar]
- 41.Williams JE, Nieto FJ, Sanford CP, Tyroler HA. Effects of an angry temperament on coronary heart disease risk: the atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(3):230–235. doi: 10.1093/aje/154.3.230. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovasc Dis. 2007;49(5):353–365. doi: 10.1016/j.pcad.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Spieker LE, Hurlimann D, Ruschitzka F, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105(24):2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 44.DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41(3 Pt 2):621–624. doi: 10.1161/01.HYP.0000047205.52509.8A. [DOI] [PubMed] [Google Scholar]
- 45.Auchincloss AH, Diez Roux AV, Brown DG, O’Meara ES, Raghunathan TE. Association of insulin resistance with distance to wealthy areas: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2007;165(4):389–397. doi: 10.1093/aje/kwk028. [DOI] [PubMed] [Google Scholar]
- 46.Diez Roux AV, Jacobs DR, Kiefe CI. Coronary artery risk developoment in young adults S. Neighborhood characteristics and components of the insulin resistance syndrome in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2002;25(11):1976–1982. doi: 10.2337/diacare.25.11.1976. [DOI] [PubMed] [Google Scholar]
- 47.Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17(5):350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 48.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips DI, Jones A, Goulden PA. Birth weight, stress, and the metabolic syndrome in adult life. Ann N Y Acad Sci. 2006;1083:28–36. doi: 10.1196/annals.1367.027. [DOI] [PubMed] [Google Scholar]
- 50.Behar DM, Rosset S, Tzur S, et al. African ancestry allelic variation at the MYH9 gene contributes to increased susceptibility to non-diabetic end-stage kidney disease in Hispanic Americans. Hum Mol Genet. 2010;19(9):1816–1827. doi: 10.1093/hmg/ddq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Genovese G, Tonna SJ, Knob AU, et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int. 2010;78(7):698–704. doi: 10.1038/ki.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicks PJ, Staten JL, Palmer ND, et al. Association analysis of the ephrin-B2 gene in African-Americans with end-stage renal disease. Am J Nephrol. 2008;28(6):914–920. doi: 10.1159/000141934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keene KL, Mychaleckyj JC, Leak TS, et al. Exploration of the utility of ancestry informative markers for genetic association studies of African Americans with type 2 diabetes and end stage renal disease. Hum Genet. 2008;124(2):147–154. doi: 10.1007/s00439-008-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40(10):1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipkowitz MS, Freedman BI, Langefeld CD, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luft FC. Renalase, a catecholamine-metabolizing hormone from the kidney. Cell Metab. 2005;1(6):358–360. doi: 10.1016/j.cmet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Li G, Wang P, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115(5):1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szanton SL, Thorpe RJ, Whitfield K. Life-course financial strain and health in African-Americans. Soc Sci Med. 2010;71(2):259–265. doi: 10.1016/j.socscimed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorpe RJ, Kelly-Moore J. Life course theories of race disparities: a comparison of cumulative dis/advantage perspective and the weathering hypothesis. In: LaVeist TA, Issac L, editors. Race, Ethnicity, and Health. 2. San Francisco, CA: Jossey-Bass; 2013. pp. 355–375. [Google Scholar]
- 66.Lohan M. How might we understand men’s health better? Integrating explanations from critical studies on men and inequalities in health. Soc Sci Med. 2007;65(3):493–504. doi: 10.1016/j.socscimed.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 67.Dannefer D. Cumulative advantage/disadvantage and the life course: cross-fertilizing age and social science theory. J Gerontol B Psychol Sci Soc Sci. 2003;58(6):S327–S337. doi: 10.1093/geronb/58.6.s327. [DOI] [PubMed] [Google Scholar]
- 68.Ferraro KF, Farmer MM, Wybraniec JA. Health trajectories: long-term dynamics among black and white adults. J Health Soc Behav. 1997;38(1):38–54. [PubMed] [Google Scholar]
- 69.Ferraro KF, Kelley-Moore JA. Cumulative disadvantage and health: long-term consequences of obesity? Am Sociol Rev. 2003;68(5):707–729. [PMC free article] [PubMed] [Google Scholar]
- 70.O’Rand AM. The precious and the precocious: understanding cumulative disadvantage and cumulative advantage over the life course. Gerontologist. 1996;36(2):230–238. doi: 10.1093/geront/36.2.230. [DOI] [PubMed] [Google Scholar]
- 71.Ferraro KF. Health and aging. In: Binstock RH, George LK, editors. Handbook of Aging and the Social Sciences. 6. Waltham, MA: Elsevier; 2006. pp. 238–256. [Google Scholar]
- 72.Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among African-Americans. Soc Sci Med. 1998;47(9):1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 73.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 74.Geronimus AT, Bound J, Waidmann TA, Colen CG, Steffick D. Inequality in life expectancy, functional status, and active life expectancy across selected black and white populations in the United States. Demography. 2001;38(2):227–251. doi: 10.1353/dem.2001.0015. [DOI] [PubMed] [Google Scholar]
- 75.Geronimus AT, Bound J, Waidmann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. N Engl J Med. 1996;335(21):1552–1558. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- 76.Williams DR. Racism and health: a research agenda. Ethn Dis. 1996;6(1–2):1–8. [PubMed] [Google Scholar]
- 77.Williams DR, Williams-Morris R. Racism and mental health: the African American experience. Ethn Health. 2000;5(3–4):243–268. doi: 10.1080/713667453. [DOI] [PubMed] [Google Scholar]
- 78.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49(3):800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 79.Iseki K. Gender differences in chronic kidney disease. Kidney Int. 2008;74(4):415–417. doi: 10.1038/ki.2008.261. [DOI] [PubMed] [Google Scholar]
- 80.Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33(5):383–392. doi: 10.1159/000320389. [DOI] [PubMed] [Google Scholar]
- 81.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5(suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Robertson S. “I’ve been like a coiled spring this last week”: embodied masculinity and health. Sociol Health Illn. 2006;28(4):433–456. doi: 10.1111/j.1467-9566.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 83.Creighton G, Oliffe JL. Theorising masculinities and men’s health: a brief history with a view to practice. Health Sociol. 2010;19(4):409–418. [Google Scholar]
- 84.Coles T. Negotiating the field of masculinity. Men Masc. 2009;12(1):30–44. [Google Scholar]
- 85.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50(10):1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 86.Griffith DM, Ellis KR, Allen JO. An intersectional approach to social determinants of stress for African American men: men’s and women’s perspectives. Am J Mens Health. 2013;7(suppl 4):19S–30S. doi: 10.1177/1557988313480227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammond WP. Taking it like a man: masculine role norms as moderators of the racial discrimination-depressive symptoms association among African American men. Am J Public Health. 2012;102(suppl 2):S232–S241. doi: 10.2105/AJPH.2011.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hooker SP, Wilcox S, Burroughs EL, Rheaume CE, Courtenay W. The potential influence of masculine identity on health-improving behavior in midlife and older African American men. J Mens Health. 2012;9(2):79–88. doi: 10.1016/j.jomh.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mankowski ES, Maton KI. A community psychology of men and masculinity: historical and conceptual review. Am J Community Psychol. 2010;45(1–2):73–86. doi: 10.1007/s10464-009-9288-y. [DOI] [PubMed] [Google Scholar]
- 90.Kershaw K, Rafferty J, Abdou C, Colbert S, Knight K, Jackson JS. Chronic stress and the role of coping behaviors in health inequalities. Annu Rev Gerontol Geriatr. 2009;29(1):161. [Google Scholar]
- 91.McLeod J, Lively K. Social psychology and stress research. In: McLeod J, Pescosolido B, editors. Mental Health, Social Mirror. New York, NY: Springer; 2007. pp. 275–303. [Google Scholar]
- 92.Bruce MA, Beech BM, Crook ED, et al. Sex, weight status, and chronic kidney disease among African Americans: the Jackson Heart Study. J Investig Med. 2013;61(4):701–707. doi: 10.231/JIM.0b013e3182880bf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Evans J, Frank B, Oliffe JL, Gregorty D. Health, illness, men, and masculinities (HIMM): a theoretical framework for understanding men and their health. J Mens Health. 2011;8(1):7–15. [Google Scholar]
- 94.Norris K, Mehrotra R, Nissenson AR. Racial differences in mortality and ESRD. Am J Kidney Dis. 2008;52(2):205–208. doi: 10.1053/j.ajkd.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African Americans versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 96.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sims M, Sims TL, Bruce MA. Race, ethnicity, concentrated poverty, and low birth weight disparities. J Natl Black Nurses Assoc. 2008;19(1):12–18. [PMC free article] [PubMed] [Google Scholar]
- 98.Sims M, Sims TL, Bruce MA. Urban poverty and infant mortality rate disparities. J Natl Med Assoc. 2007;99(4):349–356. [PMC free article] [PubMed] [Google Scholar]
- 99.Collins CA. Racism and health: segregation and causes of death amenable to medical intervention in major U.S. cities. Ann N Y Acad Sci. 1999;896:396–398. doi: 10.1111/j.1749-6632.1999.tb08152.x. [DOI] [PubMed] [Google Scholar]
- 100.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146(7):493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 101.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood) 2005;24(2):343–352. doi: 10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- 102.LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health. 2005;82(2 suppl 3):iii26–iii34. doi: 10.1093/jurban/jti061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams D, Yu Y, Jackson J, Anderson N. Racial differences in physical and mental health: socioeconomic status, stress, and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 104.Krieger N. Does racism harm health? Did child abuse exist before 1962? On explicit questions, critical science, and current controversies: an ecosocial perspective. Am J Public Health. 2003;93(2):194–199. doi: 10.2105/ajph.93.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones C. The impact of racism on health. Ethn Dis. 2002;12(1):S2, S10–S13. [PubMed] [Google Scholar]
- 106.Jones C. Levels of racism: a theoretic framework and a Gardener’s tale. Am J Public Health. 2000;90(8):1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feagin JR, Sikes MP. Living with Racism: The Black Middle Class Experience. Boston, MA: Beacon; 1994. [Google Scholar]
- 108.Feagin JR. The continuing significance of race: antiblack discrimination in public places. Am Sociol Rev. 1991;56(1):101–116. [Google Scholar]
- 109.Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315–331. doi: 10.1097/00000441-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 110.Williams DR, Neighbors HW. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis. 2001;2001(11):800–816. [PubMed] [Google Scholar]
- 111.McNeilly MD, Anderson NB, Armstead CA, et al. The Perceived Racism Scale: a multidimensional assessment of the experience of white racism among African Americans. Ethn Dis. 1996;6(1–2):154–166. [PubMed] [Google Scholar]
- 112.Wilson WJ. The Truly Disadvantaged: The Inner City, the Underclass and Public Policy. Chicago, IL: University of Chicago Press; 1987. [Google Scholar]
- 113.Massey DS. Segregation and stratification. Du Bois Rev. 2004;1(1):7–25. [Google Scholar]
- 114.LaViest TA, Wallace JM. Health risk and inequitable distribution of liquor stores in African American neighborhoods. Soc Sci Med. 2000;51(4):613–617. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 115.Guyll M, Matthews KA, Bromberger JT. Discrimination and unfair treatment: relationship of cardiovascular reactivity among African American and European women. Health Psychol. 2001;20(5):315–325. doi: 10.1037//0278-6133.20.5.315. [DOI] [PubMed] [Google Scholar]
- 116.Snow RC. Sex, gender, and vulnerability. Glob Public Health. 2008;3(suppl 1):58–74. doi: 10.1080/17441690801902619. [DOI] [PubMed] [Google Scholar]
- 117.Griffith DM. An intersectional approach to men’s health. J Mens Health. 2012;9(2):106–112. [Google Scholar]
- 118.McLeod J. The meanings of stress expanding the stress process model. Soc Ment Health. 2012;2(3):172–186. [Google Scholar]
- 119.Aldwin C. Stress, Coping, and Development: An Integrated Perspective. New York, NY: The Guiford Press; 2007. [Google Scholar]
- 120.Pieterse AL, Carter RT. An examination of the relationship between general life stress, racism-related stress, and psychological health among black men. J Couns Psychol. 2007;54(1):101–109. [Google Scholar]
- 121.Treadwell HM, Ro M. Poverty, race, and the invisible men. Am J Public Health. 2003;93(5):705–707. doi: 10.2105/ajph.93.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Treadwell HM, Young AM, Rosenberg MT. Want of a place to stand: social determinants and Men’s health. J Mens Health. 2012;9(2):104–105. [Google Scholar]
- 123.Treadwell HM, Braithwaite K. Men’s health: a myth or a possibility. J Mens Health Gend. 2005;2(3):382–386. [Google Scholar]
- 124.Pease B. Racialised masculinities and the health of immigrant and refugee men. In: Broom A, Tovey P, editors. Men’s Health: Body Identity, and Context. Hobboken, NJ: John Wiley and Sons; 2009. [Google Scholar]
- 125.Young AM. Poverty and men’s health: global implications for policy and practice. J Mens Health. 2009;6(3):279. [Google Scholar]
- 126.Young AM, Meryn S, Treadwell HM. Poverty and men’s health. J Mens Health. 2008;5(3):184–188. [Google Scholar]
- 127.Xanthos C, Treadwell HM, Holdern KB. Social determinants of health among African American men. J Mens Health. 2010;7(1):11–19. [Google Scholar]
- 128.Warner DF, Brown TH. Understanding how race/ethnicity and gender define age-trajectories of disability: an intersectionality approach. Soc Sci Med. 2011;72(8):1236–1248. doi: 10.1016/j.socscimed.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kimmel PL, Cohen SD, Peterson RA. Depression in patients with chronic renal disease: where are we going? J Ren Nutr. 2008;18(1):99–103. doi: 10.1053/j.jrn.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 130.Jackson JS, Knight KM. Race and self-regulatory health behaviors: the role of the stress response and the HPA axis. In: Schaie KW, Carstensten LL, editors. Social Structure, Aging and Self-regulation in the Elderly. New York, NY: Springer; 2006. pp. 189–240. [Google Scholar]
- 131.Merritt MM, Bennett GG, Williams RB, Sollers JJ, III, Thayer JF. Low educational attainment, John Henryism, and cardiovascular reactivity to and recovery from personally relevant stress. Psychosom Med. 2004;66(1):49–55. doi: 10.1097/01.psy.0000107909.74904.3d. [DOI] [PubMed] [Google Scholar]
- 132.Turner RJ. Understanding health disparities: the promise of the stress process model. In: Avison WR, editor. Advances in the Conceptualization of the Stress Process: Essays in Honor of Leonard I. Pearlin. New York, NY: Springer; 2009. pp. 3–21. [Google Scholar]
- 133.Turner RJ, Avison WR. Status variations in stress exposure: implications for the interpretation of research on race, socioeconomic status, and gender. J Health Soc Behav. 2003;44(4):488–505. [PubMed] [Google Scholar]
- 134.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Svetkey LP, Ard JD, Stevens VJ, et al. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity (Silver Spring) 2012;20(9):1820–1828. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Svetkey LP, Erlinger TP, Vollmer WM, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. J Hum Hypertens. 2005;19(1):21–31. doi: 10.1038/sj.jhh.1001770. [DOI] [PubMed] [Google Scholar]
- 137.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 138.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16(6):1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]